Significance
Periodic pigment patterns, such as spots and stripes, are often observed in animal skin; however, we should learn more the molecular and cellular mechanisms for patterning. Here, we examined the black and yellow stripes in the Japanese quail avian model. Using melanocyte transplantation, we found that the periodic pattern is formed by melanocytes cell autonomously. Genetic modulations of gap junction channels in melanocytes alter the periodic pattern. Furthermore, the melanocytes instruct the dermal cells to express agouti signaling protein, which induces pheomelanin (yellow pigment) by antagonizing the melanocortin 1 receptor on melanocytes. Our study suggests that melanocytes play an instructive role in melanocyte periodic patterning and that melanocytes and dermal cells may interact further to produce the final stable pigment pattern.
Keywords: Japanese quail, stripe pattern, melanocytes, ASIP, gap junction
Abstract
Animal skin pigment patterns are excellent models to study the mechanism of biological self-organization. Theoretical approaches developed mathematical models of pigment patterning and molecular genetics have brought progress; however, the responsible cellular mechanism is not fully understood. One long unsolved controversy is whether the patterning information is autonomously determined by melanocytes or nonautonomously determined from the environment. Here, we transplanted purified melanocytes and demonstrated that melanocytes could form periodic pigment patterns cell autonomously. Results of heterospecific transplantation among quail strains are consistent with this finding. Further, we observe that developing melanocytes directly connect with each other via filopodia to form a network in culture and in vivo. This melanocyte network is reminiscent of zebrafish pigment cell networks, where connexin is implicated in stripe formation via genetic studies. Indeed, we found connexin40 (cx40) present on developing melanocytes in birds. Stripe patterns can form in quail skin explant cultures. Several calcium channel modulators can enhance or suppress pigmentation globally, but a gap junction inhibitor can change stripe patterning. Most interestingly, in ovo, misexpression of dominant negative cx40 expands the black region, while overexpression of cx40 expands the yellow region. Subsequently, melanocytes instruct adjacent dermal cells to express agouti signaling protein (ASIP), the regulatory factor for pigment switching, which promotes pheomelanin production. Thus, we demonstrate Japanese quail melanocytes have an autonomous periodic patterning role during body pigment stripe formation. We also show dermal agouti stripes and how the coupling of melanocytes with dermal cells may confer stable and distinct pigment stripe patterns.
Animal skin pigment patterns, such as periodic leopard spots and zebra stripes, represent some of the most amazing phenomena observed in nature, which have fascinated biologists and nonbiologists. The mechanisms of pigment patterning have been studied by mathematical and empirical approaches. Studies on zebrafish stripe patterning have led investigators to propose that the pattern is formed by pigment cell interactions that satisfy a Turing-type model (1, 2). Mammalian genetic studies performed on horses, zebras, cheetahs, and chipmunks have identified some of the molecules involved in this process (3–5). Although theoretical models and the genetic backgrounds in the pigment patterning are well studied, how the pigment-related genes control the cell–cell interactions that generate the pigment pattern is largely unknown. Avian species present an excellent model system to answer these questions because of their extraordinarily diverse micropigment patterns within feathers (6) and embryonic manipulability that allows analyses of cell–cell interactions leading to macropigment patterning throughout the body.
Japanese quail (JQ), a model avian system, develop longitudinal black and yellow stripes on their back skin during embryonic stages (Fig. 1A and SI Appendix, Fig. S1A). Black stripes and yellow stripes are composed of melanocytes with eumelanin and melanocytes with pheomelanin, respectively (7) (Fig. 1B). Previous studies asked whether the melanocyte population contains the patterning information autonomously or if it obtains patterning information nonautonomously from the environment, such as keratinocytes and dermal cells, for example. To this end, neural crest lineage cells (NCCs), which give rise to melanocyte lineage cells, have been transplanted between different species of birds. One group transplanted JQ NCCs into White Leghorn (WL) chickens as the host, which exhibit no pigment pattern. The chimeras exhibited a JQ-like stripe pattern on their back skin, suggesting that NCCs contain patterning information (8). Another group performed similar experiments with different observations; they showed JQ wing buds grafted to guineafowl embryos before NCC invasion can exert the JQ pigmentation pattern, thus suggesting that environmental tissues can provide patterning information (9, 10). Because NCCs differentiate into a variety of cell types, such as sensory neurons and Schwann cells, in addition to melanocytes, and because transplantation conditions may not be identical, it is important to use purified melanocytes for transplantation to investigate their role in the pigment patterning.
Fig. 1.
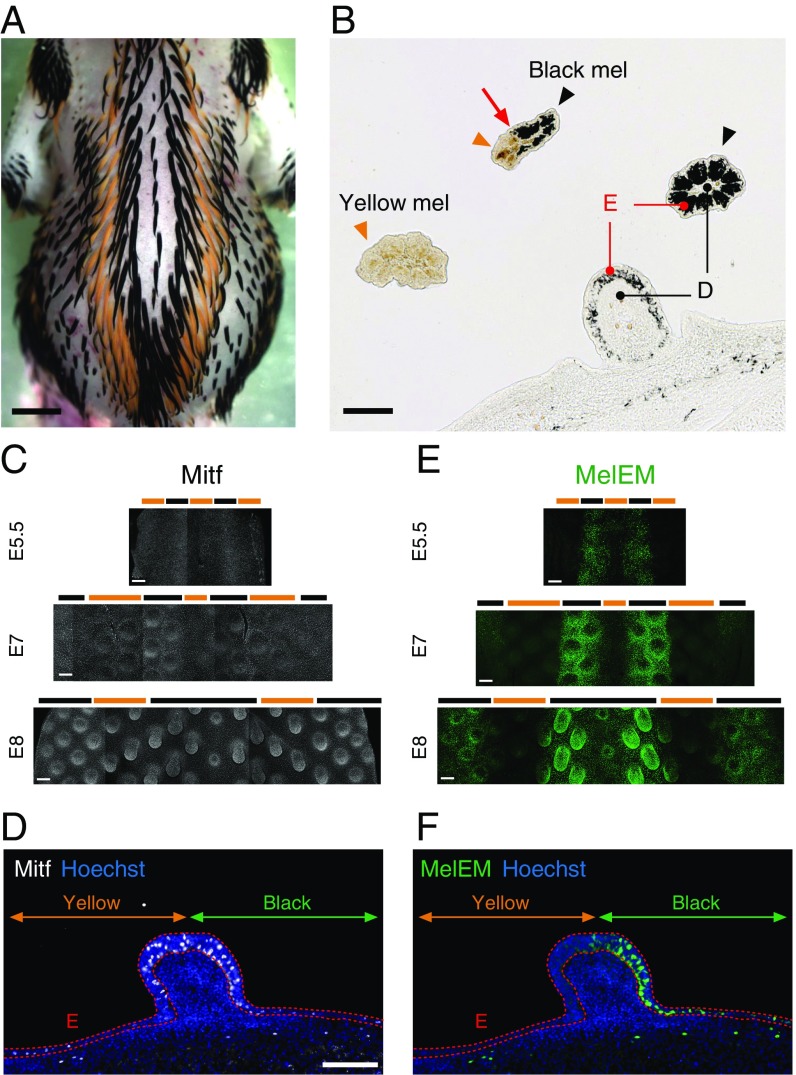
There are melanin, pheomelanin, and MelEM longitudinal stripes in JQ. (A) Dorsal view of an E10 JQ embryo shows multiple longitudinal melanin and pheomelanin stripes. (B) Transverse section image of skin and feathers in an E10 JQ. Black arrowheads and yellow arrowheads indicate melanocytes with eumelanin (Black mel) and pheomelanin (Yellow mel), respectively. The red arrow indicates the color boundary. D, dermis; E, epidermis. Immunofluorescent images of Mitf (C) and MelEM (E) during E5.5–E8 JQ skin development. Black and yellow bars represent the prospective black and yellow regions, respectively. (D and F) Immunofluorescent images of Mitf (white) and MelEM (green) in the transverse section of an E8 JQ. Dotted red lines delineate the epidermal layer (E). Green and yellow lines represent the prospective black (MelEM+) and yellow (MelEM−) stripes. (Scale bars: A, 2 mm; B and D, 100 μm; C and E, 200 μm.)
In this study, we purified a melanocyte population in vitro for use in transplantation to investigate its cell autonomy in periodic pigment patterning. WL embryos transplanted with JQ melanocytes showed a periodic pigment pattern. Furthermore, genetic disruption of connexin40 (cx40), a component of gap junctional channels, in the melanocytes resulted in pigment pattern changes. Finally, the melanocytes were able to instruct the dermal cells to express agouti signaling protein (ASIP), a regulatory factor of melanin synthesis. These results suggest that melanocytes have an autonomous role in the generation of periodic pigment pattern.
We should clarify here that periodic pigment patterning has a broad meaning in pigment pattern formation and may manifest as spots or stripes seen in different orientations or scales [across the body or stripes within a single feather (6)]. In developing quail embryos, the longitudinal black/yellow long stripes in the trunk, periodically arranged in the anterior (A)-to-posterior (P) direction, are the dominant pigment patterns. The role of somites in the formation of these longitudinal stripes is recently reported (11). The autonomous role of melanocytes in periodic pigment patterning reported in this study contributes to the generation of these longitudinal stripes, but can also induce different periodic pigment patterns as shown by the analytical studies here.
Results
Melanoblast/Cyte Early Marker-Positive Melanocytes Reside in Black Stripes and ASIP+ Dermal Cells Are Present in Yellow Stripes.
JQ have alternating black and yellow longitudinal stripes on their back skin, which starts to develop from embryonic stages and is maintained during the juvenile period (Fig. 1A and SI Appendix, Fig. S1A). Eventually, the visible black stripes and the yellow stripes are composed of melanocytes with eumelanin and melanocytes with pheomelanin, respectively (7) (Fig. 1B). The color boundary crosses a feather bud, suggesting that the mechanism guiding striped pigment pattern formation is independent of that guiding the distribution of feather buds (SI Appendix, Fig. S1B). To differentiate whether the stripe is due to a melanocyte distribution pattern or a melanin differentiation pattern, we first investigated the distribution of melanocyte progenitor cells with immunostaining against microphthalmia-associated transcription factor (Mitf) and melanoblast/cyte early marker (MelEM). The MelEM signal first emerges in neural crest-derived melanoblasts and melanocytes at embryonic day (E) 4 and is maintained during the embryonic stage (12, 13). During E5–E8, Mitf+ cells are widely distributed over the dorsal skin (Fig. 1 C and D). However, at this time, MelEM-positive cells already form bilateral stripes at E5 (SI Appendix, Fig. S2). Later, stripes become clear and additional bilateral stripes appear. Those MelEM-positive stripes correspond to prospective black stripes (14) (Fig. 1 E and F). This suggests that these pigmented stripes are initially produced via melanogenesis differentiation control.
In mature pigment stripes, the black and yellow regions are composed of melanocytes with eumelanin and melanocytes with pheomelanin, and the switch between colors is regulated by ASIP (6, 15–17) (SI Appendix, Fig. S3). Antagonistic binding of ASIP to melanocortin 1 receptor promotes pheomelanin (yellow pigment) production. We traced ASIP expression over time and found it is highly dynamic (Fig. 2):
-
i)
First, ASIP expression was detected in the ventral part of the body at E3.
-
ii)
Around E4, two lateral wide stripes flanking the midlines (A1) appeared in the dorsal part of the body.
-
iii)
At E5, a new ASIP stripe appeared in the midline (A2), while the two earlier lateral wide stripes (A1) regressed back toward the tail transiently.
-
iv)
At E5.5, two new lateral and sharper stripes (A3) appeared between the A1 and A2 stripes. After E6, this striped expression broke into spots, which corresponds to the prospective yellow stripes.
Fig. 2.
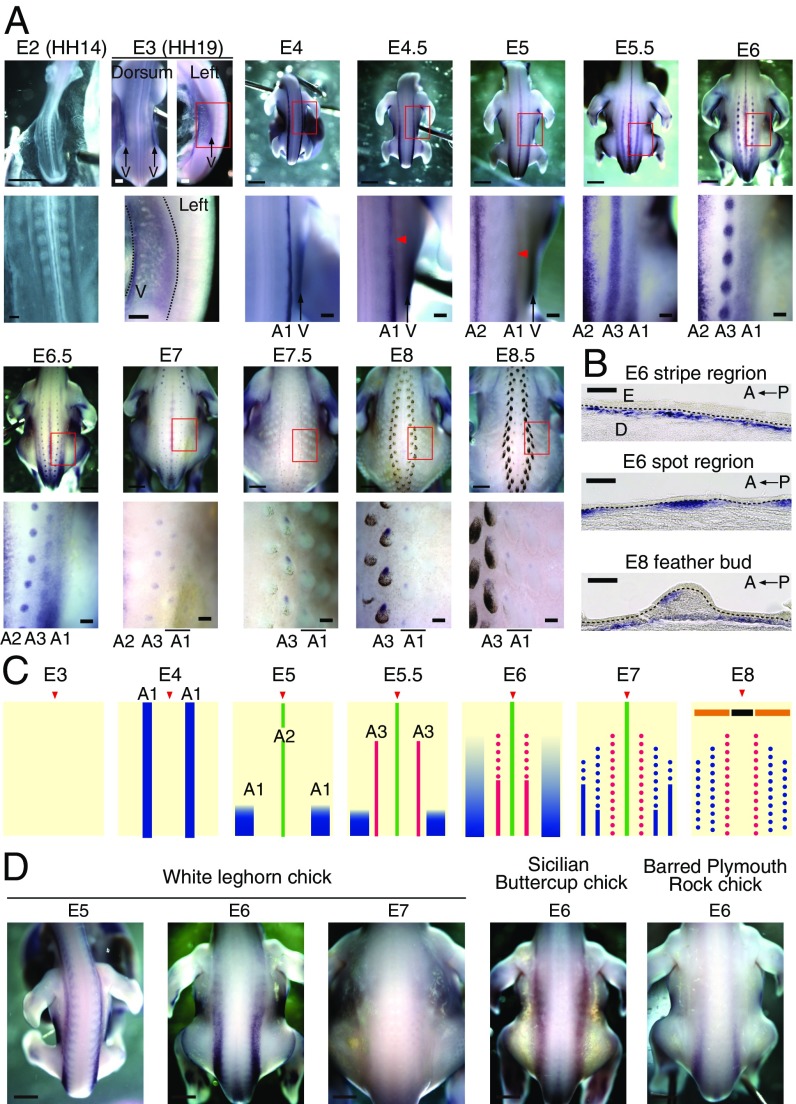
ASIP expression pattern in JQ and chicken strains. (A) Whole-mount in situ hybridization (WISH) of ASIP in developing JQ embryos. A1, first stripes of ASIP appear in the dorsal sides at E4; A2, second stripes of ASIP appear in the midline at E5; A3, third stripes of ASIP are located between A1 and A2 at E5.5; V, ventral ASIP appears at the E3 (HH19) stage. Arrowheads indicate the temporary fading of A1 expression. (B) Sagittal section of ASIP WISH in JQ. Dotted lines delineate the boundaries of the epidermis (E) and dermis (D). (C) Schematic drawings showing changes in the dorsal ASIP expression pattern during development. Arrowheads show the midline. A1 (blue lines), A2 (green lines), and A3 (red lines) are shown. Spots indicate localized ASIP expression in feather buds. (D) WISH of ASIP in chicken strains. (Scale bars: A, whole view and D, 2 mm; A, enlarged view and whole view of E3, 200 μm; B, 100 μm.)
ASIP is present in the dermis, and its expression is eventually localized to the anterior part of the feather dermis (Fig. 2B). While ASIP expression in JQ is highly dynamic (Fig. 2C), that in chicken strains is relatively static. These strains exhibited similar broader striped ASIP expression, but the expression disappeared in WL chicks after E7 (Fig. 2D).
Taken together, our data indicate dynamic changes in molecules associated with pigment pattern formation. First-round ASIP expression (A1) initiation appears be earlier than that of MelEM. The second- and third-round ASIP stripes (A2 and A3) temporally coincide with the MelEM stripes (Fig. 2A and SI Appendix, Fig. S2). To understand the mechanism regulating this patterning, we studied the relationship of these different stripes and the molecules involved in their control.
JQ Melanocytes Have an Ability to Form the Periodic Pigment Pattern.
Taking a systemic approach, we began by evaluating whether quail skin has the information to form stripe patterns. We transplanted a piece of JQ skin (E5) to a WL embryo (E6) (SI Appendix, Fig. S4). Chimeras were harvested at E11. At this stage, there is no rejection and black/yellow stripes form, although the pattern is not as regular as that seen in vivo. The skin contains keratinocytes, melanocytes, and dermal cells, and we need to sort out the contribution of each cell type to pigment patterning.
To determine the tissue controlling periodic pigment patterning, we used the epithelium mesenchymal recombined explant culture system to decide whether epithelium (which has both keratinocytes and melanocytes) or mesenchyme (dermal fibroblast) is in control of the pigment stripe patterns (Fig. 3A, schematic drawing) (18). Pigment pattern can be judged as black (eumelanin) and yellow (pheomelanin) or no color stripes, the orientation of the stripes, and their periodicity. JQ epithelium and mesenchyme were separated at E6 or E7; the epithelium was then rotated 90° relative to the mesenchyme and recombined with the mesenchyme. After 3–4 d of incubation, periodically arranged pigment stripes emerged in the explant. In the control culture, the middle black stripe (marked by broken lines in Fig. 3A) can be seen to follow the AP direction. Flanking it is a zone of much less pigmentation (faint yellow), and then a zone of pigmentation toward the periphery (Fig. 3A, Left). When the epithelium was rotated and then recombined with the dermis, the direction of the periodic black stripes followed the AP axis of the epithelium (Fig. 3A, Right). Then, the zone of yellow buds and the peripheral zone of black buds are in parallel to the AP axis of the epithelium as well. Please note that the direction of the feather bud is known to follow the AP axis of the epithelium, but this is not the issue here. At this stage, the epithelium contains keratinocyte and melanocyte progenitors. Therefore, our data suggest that keratinocytes and melanocytes contain the patterning information.
Fig. 3.
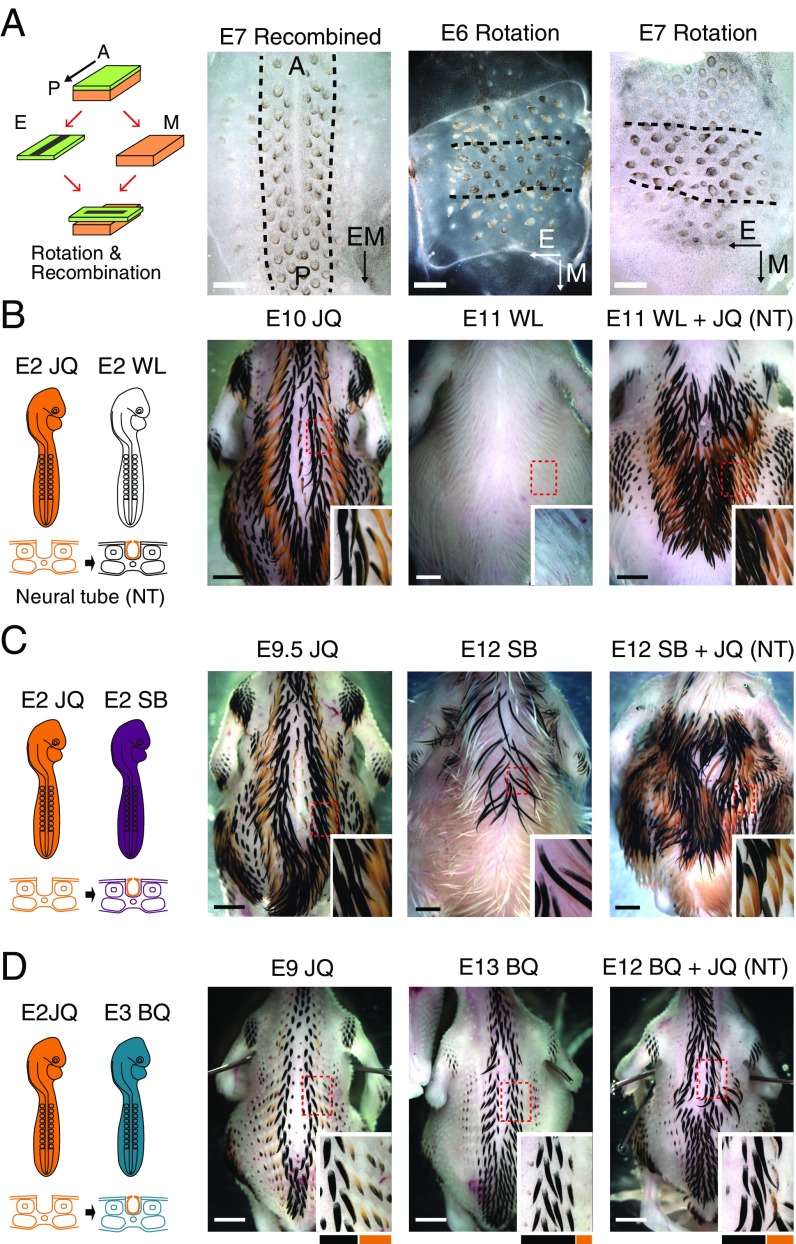
Epithelium rotation experiments in JQ and heterospecific neural tube transplantation. (A) Schematic drawing and photographs of the experiments. The epithelium (E) and mesenchyme (M) from E6 or E7 JQ skin were separated, recombined with or without 90° rotation, and then cultured for 3–4 d. We examined the direction and periodicity of these stripes (broken lines in photographs outline the middle black stripe). Black and white arrows in the lower right of each photograph indicate the AP polarity of epithelium or mesenchyme. (Left) Control recombined skin with epithelium and mesenchyme in the same orientation exhibited a pigmented stripe along the AP axis. When the epithelium was rotated 90° and then recombined, the recombinants exhibited a black stripe along the AP axis of the epithelium. Skin from an E6 embryo (Center) and an E7 embryo (Right) were used for the culture. (B–D) Transplantation of neural tube (NT) between birds with different pigmentation patterns. (B) Transplantation of JQ NT into a WL chick at E2. The chimera chicks showed a JQ-like pigment pattern (n = 7). (C) Transplantation of JQ NT into a SB chick at E2. The chimera chicks showed a JQ-like pigment pattern (n = 2). (D) Transplantation of E2 JQ NT into a BQ chick at E3. The chimera quail showed JQ-like thinner black stripes (n = 2). (Scale bars: A, 1 mm; B–D, 2 mm.)
However, it is possible that fate commitment of melanocyte pigmentation by dermal agouti is already finished by at least E6; thus, rotation of tissue does not change the stripe polarity of epithelium. We then performed neural tube transplantations from quail (E2) to chickens (E2) (19). This enables us to evaluate whether the NCCs, including melanocytes, are involved in the patterning. Embryos harvested at E11 show clear black and yellow stripes (Fig. 3B). To further verify whether NCCs indeed have an ability to form the stripe pattern, we performed transplantation experiments between different avian species. When we grafted the JQ neural tube into Sicilian Buttercup (SB) chicks, which have a different pigment pattern from JQ, the host SB chicks showed JQ-like pigment patterning (Fig. 3C). Furthermore, we transplanted the JQ neural tube into bobwhite quail (BQ), which have broader black stripes than those of JQ. The host BQ showed thinner black stripes similar to JQ in the area affected by the transplantation (Fig. 3D). These results suggest that donor NCCs contain patterning information.
NCCs give rise not only to melanocytes but also to sensory neurons or Schwann cells, which are observed in the skin tissue (20). To exclude the possibility that those cells contribute to the pigment patterning, we isolated the melanocyte population for transplantation experiments (21) (Fig. 4A). Melanocyte lineage cells were labeled by the electroporation of Tol2 plasmid expressing LynEGFP and neomycin resistance genes under the control of a ubiquitous promoter (CAGGS) (22, 23). Then, dissociated cells of E8 skin were incubated in medium 254, which is optimized for melanocyte growth (21). All surviving cells in vitro have gray pigmentation, most of which showed a Mitf+ signal (>97%), demonstrating the success of the melanocyte isolation (Fig. 4 B and C). In this culture, there are two melanocyte subpopulations defined by MelEM intensity (Fig. 4D). The melanocytes were made into small aggregates and transplanted between the E2 WL chick neural tube and presomitic mesoderm. The chimera exhibited a black and yellow periodic pattern (Fig. 4E) in which the transplanted melanocytes successfully differentiated into black and yellow types of melanocytes that sorted to become spatially segregated and formed repeated patterns (Fig. 4 F and G). Transplanted melanocytes covered both feathers and skin in the host epidermis and were preferentially pigmented in feather buds in the same way as in normal JQ (Fig. 4 H and I and SI Appendix, Fig. S5). The chimeras exhibited the periodic pigment pattern with individual variations, suggesting prepatterning in the dermis and/or epidermis of the host is unlikely (SI Appendix, Fig. S6). Unlike intact JQ skin with longitudinal stripes, most chimeras exhibited unclear AP polarity in the pigment pattern, suggesting a melanocyte-autonomous role in periodic patterning. Hereafter, we call a periodic pattern without polarity a “periodic pattern” and a periodic pattern with polarity a “stripe” in this article.
Fig. 4.
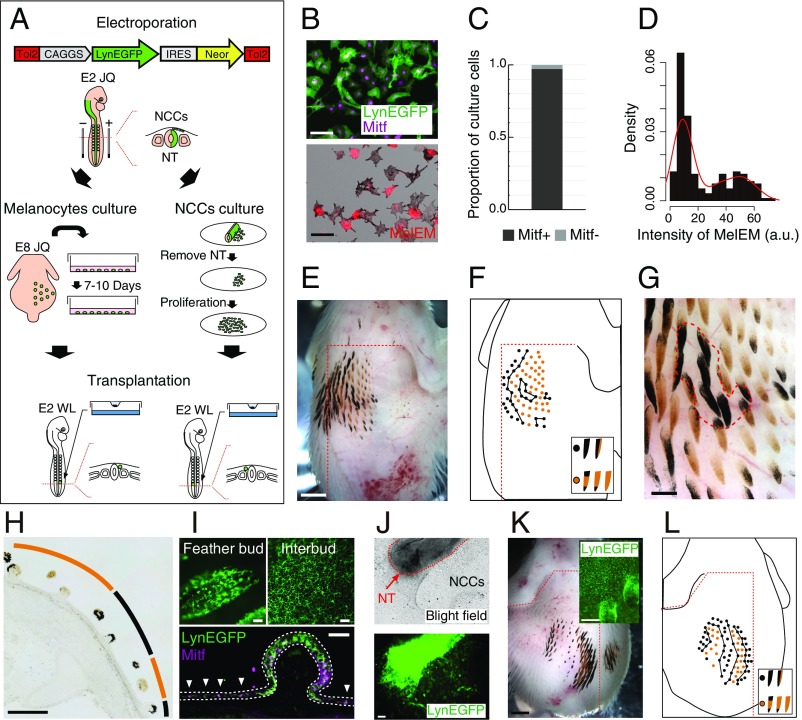
JQ melanocyte lineage cells can form periodic pigment patterns. (A) Schematic diagram describing the culture of melanocytes and NCCs for transplantation. NT, neural tube. (B) Immunofluorescent images of Mitf (purple) and MelEM (red) in JQ melanocytes incubated for 8 d. (C) Proportion of cells in the Mitf± state in cultured JQ melanocytes [n = 3,036 (Mitf+) and n = 90 (Mitf−) from three embryos]. (D) Intensity of anti-MelEM immunofluorescence from JQ melanocytes (n = 190). (E) Pigment pattern of an E11 WL chick grafted with JQ melanocytes (n = 21). (F) Feather color type distribution of E. (Inset) Feather color classification criteria. (G) Enlarged view of E. (H) Transverse section of E. (I, Upper) Transplanted melanocytes expressing LynEGFP (membrane-tethered EGFP) were observed in the E11 WL feather bud and interbud. (I, Lower) Transverse section of the chimera stained with Mitf antibody. White dotted lines indicate the epidermal layer, and arrowheads indicate melanocytes in interbuds. (J) JQ NCCs emigrated from NT expressing LynEGFP. (K) Pigment pattern of E11 WL chick grafted with JQ NCCs (n = 3). (Inset) LynEGFP signal was detected in melanocytes. (L) Feather color type distribution of K. (Scale bars: E and K, whole view, 2 mm; K, Insets, 200 μm; B and J, 100 μm; G and H, 500 μm; I, 50 μm.)
In the above experiments, melanocytes isolated from E8 skin were already exposed to the ASIP signal from E4, and they may be committed to pheomelanin production. To rule out that this early event could guide the pigment patterning, we isolated melanocytes from the neural tube of E2 embryos before ASIP expression for transplantation (Fig. 4A). NCCs (melanocyte lineage cells) emigrating from the neural tube labeled with LynEGFP-neomycin were cultured in medium 254 and grafted into E2 WL chicks (Fig. 4J). The chimera displayed a similar periodic pigment pattern to that formed by melanocytes isolated from a later stage (Fig. 4 K and L). This chimera has two yellow stripes on the left side of the body, which cannot be explained by the ASIP expression pattern of the host WL embryo (Fig. 2D). Thus, this result supports the idea of melanocyte-autonomous periodic patterning.
Furthermore, when the melanocytes derived from either Barred Plymouth Rock chickens or Black Australorp chickens, which have only black pigmentation, were grafted into WL chickens, host embryos showed black pigmentation without any patterns (SI Appendix, Fig. S7). This suggests that the pigment pattern is dependent on the genotype of melanocytes. Taken together, these transplantation experiments demonstrate JQ melanocytes have an ability to form the periodic patterns.
Gap Junctions Regulate the Pigment Pattern.
Next, we wanted to know what molecular pathway was involved in setting up the periodic pigmented stripes. We took a systemic view to compare transcriptomes (24) from black and yellow quail skin stripes (whole skin at E7). We identified several pathways differentially enriched in black and yellow stripes, as well as many that were expressed in both regions. Most pathways enriched in black or yellow stripes are those involved in melanogenesis (SI Appendix, Fig. S8). Patterning genes may not always be found in the list of differentially expressed genes, as indicated in a cheetah study (3). Since the patterning process occurs before the stripes form, some patterning genes are likely to be expressed in all melanocyte progenitors.
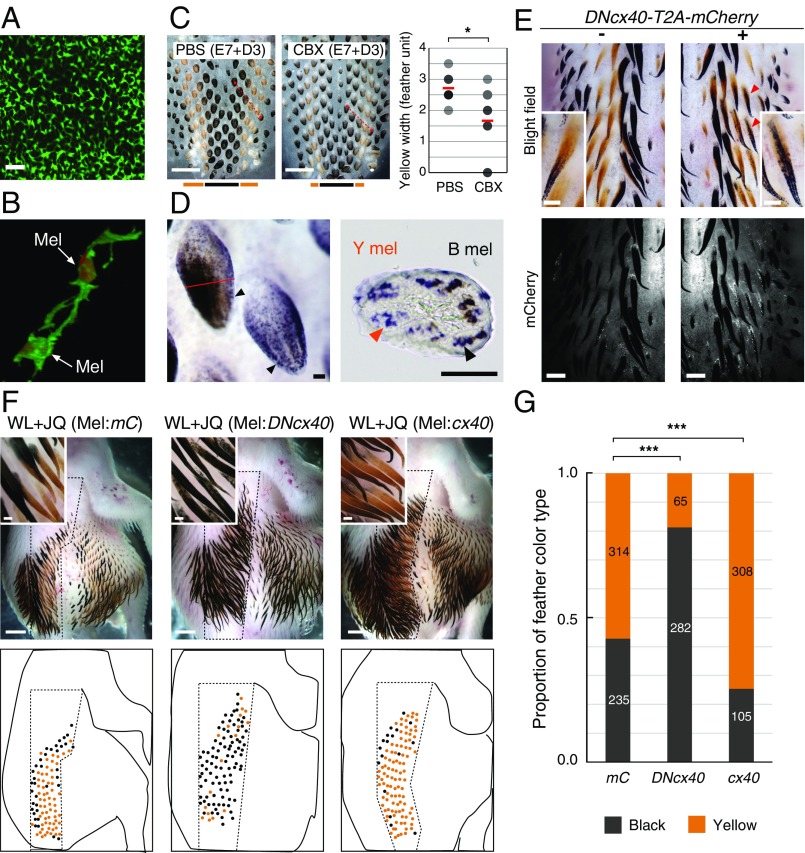
To better understand interactions that might guide pigment patterning, we performed the following studies. We observe that cultured JQ melanocytes directly connect with each other via filopodia-like structures in a network (Fig. 4B). This melanocyte network is also observed in JQ melanocytes in vivo (Fig. 5 A and B). This type of connection could be mediated with gap junction channels as seen in the pigment cells of zebrafish, where a mutant study showed that connexin is involved in stripe formation (25, 26). Therefore, we set up an explant culture system in which pigment stripes form and tested the role of ion channels and gap junctions in pigment patterning. Amiloride (Na+/Ca2+ channel blocker) suppressed pigment patterning, while conotoxin (N-type Ca2+ channel blocker) increased pigment formation (SI Appendix, Fig. S9). Most interestingly, carbenoxolone, a gap junction inhibitor, induced patterning changes that reduced the width of the yellow stripe (Fig. 5C), suggesting gap junction communication may be involved in periodic pigment patterning.
Fig. 5.
Cx40 regulates the ratio of black to yellow pigmented feathers. (A) Cell network of JQ melanocytes in E8 skin visualized with MelEM staining. (B) Direct cell–cell contact between JQ melanocytes (Mel) expressing LynEGFP with Mitf (red) staining in E8 skin. (C) E7 JQ skin explants incubated with the gap junction inhibitor for 3 d. PBS was used at a 1:1,000 ratio. CBX, carbenoxolone (50 μM). The graph shows the width of the yellow stripe measured along the red dotted line in the skin (PBS: n = 7, CBX: n = 9; *P = 0.029). (D, Left) JQ cx40 whole-mount in situ hybridization. Arrowheads indicate cx40 expression in feather buds. (D, Right) Red line indicates the plane of section. Yellow and black arrowheads indicate cx40 expression in yellow (Y mel) and black (B mel) melanocytes, respectively. (E) Effect of the DNcx40 form on E9 JQ pigment (n = 7). Plasmid DNA electroporated toward the right E2 JQ neural tube (+). Red arrowheads indicate ectopic black pigmentation. (F) E11 WL embryos with transplanted melanocytes expressing mCherry (Mel:mC; n = 6), DNcx40 (Mel:DNcx40; n = 4), or cx40 (Mel:cx40; n = 4). (G) Proportion of feather color type (***P < 0.001). Values are the sum of the number of feathers from six (mC), four (DNcx40), and four (cx40) individuals described in F. (Scale bars: A and D, 50 μm; C, 1 mm; E, whole view, 500 μm; E, Insets, 200 μm; F, whole view, 2 mm; F, Insets, 200 μm.)
We explored the role of connexin further with molecular studies. GJA5/cx40, a component of the gap junctional channel (27), was detected in both color types of JQ melanocytes (Fig. 5D and SI Appendix, Fig. S10). To determine whether cx40 inhibition changes the pigment pattern, we constructed a dominant negative form of JQ cx40 (DNcx40) by deleting six N-terminal amino acids based on the zebrafish study (25, 26). Electroporation of DNcx40 into JQ embryos produced ectopic black pigmentation, consistent with results of the inhibitor experiment (Fig. 5E). Furthermore, we analyzed the effect of cx40 activity changes on the pigment pattern by using WL embryos grafted with JQ melanocytes expressing either cx40 or DNcx40. Transplantation of melanocytes expressing DNcx40 expanded black domains in WL embryos. In contrast, melanocytes overexpressing cx40 expanded yellow-orange color domains (Fig. 5 F and G). These results suggest that gap junction channels in melanocytes can regulate the size of black and yellow domains.
ASIP Induction by Melanocytes.
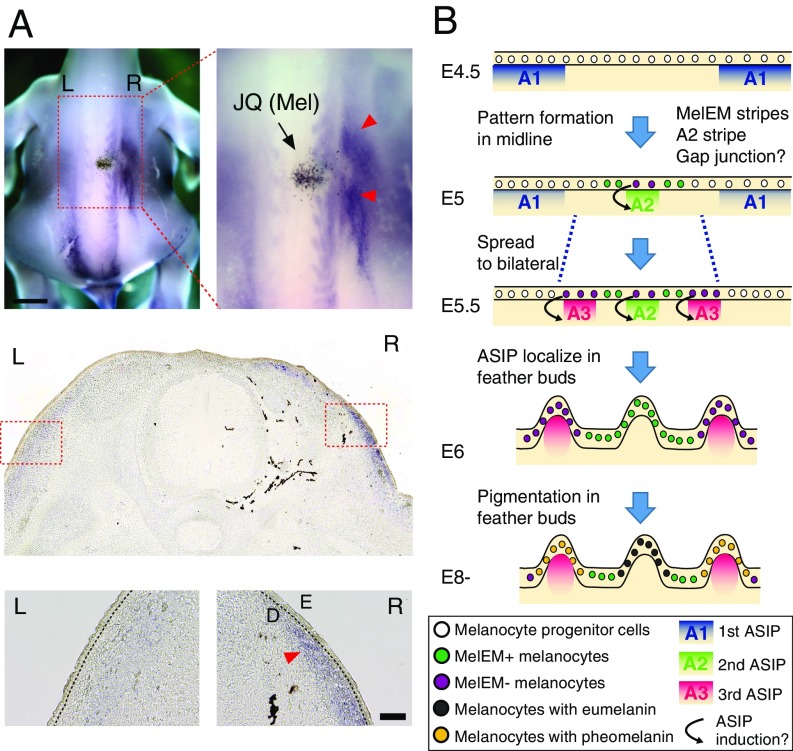
Our data suggest that the information encoding the periodic pattern is generated in the melanocyte population autonomously. If this is true, how does this information interact with the dermis expressing ASIP? When we transplant JQ melanocytes into WL host embryos, ectopic pheomelanin occurs, so melanocytes are expected to influence ASIP expression. To demonstrate this further, we examined the expression of ASIP in WL chickens with/without JQ melanocytes. WL embryos showed weak longitudinal striped ASIP expression, whereas in the WL chimera with JQ melanocytes, ectopic ASIP expression was detected in the dermis adjacent to the transplanted melanocytes (Fig. 6A). Therefore, we reasoned that ASIP expression was up-regulated by the JQ melanocyte that is most likely to become a future yellow melanocyte (MelEM-negative region). In conclusion, our results suggest that the interaction of melanocyte-autonomous patterning with dermis expressing ASIP generates the JQ pigment pattern (Fig. 6B).
Fig. 6.
ASIP up-regulation in dermis by JQ melanocyte transplantation. (A, Upper) ASIP whole-mount in situ hybridization in an E6 WL chick transplanted with JQ melanocytes. (A, Middle and Lower) Transverse section images. Red arrowheads indicate dermal (D) ectopic ASIP expression in a WL chicken (n = 3). L, left; R, right. (B) Model of JQ pigment pattern formation in a transverse section view of skin. At E4.5, the first ASIP stripes (A1) are located in the flank of the skin. From E5, the second ASIP stripe (A2) and MelEM stripes have emerged around the midline simultaneously. At E5.5, the third ASIP stripes (A3) have emerged. The MelEM-negative melanocytes may induce the ASIP (A2 and A3) stripes. Later than E6, ASIP is localized in the feather dermis, followed by pigmentation at E8. (Scale bars: A, whole view, 2 mm; A, Inset, 100 μm.)
Discussion
We began this study asking whether the pigment stripe pattern is controlled by melanocytes autonomously or by other cell types in the environment. As shown in a recent study by Haupaix et al. (11), early expression of ASIP in somite-derived dermis could be a prepattern of longitudinal stripes in juvenile galliform birds, including JQ. Such prepatterns of ASIP, as demonstrated by the heterospecific somite transplantation, surely instruct the specific pigment pattern, leading to non–melanocyte-autonomous pigment patterning. In our study, grafted JQ melanocytes could induce the periodic patterns, but not the longitudinal stripes, in most cases. Because the host WL chicken has a fading longitudinal ASIP signal (Fig. 2D), it is possible the grafted melanocytes may not be constrained by this prepattern and may manifest stochastic self-organizing periodic patterning. We observed the signaling pathway by which melanocytes instruct the dermal cells to express ASIP (Fig. 6A).
Avian periodic pigment patterns are rich with spots, stripes, or intermediates. There can be patterns across the whole body or patterns within a single feather. Stripes can be parallel to the trunk [e.g., longitudinal stripes on quail embryos studied here and in the study by Haupaix et al (11)] or horizontal to the feather axis (e.g., barred Plymouth Rock chicken). The formation of longitudinal stripes is only one of the periodic pigment patterns. In our study, we show the stripe pattern is not prefixed with several lines of experiments:
-
i)
In skin explant cultures, the spacing and width of stripes can be altered by ion channel or gap junction modulators, suggesting a local patterning mechanism takes place (SI Appendix, Fig. S9).
-
ii)
Melanocyte transplantation experiments in vivo showing that periodic pigment stripes form in an irregular orientation, not following AP orientation, suggest there is no prepattern (Fig. 4 and SI Appendix, Fig. S6).
-
iii)
Epithelium/mesenchymal explant culture with recombination and 90° rotation show that the epithelium, which contains keratinocytes and melanocytes at this stage, is in control of the orientation and spacing of periodic pigment stripes of the explants (Fig. 3A).
The patterning roles of the melanocytes and dermis (11) do not have to be in conflict. Recently, propagating periodic formation of feather buds has been shown to result from a combination of a local Turing pattern and a global ectodysplasin A (EDA)/FGF20/cell aggregation-mediated mechanochemical wave (28, 29). It is possible the autonomous melanocyte patterning signaling here may work with the somite/dermis-mediated agouti patterning signals (11) to fine-tune, interact, or stabilize the final pattern. In the study by Haupaix et al. (11), it was suggested that the stripe width is correlated with the dosage of agouti. In our study, we propose the size of pigment domains is modulated by the gap junction control. We think autonomous periodic pigment patterns generated by melanocytes (via gap junction communication) and by dermal fibroblasts (via agouti) may cross-talk to establish the final pigment pattern on the skin, just like epithelial and mesenchymal tissues interact to establish the final hair/feather patterns. With the dynamic expression of agouti, it is possible the A1 agouti pattern may disappear and new agouti in dermis may be induced by melanocytes. The final avian pigment patterns may be regulated by both dermal cells (via ASIP) and melanocytes (via gap junction).
Acknowledging that there are many coexisting, nonexclusive, patterning mechanisms may help us appreciate the “controversies,” such as those shown in the roles of the neural crest (8) versus the wing environment (10). Given the extraordinary diversity of pigment patterns in avian species, it is reasonable to consider that both extrinsic and intrinsic factors may contribute, separately, combinatorically, or interactively to set up the pattern.
So far, only a few genes regulating avian pigment pattern formation have been reported (6, 30). Here, we report the role of gap junction involvement in regulating the size of the periodic pigment pattern in JQ. While involvement of gap junctions in pigment stripe patterning has been reported in the zebrafish, the pigment cell type and developmental lineage are different between birds (only melanocytes) and fish (multiple pigment cell types) (31). However, molecular signaling involved in regulating the periodic patterning of melanocytes might be conserved among vertebrates. Indeed, many second messengers (cAMP, IP3, or calcium ions) that can pass through gap junctions are used in cell–cell communications. Those molecules could serve as long-range factors to transmit information contributing to Turing-type patterning mechanisms (32, 33).
The finding here that pigment patterns can be mediated by a mechanism involving gap junctions provides a platform to study pattern formation across the body surface, which can lead to an understanding of the fundamental processes of morphogenesis and pattern formation.
Materials and Methods
Specific pathogen-free WL eggs were purchased from Charles River Laboratories, and JQ eggs were from AA Laboratories. Animal care and experiments were conducted according to the guidelines established by the University of Southern California Institutional Animal Care and Use Committee. Molecular analysis, neural crest/melanocyte culture, transplantation, in ovo electroporation, lentivirus infection, and RNA sequencing are detailed in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank L. Anderson for critical comments on the manuscript and K. Kawakami and Y. Takahashi for providing Tol2 plasmids. We thank the University of Southern California Stem Cell Microscopy Core Facility and the Cell and Tissue Imaging Core. This work is supported by the US NIH (Grants AR47364, AR60306, and GM125322). M.I. is supported by the Human Frontier Science Program (Grant LT001148/2015-L) and a Japan Society for the Promotion of Science fellowship (KAKENHI 13J06412).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE119008).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816107116/-/DCSupplemental.
References
- 1.Watanabe M, Kondo S. Is pigment patterning in fish skin determined by the Turing mechanism? Trends Genet. 2015;31:88–96. doi: 10.1016/j.tig.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Kondo S. An updated kernel-based Turing model for studying the mechanisms of biological pattern formation. J Theor Biol. 2017;414:120–127. doi: 10.1016/j.jtbi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Kaelin CB, et al. Specifying and sustaining pigmentation patterns in domestic and wild cats. Science. 2012;337:1536–1541. doi: 10.1126/science.1220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallarino R, et al. Developmental mechanisms of stripe patterns in rodents. Nature. 2016;539:518–523. doi: 10.1038/nature20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imsland F, et al. Regulatory mutations in TBX3 disrupt asymmetric hair pigmentation that underlies Dun camouflage color in horses. Nat Genet. 2016;48:152–158. doi: 10.1038/ng.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SJ, et al. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science. 2013;340:1442–1445. doi: 10.1126/science.1230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiojiri N, Niwa T, Wakamatsu K, Ito S, Nakamura A. Chemical analysis of melanin pigments in feather germs of Japanese quail Bh (black at hatch) mutants. Pigment Cell Res. 1999;12:259–265. doi: 10.1111/j.1600-0749.1999.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 8.Kinutani M, Le Douarin NM. Avian spinal cord chimeras. I. Hatching ability and posthatching survival in homo- and heterospecific chimeras. Dev Biol. 1985;111:243–255. doi: 10.1016/0012-1606(85)90449-x. [DOI] [PubMed] [Google Scholar]
- 9.Richardson MK, Hornbruch A. Quail neural crest cells cannot read positional values in the dorsal trunk feathers of the chicken embryo. Rouxs Arch Dev Biol. 1991;199:397–401. doi: 10.1007/BF01705849. [DOI] [PubMed] [Google Scholar]
- 10.Richardson MK, Hornbruch A, Wolpert L. Pigment patterns in neural crest chimeras constructed from quail and Guinea fowl embryos. Dev Biol. 1991;143:309–319. doi: 10.1016/0012-1606(91)90082-e. [DOI] [PubMed] [Google Scholar]
- 11.Haupaix N, et al. The periodic coloration in birds forms through a prepattern of somite origin. Science. 2018;361:eaar4777. doi: 10.1126/science.aar4777. [DOI] [PubMed] [Google Scholar]
- 12.Nataf V, Mercier P, Ziller C, Le Douarin NM. Novel markers of melanocyte differentiation in the avian embryo. Exp Cell Res. 1993;207:171–182. doi: 10.1006/excr.1993.1177. [DOI] [PubMed] [Google Scholar]
- 13.Nataf V, et al. Melanoblast/melanocyte early marker (MelEM) is a glutathione S-transferase subunit. Exp Cell Res. 1995;218:394–400. doi: 10.1006/excr.1995.1171. [DOI] [PubMed] [Google Scholar]
- 14.Niwa T, Mochii M, Nakamura A, Shiojiri N. Plumage pigmentation and expression of its regulatory genes during quail development–Histochemical analysis using Bh (black at hatch) mutants. Mech Dev. 2002;118:139–146. doi: 10.1016/s0925-4773(02)00256-3. [DOI] [PubMed] [Google Scholar]
- 15.Millar SE, Miller MW, Stevens ME, Barsh GS. Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development. 1995;121:3223–3232. doi: 10.1242/dev.121.10.3223. [DOI] [PubMed] [Google Scholar]
- 16.Manceau M, Domingues VS, Mallarino R, Hoekstra HE. The developmental role of Agouti in color pattern evolution. Science. 2011;331:1062–1065. doi: 10.1126/science.1200684. [DOI] [PubMed] [Google Scholar]
- 17.Yoshihara C, et al. Elaborate color patterns of individual chicken feathers may be formed by the agouti signaling protein. Gen Comp Endocrinol. 2012;175:495–499. doi: 10.1016/j.ygcen.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Chuong CM, Widelitz RB, Ting-Berreth S, Jiang TX. Early events during avian skin appendage regeneration: Dependence on epithelial-mesenchymal interaction and order of molecular reappearance. J Invest Dermatol. 1996;107:639–646. doi: 10.1111/1523-1747.ep12584254. [DOI] [PubMed] [Google Scholar]
- 19.Teillet M-A, Ziller C, Le Douarin NM. Quail-chick chimeras. Methods Mol Biol. 2008;461:337–350. doi: 10.1007/978-1-60327-483-8_24. [DOI] [PubMed] [Google Scholar]
- 20.Le Douarin N, Kalcheim C. The Neural Crest. 2nd Ed Cambridge Univ Press; Cambridge, UK: 2009. [Google Scholar]
- 21.Murai H, Tadokoro R, Sakai K, Takahashi Y. In ovo gene manipulation of melanocytes and their adjacent keratinocytes during skin pigmentation of chicken embryos. Dev Growth Differ. 2015;57:232–241. doi: 10.1111/dgd.12201. [DOI] [PubMed] [Google Scholar]
- 22.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami K. Tol2: A versatile gene transfer vector in vertebrates. Genome Biol. 2007;8(Suppl 1):S7. doi: 10.1186/gb-2007-8-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inaba M, Liang Y. 2019 Japanese quail embryonic skin samples. Gene Expression Omnibus (GEO) database. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119008. Deposited August 24, 2018.
- 25.Watanabe M, Kondo S. Changing clothes easily: Connexin41.8 regulates skin pattern variation. Pigment Cell Melanoma Res. 2012;25:326–330. doi: 10.1111/j.1755-148X.2012.00984.x. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M, Sawada R, Aramaki T, Skerrett IM, Kondo S. The physiological characterization of connexin41.8 and connexin39.4, which are involved in the striped pattern formation of zebrafish. J Biol Chem. 2016;291:1053–1063. doi: 10.1074/jbc.M115.673129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai D. Atrial fibrillation-linked GJA5/connexin40 mutants impaired gap junctions via different mechanisms. FEBS Lett. 2014;588:1238–1243. doi: 10.1016/j.febslet.2014.02.064. [DOI] [PubMed] [Google Scholar]
- 28.Ho WKW, et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 2019;17:e3000132. doi: 10.1371/journal.pbio.3000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaba M, Harn HI-C, Chuong C-M. Turing patterning with and without a global wave. PLoS Biol. 2018 doi: 10.1371/journal.pbio.3000195. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwochow Thalmann D, et al. The evolution of sex-linked barring alleles in chickens involves both regulatory and coding changes in CDKN2A. PLoS Genet. 2017;13:e1006665. doi: 10.1371/journal.pgen.1006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelsh RN, Harris ML, Colanesi S, Erickson CA. Stripes and belly-spots–A review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol. 2009;20:90–104. doi: 10.1016/j.semcdb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin M. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol. 2007;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cervera J, Pietak A, Levin M, Mafe S. Bioelectrical coupling in multicellular domains regulated by gap junctions: A conceptual approach. Bioelectrochemistry. 2018;123:45–61. doi: 10.1016/j.bioelechem.2018.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.