Significance
Stress hormones are known to facilitate memory of emotionally arousing experiences, an effect that is mediated by the basolateral amygdala (BLA) and its interaction with other brain regions. Brain activation studies also indicate that emotional arousal activates several functional connections beyond the BLA. However, it is largely unknown whether BLA activity influences these connections. Here, we report that BLA activity enables functional interactions between the prelimbic cortex, anterior insular cortex, and dorsal hippocampus in regulating glucocorticoid hormone effects on recognition memory. These findings increase our understanding of how BLA activity affects emotional memory within large brain networks.
Keywords: basolateral amygdala, medial prefrontal cortex, anterior insular cortex, dorsal hippocampus, norepinephrine
Abstract
Extensive evidence indicates that the basolateral amygdala (BLA) interacts with other brain regions in mediating stress hormone and emotional arousal effects on memory consolidation. Brain activation studies have shown that arousing conditions lead to the activation of large-scale neural networks and several functional connections between brain regions beyond the BLA. Whether such distal interactions on memory consolidation also depend on BLA activity is not as yet known. We investigated, in male Sprague–Dawley rats, whether BLA activity enables prelimbic cortex (PrL) interactions with the anterior insular cortex (aIC) and dorsal hippocampus (dHPC) in regulating glucocorticoid effects on different components of object recognition memory. The glucocorticoid receptor (GR) agonist RU 28362 administered into the PrL, but not infralimbic cortex, immediately after object recognition training enhanced 24-hour memory of both the identity and location of the object via functional interactions with the aIC and dHPC, respectively. Importantly, posttraining inactivation of the BLA by the noradrenergic antagonist propranolol abolished the effect of GR agonist administration into the PrL on memory enhancement of both the identity and location of the object. BLA inactivation by propranolol also blocked the effect of GR agonist administration into the PrL on inducing changes in neuronal activity within the aIC and dHPC during the postlearning consolidation period as well as on structural changes in spine morphology assessed 24 hours later. These findings provide evidence that BLA noradrenergic activity enables functional interactions between the PrL and the aIC and dHPC in regulating stress hormone and emotional arousal effects on memory.
Stressful and emotional experiences activate hormonal and brain systems that create strong memories (1, 2). Extensive evidence indicates that noradrenergic activation of the basolateral amygdala (BLA), induced by emotional arousal, is crucially involved in strengthening the consolidation of long-term memory (2). We have reported extensive evidence indicating that noradrenergic activation of the BLA also plays a critical role in enabling the enhancing effects of adrenal stress hormones, that is, epinephrine and glucocorticoids, on memory consolidation (3–5). Many previous studies have investigated how such BLA activation enhances the consolidation of memory by influencing neural plasticity and information storage processes within specific target regions, such as the hippocampus (2, 6–10). Accumulating evidence from brain activation studies indicates that arousing conditions increase activation of large-scale neural networks (11–13) and also affect numerous functional interactions between brain regions beyond the amygdala (14, 15). However, it is not known whether BLA activity is required for regulating emotional arousal effects on functional interactions between such distal brain regions.
We investigated whether BLA noradrenergic activity enables functional interactions between the medial prefrontal cortex (mPFC) and the anterior insular cortex (aIC) and dorsal hippocampus (dHPC) in mediating glucocorticoid hormone enhancement of recognition memory for objects and their location. Previous findings indicate that the mPFC contributes to memory through cognitive or strategic control over memory processes within other brain areas (16, 17) and that the aIC is involved in memory for the identity of an object (18). Functional interactions between the mPFC and aIC might occur when emotionally salient information, including novel objects, is detected (19, 20). Previous findings also indicate that the dHPC is involved in associating an object with its context or place (21–23) and that the processing of contextual information engages a functional cross talk between the mPFC and dHPC (24–26). Importantly, the mPFC (27), aIC (28), and dHPC (29) all receive strong inputs from the BLA. We show that a glucocorticoid receptor (GR) agonist administered into the prelimbic cortex (PrL) but not the infralimbic cortex (IL) of the mPFC after an object recognition training experience enhances the consolidation of memory of both the identity and location of an object via functional cross talk with the aIC and dHPC, respectively. Most importantly, we show that BLA noradrenergic activity drives functional interactions between these distal brain regions. These findings indicate that the BLA influences large-scale brain networks in regulating stress hormone and emotional arousal effects on memory (13).
Results
Effect of GR Agonist Administration into the PrL and IL on the Consolidation of Object Recognition Memory and Object Location Memory.
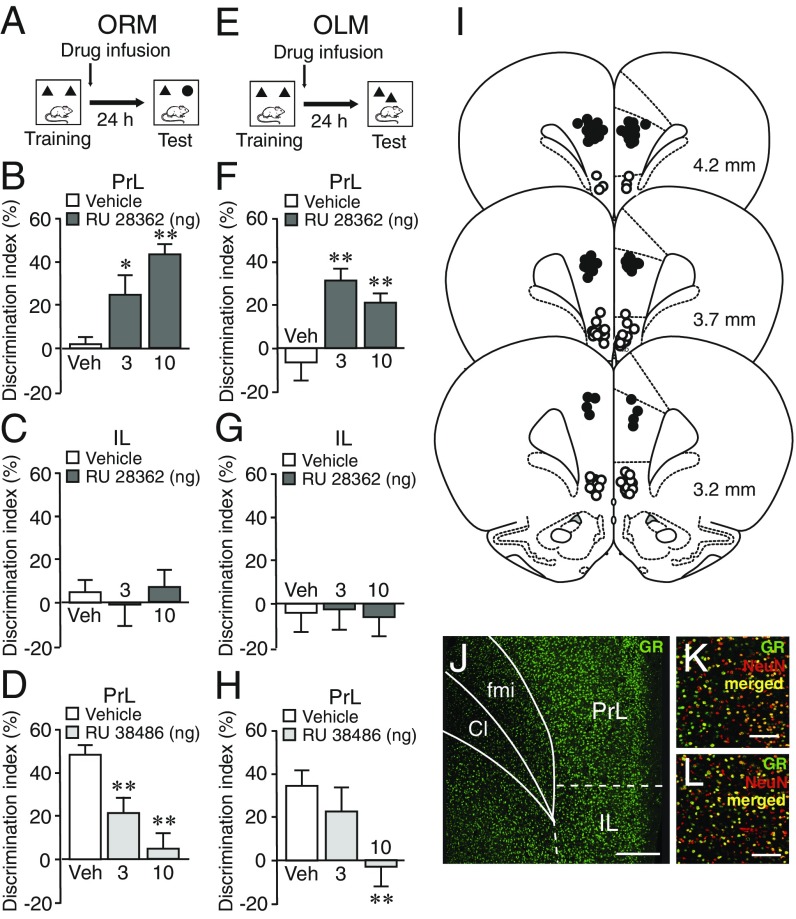
We investigated whether GR agonist administration into the PrL and IL, two subregions of the mPFC, enhances the consolidation of memory of different components of object recognition training. Male Sprague–Dawley rats were trained on a 3-min object recognition trial during which they could freely explore two identical objects, immediately followed by bilateral microinfusions of the GR agonist RU 28362 (3 or 10 ng in 0.5 μL) or vehicle administered into either the PrL or IL (Fig. 1I and SI Appendix, Fig. S1 for infusion sites). Other groups of rats were trained on a 10-min training trial, which induces robust long-term memory (23, 30), and we examined whether a blockade of GR activity with posttraining infusions of the GR antagonist RU 38486 (3 or 10 ng in 0.5 μL) impairs memory of that training. To determine whether animals exhibit a memory for the identity of the object (object recognition memory, ORM), some rats were given a 24-h retention test in which one object was familiar and the other object was novel. This protocol is depicted in Fig. 1A. To determine whether animals exhibit a memory for the location of the object (object location memory, OLM), other rats were given a 24-h retention test in which both objects were familiar but one was placed in a novel location (Fig. 1E).
Fig. 1.
Posttraining GR agonist and GR antagonist administration into the PrL but not the IL modulate ORM and OLM. (A) Experimental design of the ORM task (SI Appendix, Supplementary Methods). (B) The GR agonist RU 28362 (3 or 10 ng in 0.5 μL) administered into the PrL after a 3-min training trial enhanced 24-h ORM. Data are presented as discrimination index (mean ± SEM; see Methods). n = 11–13 rats/group. (C) No effect of GR agonist administration into the IL after a 3-min training trial on 24-h ORM. n = 12 rats/group. (D) The GR antagonist RU 38486 (3 or 10 ng in 0.5 μL) administered into the PrL after a 10-min training trial impaired 24-h ORM. n = 10–13 rats/group. (E) Experimental design of the OLM task. (F) The GR agonist administered into the PrL after a 3-min training trial enhanced 24-h OLM. n = 9–11 rats/group. (G) No effect of GR agonist administration into the IL after a 3-min training trial on 24-h OLM. n = 10–12 rats/group. (H) The GR antagonist administered into the PrL after a 10-min training trial impaired 24-h OLM. n = 11–13 rats/group. *P < 0.05; **P < 0.01. (I) Infusion sites in the PrL (solid circles) and IL (open circles) of rats included in Fig. 1 B and C. (J) Fluorescence photomicrograph showing similar GR expression levels within the PrL and IL. Cl, claustrum; fmi, forceps minor of the corpus callosum. (Scale bar, 300 µm.) (K) Detailed picture showing coexpression of GR (green) and the neuron marker NeuN (red) in the PrL. (Scale bar, 100 µm.) (L) Detailed picture showing GR (green) and NeuN (red) coexpression in the IL. (Scale bar, 100 µm.)
ORM task.
GR agonist administration into the PrL immediately after a 3-min training trial enhanced 24-h memory for the identity of the object (one-way ANOVA for discrimination index: F2,33 = 10.02, P = 0.0004). As shown in Fig. 1B, vehicle-treated control rats did not express 24-h retention of the object (one-sample t test: t11 = 0.40, P = 0.69), and both doses of the GR agonist significantly enhanced retention (3 ng: P < 0.05; 10 ng: P < 0.01 vs. vehicle). In contrast, GR agonist infusions administered into the IL did not affect 24-h retention (F2,33 = 0.25, P = 0.78; Fig. 1C). As shown in Fig. 1D, GR antagonist administration into the PrL after a 10-min training session significantly impaired 24-h memory for the identity of the object (F2,32 = 13.14, P < 0.0001). Vehicle-treated rats expressed significant retention (t12 = 12.08, P < 0.0001), and both doses of the GR antagonist impaired retention performance (3 and 10 ng: P < 0.01). The treatment groups did not differ in total exploration time of the two objects during either training or 24-h retention test (SI Appendix, Fig. S2).
OLM task.
GR agonist administration into the PrL also enhanced 24-h memory for the location of the object (F2,28 = 9.69, P = 0.0006). As shown in Fig. 1F, vehicle-treated control rats did not express significant memory of the 3-min training trial (one-sample t test: t10 = −0.80, P = 0.44), and both doses of the GR agonist enhanced retention (3 and 10 ng: P < 0.01). GR agonist administration into the IL did not affect 24-h retention (F2,30 = 0.17, P = 0.84; Fig. 1G). As shown in Fig. 1H, the GR antagonist administered into the PrL after a 10-min training session significantly impaired memory for the location of the object (F2,32 = 4.30, P = 0.02). Vehicle-treated rats expressed significant 24-h retention (t12 = 4.57, P = 0.0006), and the higher dose of the GR antagonist impaired retention performance (10 ng: P < 0.01). The treatment groups did not differ in total exploration time of the two objects during either training or 24-h retention test (SI Appendix, Fig. S2). These findings thus indicate that GR agonist administration into the PrL but not the IL, despite similar GR expression levels (Fig. 1J), enhances the consolidation of memory of both the “what” (object identity) and “where” (object location) components of object recognition memory.
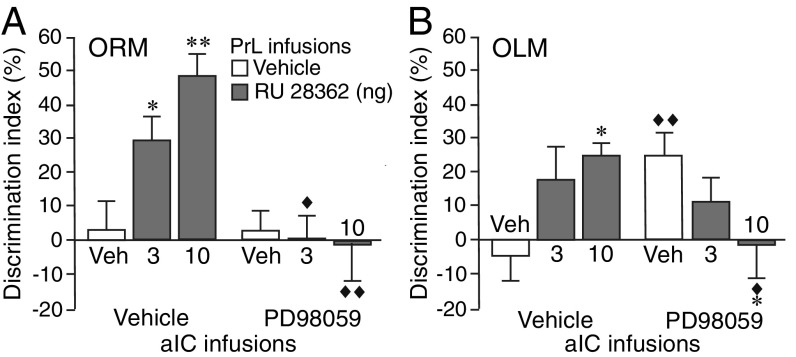
PrL Interactions with the aIC in Regulating GR Agonist Effects on ORM.
We investigated interactions between the PrL and aIC in mediating GR agonist effects on memory in the ORM and OLM tasks. The PrL, but not the IL, sends direct anatomical projections to the aIC (31), and the aIC is implicated in memory for the identity but not location of an object (22, 23). We examined whether a functional blockade of the aIC by inhibition of phosphorylated extracellular signal-regulated kinase1/2 (pERK1/2), a signaling cascade critically implicated in neuronal activity and synaptic plasticity (32), prevents the effect of intra-PrL GR agonist administration on memory enhancement in both the ORM and OLM tasks. As shown in Fig. 2A, posttraining GR agonist (3 or 10 ng in 0.5 μL) administration into the left PrL (see SI Appendix, Fig. S3A for infusion sites) enhanced 24-h memory for the identity of the object in the ORM task (3 ng: P < 0.05; 10 ng: P < 0.01). The MEK inhibitor PD98059 (50 ng in 0.5 μL) administered into the ipsilateral aIC after the training blocked the GR agonist effect on memory enhancement for the identity of the object (3 ng: P < 0.05; 10 ng: P < 0.01). In contrast, functional blockade of the aIC with this dose of the MEK inhibitor did not prevent the modulatory effect of GR agonist administration into the PrL on memory for the location of the object in the OLM task. As shown in Fig. 2B, MEK inhibitor administration into the aIC alone enhanced 24-h retention of the location of the object (P < 0.01), and, in this condition, GR agonist administration into the PrL induced significant memory impairment (10 ng: P < 0.05 vs. vehicle). The treatment groups did not differ in total exploration time of the two objects during either training or 24-h retention test (SI Appendix, Fig. S3 B and C). These findings indicate that the PrL interacts with the aIC in regulating GR agonist effects on memory for the identity but not location of the object.
Fig. 2.
Inhibition of aIC activity with a MEK inhibitor selectively blocks the effect of GR agonist treatment into the PrL on enhancement of ORM. Rats were given a 3-min training trial followed by MEK inhibitor (PD98059, 50 ng in 0.5 μL) or vehicle administration into the left aIC together with the GR agonist (RU 28362, 3 or 10 ng in 0.5 μL) or vehicle into the ipsilateral PrL. ORM or OLM was tested 24 h later. (A) PD98059 administration into the aIC blocked enhancement of ORM induced by GR agonist administration into the PrL (n = 8–11 rats/group, two-way ANOVA: RU 28362 F2,51 = 3.44, P = 0.04; PD98059 F1,51 = 16.45, P = 0.0002; interaction F2,51 = 5.03, P = 0.01). (B) PD98059 administration into the aIC alone enhanced OLM, but this did not prevent memory modulation induced by GR agonist administration into the PrL (n = 9–14 rats/group, two-way ANOVA: RU 28362 F2,60 = 0.13, P = 0.88; PD98059 F1,60 = 0.02, P = 0.88; interaction F2,60 = 5.38, P = 0.007). *P < 0.05, **P < 0.01 vs. vehicle; ◆P < 0.05, ◆◆P < 0.01 vs. vehicle or RU 28362 alone.
PrL Interactions with the dHPC in Regulating GR Agonist Effects on OLM.
Next, we examined functional interactions between the PrL and dHPC in mediating GR agonist effects on memory in the ORM and OLM tasks. Although a role of the dHPC in familiarity discrimination remains controversial (33), several findings indicate that an object’s association with its context or place does require the dHPC (22, 23). As shown in Fig. 3A, GR agonist (3 or 10 ng in 0.5 μL) administration into the left PrL after the training trial enhanced 24-h memory for the identity of the object in the ORM task (10 ng: P < 0.01), and concomitant blockade of the ipsilateral dHPC with the MEK inhibitor PD98059 (50 ng in 0.5 μL) (see SI Appendix, Fig. S4A for infusion sites) did not prevent this GR agonist effect (3 and 10 ng: P < 0.01 vs. vehicle). In contrast, dHPC inactivation completely blocked the GR agonist effect on memory enhancement for the location of the object in the OLM task. As shown in Fig. 3B, GR agonist administration into the PrL enhanced 24-h retention of the location of the object (10 ng: P < 0.01), and this effect was blocked following MEK inhibitor administration into the dHPC (3 ng: P < 0.05; 10 ng: P < 0.01 vs. GR agonist). The treatment groups did not differ in total exploration time of the two objects during training or 24-h retention test (SI Appendix, Fig. S4 B and C). Thus, these findings indicate that the PrL interacts with the dHPC in regulating GR agonist effects on memory for the location but not the identity of the object.
Fig. 3.
Inhibition of dHPC activity with a MEK inhibitor selectively blocks the effect of GR agonist treatment into the PrL on enhancement of OLM. Rats were given a 3-min training trial followed by MEK inhibitor (PD98059, 50 ng in 0.5 μL) or vehicle administration into the left dHPC together with the GR agonist (RU 28362, 3 or 10 ng in 0.5 μL) or vehicle into the ipsilateral PrL. ORM or OLM was tested 24 h later. (A) PD98059 administration into the dHPC did not block the enhancement of ORM induced by GR agonist administration into the PrL (n = 9–13 rats/group, two-way ANOVA: RU 28362 F2,64 = 12.91, P < 0.0001; PD98059 F1,64 = 0.20, P = 0.66; interaction F2,64 = 0.10, P = 0.91). (B) PD98059 administration into the dHPC blocked enhancement of OLM induced by GR agonist administration into the PrL (n = 9–13 rats/group, two-way ANOVA: RU 28362 F2,61 = 1.33, P = 0.27; PD98059 F1,61 = 14.66, P = 0.0003; interaction F2,61 = 3.31, P = 0.04). **P < 0.01 vs. vehicle; ◆P < 0.05, ◆◆P < 0.01 vs. RU 28362 alone.
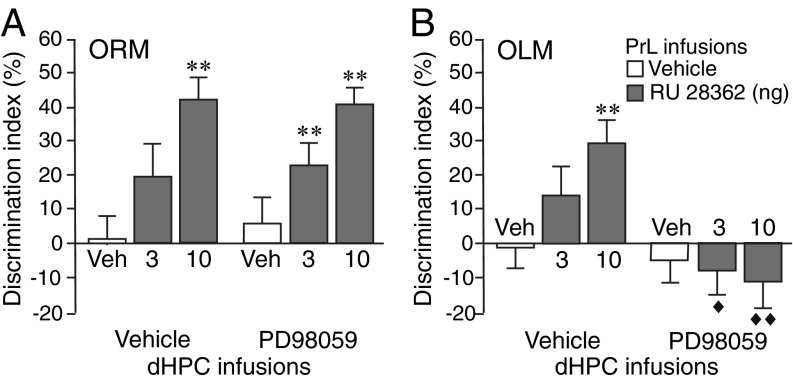
Impact of BLA Noradrenergic Activity on the Effect of GR Agonist Administration into the PrL on ORM and OLM.
To determine whether BLA noradrenergic activity is critically involved in regulating the effect of GR agonist administration into the PrL on memory in the ORM and OLM tasks, the GR agonist (3 or 10 ng in 0.5 µL) was administered into the left PrL, and the β-adrenoceptor antagonist propranolol (0.3 µg in 0.2 µL) was administered into the ipsilateral BLA (see SI Appendix, Fig. S5A, for infusion sites) immediately after the training trial. As shown in Fig. 4A, GR agonist administration into the PrL enhanced 24-h memory for the identity of the object in the ORM task (3 and 10 ng: P < 0.01), and propranolol administration into the BLA blocked this GR agonist effect (3 ng: P < 0.05; 10 ng: P < 0.01 vs. GR agonist alone). As shown in Fig. 4B, GR agonist administration into the PrL also enhanced 24-h memory for the location of the object in the OLM task (3 and 10 ng: P < 0.01), and propranolol administration into the BLA also blocked this GR agonist effect (10 ng: P < 0.01 vs. GR agonist alone). The treatment groups did not differ in total exploration time of the two objects during either training or 24-h retention test (SI Appendix, Fig. S5 B and C). Thus, these findings indicate that BLA noradrenergic activity is required for enabling the effect of GR agonist administration into the PrL on memory enhancement of both aIC-dependent ORM and dHPC-dependent OLM.
Fig. 4.
Blockade of BLA noradrenergic activity prevents the effect of GR agonist administration into the PrL on enhancement of both ORM and OLM. Rats were given a 3-min training trial followed by β-adrenoceptor antagonist propranolol (0.3 μg in 0.2 μL) or saline administration into the left BLA together with the GR agonist RU 28362 (3 or 10 ng in 0.5 μL) or vehicle into the ipsilateral PrL. ORM or OLM was tested 24 h later. (A) Propranolol administration into the BLA blocked enhancement of ORM induced by GR agonist administration into the PrL (n = 10–11 rats/group, two-way ANOVA: RU 28362 F2,59 = 6.40, P = 0.003; propranolol F1,59 = 11.41, P = 0.001; interaction F2,59 = 3.82, P = 0.03). (B) Propranolol administration into the BLA also blocked enhancement of OLM induced by GR agonist administration into the PrL (n = 10–13 rats/group, two-way ANOVA: RU 28362 F2,63 = 3.21, P = 0.047; propranolol F1,63 = 12.30, P = 0.0008; interaction F2,63 = 5.51, P = 0.006). **P < 0.01 vs. vehicle; ◆P < 0.05, ◆◆P < 0.01 vs. RU 28362 alone.
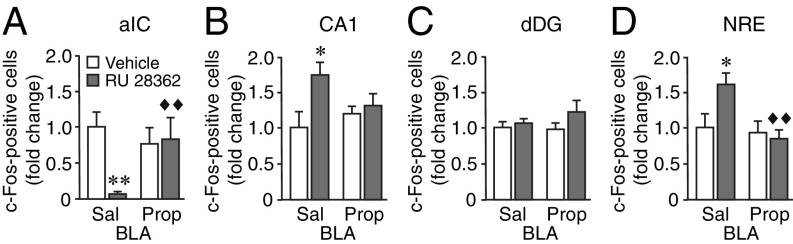
Impact of BLA Noradrenergic Activity on the Effect of GR Agonist Administration into the PrL on Neuronal Activity Changes Within the aIC and dHPC.
To further examine whether BLA noradrenergic activity enables functional interactions between the PrL and the aIC and dHPC, we investigated whether GR agonist administration into the PrL after object recognition training induces activity-dependent neuronal activity changes within the aIC and dHPC during the postlearning consolidation period (34) and, most importantly, whether these effects are dependent on BLA noradrenergic activity. Neuronal activity was assessed, 1 h after training and drug treatment, by immunofluorescence for c-Fos (35), an immediate early gene product that is a well-established molecular marker for identifying recently activated cells (36) and the mapping of neuronal ensembles that underwent increased task-related activity (37).
Within the aIC, c-Fos labeling was determined within cortical layers II/III of its three main subdivisions (SI Appendix, Fig. S6A). Vehicle-treated trained rats showed a high numerical density of c-Fos–positive cells, which was highest within the agranular and dysgranular subdivisions (SI Appendix, Fig. S6B). As shown in Fig. 5A, GR agonist (10 ng in 0.5 μL) administration into the left PrL led to a significant reduction in the numerical density of c-Fos–positive cells within the ipsilateral aIC (P < 0.01) (see SI Appendix, Fig. S6B, for analysis per subdivision). Double staining for c-Fos and for glutamic acid decarboxylase 67 (GAD67), a marker for GABAergic inhibitory neurons (38), revealed that c-Fos was predominantly expressed in excitatory, that is, GAD67-negative, neurons and that GR agonist administration did not significantly alter the ratio of c-Fos–positive excitatory vs. inhibitory cells (SI Appendix, Fig. S6C). To test for changes in GABAergic synapse numbers, we also quantified the number of GAD67-positive puncta that were localized at the somatic circumference of excitatory neurons (SI Appendix, Fig. S6D) (39). GR agonist administration into the PrL did not affect the number of GAD67-positive puncta per neuron soma (SI Appendix, Fig. S6E), indicating that the reduced aIC neuronal activity was not mediated via an up-regulation of GABAergic inhibitory tone. Rather, these findings suggest that GR agonist administration into the PrL reduces the level of excitatory input to the aIC. Most importantly, although propranolol (0.3 µg in 0.2 µL) administration into the BLA by itself did not significantly affect the numerical density of c-Fos–positive cells within the aIC, it completely blocked the c-Fos expression changes induced by the GR agonist (P < 0.01). These findings thus indicate that BLA noradrenergic activity is essential for enabling the GR agonist effect on activity-dependent neuronal activity changes within the PrL-aIC pathway during the postlearning consolidation period.
Fig. 5.
Blockade of BLA noradrenergic activity prevents the effect of GR agonist treatment into the PrL on neuronal activity changes within the aIC and dHPC. Rats were given a 3-min training trial followed by β-adrenoceptor antagonist propranolol (Prop) (0.3 μg in 0.2 μL) or saline (Sal) administration into the left BLA together with the GR agonist RU 28362 (10 ng in 0.5 μL) or vehicle into the ipsilateral PrL. c-Fos immunofluorescence was assessed 1 h later. (A) Numerical density of c-Fos–positive cells within cortical layers II/III of aIC. Data are presented as fold change (mean ± SEM) (n = 6–9 rats/group, two-way ANOVA: RU 28362 F1,26 = 5.10, P = 0.03; propranolol F1,26 = 1.75, P = 0.20; interaction F1,26 = 6.63, P = 0.02). (B) Numerical density of c-Fos–positive cells within the pyramidal cell layer of CA1 (two-way ANOVA: RU 28362 F1,25 = 5.06, P = 0.03; propranolol F1,25 = 0.41, P = 0.53; interaction F1,25 = 2.73, P = 0.11). (C) Numerical density of c-Fos–positive cells within the granule cell layer of dDG (two-way ANOVA: RU 28362 F1,25 = 2.04, P = 0.17; propranolol F1,25 = 0.28, P = 0.60; interaction F1,25 = 0.84, P = 0.37). (D) Numerical density of c-Fos–positive cells within the NRE (two-way ANOVA: RU 28362 F1,26 = 2.32, P = 0.14; propranolol F1,26 = 6.58, P = 0.02; interaction F1,26 = 4.24, P = 0.049). *P < 0.05, **P < 0.01 vs. vehicle; ◆◆P < 0.01 vs. RU 28362 alone.
Within the dHPC, c-Fos immunoreactivity was assessed within both the CA1 and dorsal blade of the dentate gyrus (dDG). As shown in Fig. 5B, posttraining GR agonist administration into the PrL significantly increased the numerical density of c-Fos–positive cells within the pyramidal cell layer of CA1 (P < 0.05). c-Fos labeling within the dDG granule cell layer was not significantly affected (Fig. 5C). There are no direct monosynaptic projections from the PrL to the dHPC, and the nucleus reuniens (NRE) of the ventral midline thalamus is an important anatomical link between the PrL and dHPC (40–42). The NRE directly innervates the CA1 (stratum lacunosum-moleculare) but not dDG (43). We found that GR agonist administration into the PrL also increased the numerical density of c-Fos–positive cells within the NRE (P < 0.05), supporting the view that the NRE forms an interface between the PrL and dHPC (41, 44). Most importantly, propranolol administration into the BLA completely abolished the effect of intra-PrL GR agonist administration on increased c-Fos labeling within both the CA1 (P = 0.59 vs. vehicle) and NRE (P = 0.58 vs. vehicle). These findings show that BLA noradrenergic activity is also essential for enabling functional interactions between the PrL and dHPC induced by object recognition training and posttraining GR agonist administration.
Impact of BLA Noradrenergic Activity on the Effect of GR Agonist Administration into the PrL on Dendritic Plasticity Within the aIC.
After learning, memories are created by alterations in neural activity and synaptic transmission; in excitatory neurons, these modifications are then actively stabilized by structural changes at postsynaptic sites on dendritic spines (45). We investigated whether GR agonist administration into the PrL after object recognition training induces long-term structural changes in dendritic morphology and spine density within the aIC, and whether this neural plasticity within the PrL-aIC pathway is also dependent on BLA noradrenergic activity. The GR agonist (10 ng in 0.5 µL) was administered into the left PrL, and propranolol (0.3 µg in 0.2 µL) was administered into the left BLA immediately after the training. Dendritic morphology and spine density of pyramidal neurons within cortical layers II/III of the ipsilateral granular aIC were assessed 24 h later. Images of Golgi-impregnated neurons were captured under a bright-field microscope (Fig. 6A). Three-dimensional reconstructions of their somatodendritic organization and dendritic branching order-dependent quantification of spine type and spine density were produced (Fig. 6B; see SI Appendix, Supplementary Methods).
Fig. 6.
Blockade of BLA noradrenergic activity prevents the effect of GR agonist treatment into the PrL on spine morphology changes within the aIC, assessed 24 h after object recognition training. (A) Image of a Golgi-impregnated neuron. (B) Dendrite showing different spine types (100× magnification). (C) Spine densities for long thin, stubby, mushroom, and branched spine types in apical dendrites. Data are presented as the number of spines per micrometer (mean ± SEM) [n = 15–20 cells (from five rats)/group: two-way ANOVA, mushroom spines: RU 28362 F1,69 = 7.72, P = 0.007; propranolol F1,69 = 1.38, P = 0.24; interaction F1,69 = 4.40, P = 0.04; branched spines: RU 28362 F1,69 = 6.40, P = 0.01; propranolol F1,69 = 2.63, P = 0.11; interaction F1,69 = 9.21, P = 0.003]. (D) Spine densities in basal dendrites [n = 15–18 cells (from five rats)/group: two-way ANOVA, mushroom spines: RU 28362 F1,62 = 1.46, P = 0.23; propranolol F1,62 = 1.84, P = 0.18; interaction F1,62 = 5.71, P = 0.02; branched spines: RU 28362 F1,62 = 5.75, P = 0.02; propranolol F1,62 = 2.99, P = 0.09; interaction F1,62 = 4.24, P = 0.04]. **P < 0.01 vs. vehicle; ◆◆P < 0.01 vs. RU 28362 alone.
Total spine density did not differ between treatment groups (SI Appendix, Fig. S7B). To examine whether the GR agonist treatment induced a shift in spine type, we assessed the density of four different spine types (46). Densities of long thin and stubby spines were not affected. However, mushroom spine density, which has been associated with long-term information storage and long-term potentiation (47), was significantly decreased in both apical (P < 0.01) and basal (P < 0.01) dendrites 24 h following training and GR agonist administration (Fig. 6 C and D). In contrast, the density of branched spines, which emerge after the splitting of spines (48), was significantly increased (P < 0.01). As mushroom spines in particular exhibit a high sensitivity to glutamate and have a high number of AMPA-sensitive glutamate receptors (49), the observed reduction in mushroom spine density would be consistent with the decreased c-Fos labeling and neuronal excitability within the aIC after GR agonist administration. Most importantly, propranolol administration into the BLA blocked the changes in mushroom (P < 0.01) and branched (P < 0.01) spine densities induced by the GR agonist infusion. Thus, these findings demonstrate that BLA noradrenergic activity is necessary for enabling the PrL effect on inducing structural plasticity changes within the aIC following object recognition training and memory-enhancing GR agonist treatment.
Discussion
The present study investigated whether BLA noradrenergic activity regulates PrL interactions with the aIC and dHPC in mediating glucocorticoid effects on different components of object recognition memory. This question stems from previous findings that BLA activity facilitates the consolidation of memory of emotionally arousing experiences via interactions with other brain regions (1, 2). However, accumulating evidence, particularly from human neuroimaging studies, indicates that emotional arousal induces the activation of large-scale neural networks with several functional connections beyond the BLA (12). Whether BLA activity modulates emotional arousal-associated changes in connectivity between such distal brain regions had not been investigated.
In the present study, we found that posttraining activation of GRs within the PrL but not the IL enhanced long-term memory of both the identity and location of the object in the ORM and OLM tasks. Conversely, antagonism of GRs within the PrL impaired memory consolidation in both tasks. These findings provide additional evidence that glucocorticoid administration into the mPFC facilitates long-term storage of memory of different kinds of training experiences (50, 51). Additionally, we found that by functionally blocking aIC and dHPC activity (32, 50), the GR agonist effect on memory for the identity of the objects requires an interaction of the PrL with the aIC and that the GR agonist effect on memory for the location of the object depends on a PrL interaction with the dHPC. These findings are consistent with extensive evidence that the aIC and dHPC make distinct contributions to recognition memory. To support the full complement of memory-guided behavior, the two systems must interact, and the PrL may serve as a site of integration between the two systems (17). The aIC, which has been implicated in the integration of autonomic and visceral information into emotional, cognitive, and motivational functions (52), is involved in recognition memory and the processing of information about items (18, 22, 23). Our finding, however, that functional blockade of the aIC by itself induced memory enhancement for the location of the object suggests that the aIC might also contribute to spatial and contextual information processing (53). On the other hand, the dHPC is involved in the association of an object with its context or place (21–23), and the processing of contextual information is known to crucially engage a functional cross talk between the mPFC and dHPC (24–26). Considerable evidence indicates that the mPFC and dHPC become coupled via oscillatory synchrony that reflects bidirectional flow of information (54).
In further support of such functional interactions, we found that GR agonist administration into the PrL after object recognition training induces activity-dependent neuronal activity changes within the aIC and dHPC during the postlearning consolidation period as well as structural changes in spine morphology assessed 24 h later. Our finding that GR agonist-induced enhancement of recognition memory is associated with reduced c-Fos expression, and possibly neuronal activity, of the aIC is in agreement with previous findings indicating reduced aIC neuronal activity after systemic corticosterone treatment following inhibitory avoidance training in rats (55) or after stress-induced glucocorticoid release in human subjects (56). Such reduced aIC activity might be needed to facilitate local consolidation processes, possibly by temporarily increasing the threshold for the detection of salient stimuli (23, 57). Alternatively, as the aIC might work as a “switch” between different neural network systems (58), reduced aIC activity could reflect a reallocation of neural resources and therefore disinhibition of other brain systems involved in the storage of object information and higher-order cognitive processes (56). By contrast, the GR agonist administration increased the number of c-Fos–positive pyramidal cells within CA1, without affecting c-Fos expression within the dDG region of the dHPC. In support of the view that this PrL influence on the CA1 involves the indirect NRE pathway (40–42), we also found an increased number of c-Fos–positive cells within the NRE. Together, these findings provide evidence that GR agonist administration into the PrL enhances the consolidation of different components of recognition memory via functional coupling with the aIC and dHPC.
Our primary finding is that a blockade of noradrenergic transmission within the BLA blocked these intra-PrL GR agonist administration effects on memory enhancement as well as on neuronal activity and structural plasticity changes within the aIC and dHPC. In many prior studies, we reported that arousing stimulation induces the release of norepinephrine within the BLA (2, 59) and that elevated BLA norepinephrine levels enhance the consolidation of memory of different training experiences by influencing neural plasticity and information storage in distinct brain regions, including the mPFC, aIC, and dHPC (2, 7, 9, 57, 60). The present findings provide evidence that BLA noradrenergic activation is also essential for enabling functional interactions between such other brain regions and increase our understanding of the role of the BLA in regulating emotional arousal effects on large-scale brain network dynamics.
Methods
Subjects.
Male Sprague–Dawley rats (Charles River) were kept individually in a temperature-controlled (22 °C) vivarium room (0700–1900 hours lights on). Training and testing were performed during the light phase of the cycle between 1000 and 1500 hours. All experimental procedures were in compliance with European Union Directive 2010/63/EU and approved by the Institutional Animal Care and Use Committee of Radboud University, Nijmegen, The Netherlands.
Object Recognition Task.
In the training trial, rats explored two identical objects (A1 and A2) for either 3 or 10 min. Retention was tested 24 h later. For ORM, one copy of the familiar object (A3) and a new object (B) were placed in the same location as stimuli during the training trial. For OLM, one copy of the familiar object (A3) was placed in the middle of the box (novel location); the other familiar object (A4) was placed in the same location as during the training trial. A discrimination index was calculated as the difference in time exploring the novel and familiar objects (or locations), expressed as the ratio of the total time spent exploring both objects (i.e., [(time novel − time familiar)/(time novel + time familiar)] × 100%) (ref. 23; SI Appendix, Supplementary Methods).
Drug Treatment.
RU 28362 (11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20yn-3-one, 3 or 10 ng in 0.5 μL) and RU 38486 [17β-hydroxy-11β-(4-dimethylaminophenyl)-17α-(1-propynyl)-oestra-4,9-dien-3-one, 3 or 10 ng in 0.5 μL] were dissolved in 0.5% ethanol in saline (51). Propranolol (0.3 μg in 0.2 μL) was dissolved in saline (60). PD98059 [2-(2-amino-3-methoxyphenyl)-4 H-1-benzopyran-4-one, 50 ng in 0.5 μL] was dissolved in 6% DMSO in saline (ref. 50; SI Appendix, Supplementary Methods).
c-Fos Immunoreactivity.
Brain sections were incubated with primary antibodies [c-Fos (rabbit anti–c-Fos; 1:1,000; Synaptic Systems), GAD67 (mouse anti-GAD67; 1:250; Millipore), NeuN (chicken anti-NeuN; 1:500; Millipore)], followed by appropriate fluorophore-conjugated secondary antibodies. For all brain regions, three sections per animal were imaged (20× or 40×; Leica DMI 6000B). The number of immunofluorescent cells was quantified with ImageJ 1.47v software and area corrected. For GAD67-positive puncta, images were acquired at 63× magnification on a confocal microscope (Olympus FV1000), and the number of GAD67-positive puncta per NeuN-labeled excitatory neuron was counted manually (SI Appendix, Supplementary Methods).
Statistics.
One- or two-way ANOVAs were used, when appropriate, followed by post hoc comparison tests. One-sample t tests determined whether the discrimination index was different from zero and thus whether learning had occurred. P < 0.05 was accepted as statistical significance.
Supplementary Material
Acknowledgments
We thank Chantal Schoenmaker and Masoud Ramuz for technical assistance. Research was supported by a Radboud University Topfund (to B.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901513116/-/DCSupplemental.
References
- 1.McGaugh JL. Memory–A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- 4.Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 7.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 8.Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre CK, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci USA. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McReynolds JR, Anderson KM, Donowho KM, McIntyre CK. Noradrenergic actions in the basolateral complex of the amygdala modulate Arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiol Learn Mem. 2014;115:49–57. doi: 10.1016/j.nlm.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans EJ, et al. How the amygdala affects emotional memory by altering brain network properties. Neurobiol Learn Mem. 2014;112:2–16. doi: 10.1016/j.nlm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Paz R, Pelletier JG, Bauer EP, Paré D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci. 2006;9:1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- 15.Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, Alberini CM. Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci. 2017;20:52–61. doi: 10.1038/nn.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- 17.Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: The PMAT framework. Prog Brain Res. 2015;219:45–64. doi: 10.1016/bs.pbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Bermudez-Rattoni F. The forgotten insular cortex: Its role on recognition memory formation. Neurobiol Learn Mem. 2014;109:207–216. doi: 10.1016/j.nlm.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 20.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balderas I, et al. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roozendaal B, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwindel CD, McNaughton BL. Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Prog Brain Res. 2011;193:163–177. doi: 10.1016/B978-0-444-53839-0.00011-9. [DOI] [PubMed] [Google Scholar]
- 25.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khodagholy D, Gelinas JN, Buzsáki G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science. 2017;358:369–372. doi: 10.1126/science.aan6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res. 1991;564:97–101. doi: 10.1016/0006-8993(91)91357-7. [DOI] [PubMed] [Google Scholar]
- 28.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- 30.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 32.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 33.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 35.Verma IM. Proto-oncogene fos: A multifaceted gene. Trends Genet. 1986;2:93–96. [PubMed] [Google Scholar]
- 36.Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci. 2016;8:78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrin-Terrin AS, et al. The c-FOS protein immunohistological detection: A useful tool as a marker of central pathways involved in specific physiological responses in vivo and ex vivo. J Vis Exp. 2016;110:e53613. doi: 10.3791/53613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito T, Inoue K, Takada M. Distribution of glutamatergic, GABAergic, and glycinergic neurons in the auditory pathways of macaque monkeys. Neuroscience. 2015;310:128–151. doi: 10.1016/j.neuroscience.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 39.Siucinska E. GAD67-positive puncta: Contributors to learning-dependent plasticity in the barrel cortex of adult mice. Brain Res. 2006;1106:52–62. doi: 10.1016/j.brainres.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 40.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy A, Svensson FP, Mazeh A, Kocsis B. Prefrontal-hippocampal coupling by theta rhythm and by 2-5 Hz oscillation in the delta band: The role of the nucleus reuniens of the thalamus. Brain Struct Funct. 2017;222:2819–2830. doi: 10.1007/s00429-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viena TD, Linley SB, Vertes RP. Inactivation of nucleus reuniens impairs spatial working memory and behavioral flexibility in the rat. Hippocampus. 2018;28:297–311. doi: 10.1002/hipo.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: Light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 46.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 47.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 48.Calverley RK, Jones DG. Contributions of dendritic spines and perforated synapses to synaptic plasticity. Brain Res Brain Res Rev. 1990;15:215–249. doi: 10.1016/0165-0173(90)90002-6. [DOI] [PubMed] [Google Scholar]
- 49.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roozendaal B, et al. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci USA. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig AD. How do you feel–Now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 53.Bermudez-Rattoni F, Introini-Collison IB, McGaugh JL. Reversible inactivation of the insular cortex by tetrodotoxin produces retrograde and anterograde amnesia for inhibitory avoidance and spatial learning. Proc Natl Acad Sci USA. 1991;88:5379–5382. doi: 10.1073/pnas.88.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci. 2017;18:547–558. doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- 55.Fornari RV, et al. Involvement of the insular cortex in regulating glucocorticoid effects on memory consolidation of inhibitory avoidance training. Front Behav Neurosci. 2012;6:10. doi: 10.3389/fnbeh.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Leeuwen JMC, et al. At-risk individuals display altered brain activity following stress. Neuropsychopharmacology. 2018;43:1954–1960. doi: 10.1038/s41386-018-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Barsegyan A, Nadif Kasri N, Roozendaal B. Basolateral amygdala noradrenergic activity is required for enhancement of object recognition memory by histone deacetylase inhibition in the anterior insular cortex. Neuropharmacology. 2018;141:32–41. doi: 10.1016/j.neuropharm.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 60.Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.