Abstract
Surgery is the only known curative treatment option for cholangiocarcinoma. Ex situ liver surgery and autotransplantation are promising approaches in cases that cannot be treated by conventional methods and particularly in the presence of centrally localized liver tumors as well as tumors that invade the main vascular structures. A 53-year-old female patient presented with abdominal pain and nausea. Abdominal tomography showed a tumoral mass lesion that filled the left lobe of the liver and invaded the left hepatic vein and the inferior vena cava. Cholangiocarcinoma diagnosis was reached based on biopsy findings, and the patient was scheduled for surgery as positron emission tomography did not indicate any other disease focus. The patient underwent ex situ liver resection and autotransplantation. She was discharged on the 7th postoperative day. A 68-year-old male presented with abdominal pain, weakness, and weight loss. Laboratory analysis indicated elevated carbohydrate antigen 19-9: 400 U/ml and alpha-fetoprotein (AFP): 2000 U/ml, and there was no other pathology. Abdominal tomography showed a mass that filled the center of the liver and invaded the left hepatic vein and the inferior vena cava. Pathological findings of the biopsy sample were reported as combined hepatocellular-cholangiocellular carcinoma. The patient's AFP levels continued to increase despite transcatheter arterial chemoembolization and radiofrequency ablation therapy. Surgery was decided as indocyanine green clearance test, and the result was 8.5%. He underwent ex situ liver resection and autotransplantation. Unfortunately, he died on the 4th postoperative day due to respiratory failure. Ex vivo liver resection and partial liver autotransplantation should be considered for the surgical treatment of locally advanced cholangiocarcinomas that invaded the main vascular structures.
KEYWORDS: Cholangiocarcinoma, ex situ resection, liver autotransplantation
INTRODUCTION
Currently, the only known curative treatment option for cholangiocarcinomas is complete removal of the tumor tissue with clear margins. However, surgery is not possible in the majority of patients as they present locally advanced disease and peritonitis carcinomatosis. Of all the patients who are found to be operable based on preoperative investigations, only 27%–32.8% could be taken into curative surgery. Therefore, more aggressive surgery options, including liver transplantation, gained interest as alternatives to the conventional methods.[1] As part of these studies, ex situ liver surgery and autotransplantation are now considered as promising approaches in cases that cannot be treated by conventional methods and particularly in the presence of centrally localized liver tumors as well as for tumors that invaded the main vascular structures. In the present study, we aimed to report the outcomes of our surgical treatment approaches in two cases of cholangiocarcinoma that invaded the inferior vena cava.
CASE REPORTS
Case 1
A 53-year-old female patient was referred to the hospital with complaints of abdominal pain and nausea. The patient described that her abdominal pain gradually became more severe, and she lost almost 20 kg over the last 3 months. A palpable mass was detected at abdominal midline during physical examination of the patient. The patient's medical and family history did not include any specific characteristic and her blood pressure was 100/50 mmHg and her pulse was 80 (min) at presentation. Laboratory analysis showed an increased level of carbohydrate antigen (CA) 19-9: 1500 U/ml, and there was no other pathology. Hepatitis serology was negative. Abdominal ultrasonography showed a tumoral mass of approximately 20 cm × 15 cm that almost filled the left lobe of the liver. Abdominal tomography confirmed the presence of a tumoral mass lesion that filled in the left lobe and invaded the middle and left hepatic veins and the inferior vena cava [Figure 1a]. Endoscopic examinations did not reveal any other pathology, and a diagnosis of cholangiocarcinoma was made based on preoperative investigations of the patient, and the biopsy sample obtained from the liver mass. Positron emission tomography-computed tomography (PET/CT), which was performed to scan for distant organ metastases, did not show any other disease site. Therefore, a treatment decision was made to proceed with surgery.
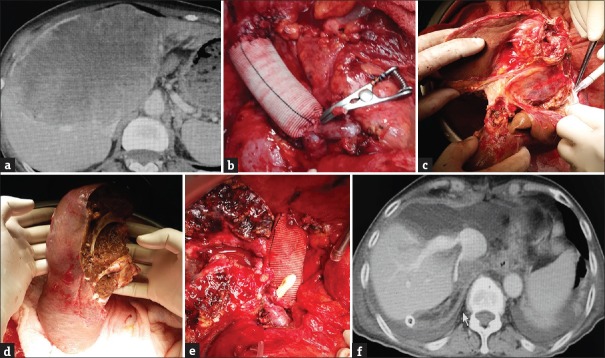
Figure 1.
(a) Abdominal tomography that shows a tumoral mass, measuring approximately 20 cm × 15 cm, which almost fills in the left liver lobe and invades the middle and left hepatic veins and the inferior vena cava. (b) Reconstruction of the inferior vena cava by a synthetic graft and appearance after insertion of a portocaval shunt. (c) Ex situ transection of the liver parenchyma by Cavitron Ultrasonic Surgical Aspirator. (d) The appearance of the remnant liver after resection of segments 1, 2, 3, 4, 5, and 8. (e) The appearance of the remnant liver after implantation. (f) Postoperative day 5 follow-up liver tomography of the first case
Case 2
A 68 year-old male patient referred with abdominal pain and weight loss. He had lost 15 kg over the last 3 months. His medical history did not include any pathology except for a smoking history of 45 pack-years. Laboratory results were as follows: aspartate aminotransferase 40 U/L, alanine aminotransferase 45 U/L, creatinine 0.7 mg/dl, CA 19-9 400 U/ml, and alpha-fetoprotein (AFP) 2000 U/ml. Hepatitis B serology was positive. Abdominal tomography of the patient showed a 12 mm × 5 mm mass that filled the central part of the liver and invaded the middle and left hepatic veins and the inferior vena cava. Biopsy findings were interpreted as combined hepatocellular-cholangiocellular carcinoma. PET/CT did not indicate any other disease site. The patient's AFP levels continued to increase despite transcatheter arterial chemoembolization and radiofrequency ablation therapy. Cell-targeted chemotherapy was initiated with sorafenib but could not be continued due to side effects. The patient's liver enzyme levels were normal during follow-up, and he underwent indocyanine green clearance test (ICG15) for surgery. The result was 8.5% (normal value, <10%). Thus, a decision was made to take the patient into surgery as there was no live donor.
Surgical technique and survival
As peritoneal deposits were not detected during abdominal extrapolation, the hepatoduodenal ligament was dissected, and the choledoc, main portal vein, and main hepatic vein were hanged [Figure 2a]. The intracaval ligaments were connected after the left and right lobes of the liver were released. After the gastroduodenal artery was connected and cut, the hepatic artery was transected from the point of connection. The choledoc was transected just above the cystic duct intersection. After the main portal vein was transected, suprarenal and suprahepatic vena cava were clamped, and the liver was taken out from the abdomen. Outside the abdomen, in a preprepared flash ice bath, the liver was perfused from the portal vein by 5% ringer lactate solution containing 2% albumin in the first case and by histidine-tryptophan-ketoglutarate solution in the second case until a clean solution came out from the hepatic vein. The inferior vena cava was reconstructed using a synthetic graft, and then, a temporary portocaval shunt was placed [Figures 1b and 2b]. Both patients were hemodynamically stable during the procedures. Liver parenchyma was transected using Cavitron Ultrasonic Surgical Aspirator (Sonoca 300; Söring GmbH, Quickborn, Germany) [Figures 1c and 2c]. Liver segments 1, 2, 3, 4, 5, and 8 were resected in the first case, while segments 4a, 8, and partially segment 5 were resected in the second case [Figures 1d and 2d]. After the remnant liver was transferred to the site, the portocaval shunt was canceled by total clamping. Vena cava was anastomized by 5/0 prolene, the port was anastomized by 6/0 prolene, and the hepatic artery was anastomized by 7/0 prolene. The bile ducts were closed by 4/0 absorbable sutures [Figures 1e and 2e]. Instant hepatic phases were recorded as 65 and 57 min in the first and second cases, respectively. Intraoperative Doppler ultrasonography was used to monitor the openness of vascular structures. There was no major surgical complication in the first case, and the patient was discharged on the 7th postoperative day [Figure 1f]. The second case developed adult respiratory failure on the 2nd postoperative day, and the patient died on the 4th postoperative day with functional liver [Figure 2f].
Figure 2.
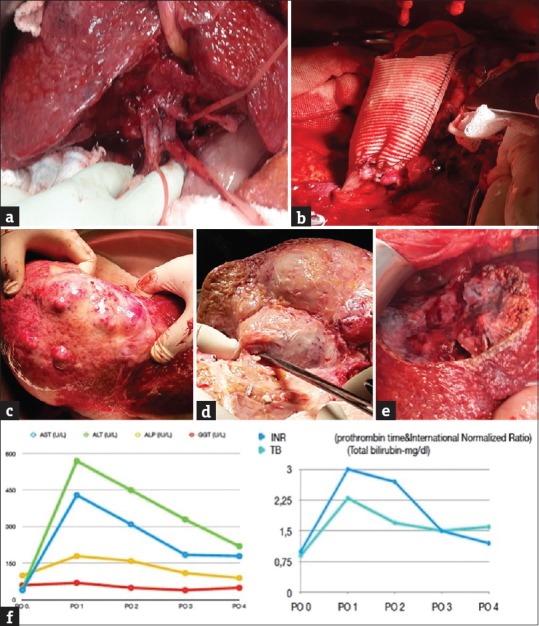
(a) The appearance of the second case ready for the explanation after hepatoduodenal ligament dissection. (b) Reconstruction of the inferior vena cava and appearance of the portocaval shunt in the second case. (c) The appearance of the malignant mass after explantation. (d) Ex situ transection of segments 4, 8, and 5 by Cavitron Ultrasonic Surgical Aspirator. (e) The appearance of the remnant liver after implantation in the second case. (f) The liver functional tests of second case after surgery
DISCUSSION
Ex situ liver surgery and partial autotransplantation (EPOT) were first defined in 1988 by Pichlmayr.[2] Hannoun and Sauvanet are among the researchers, who spent efforts to improve the ex situ technique.[3] While the technique rapidly gained acceptance, it did not turn into a commonly performed procedure due to the difficulties in implementation. The aim of EPOT technique is to increase the number of patients eligible for Ro surgery by decreasing the risk of intraoperative bleeding and liver ischemia-reperfusion damage as well as to reduce the need for liver transplantation in specific patient groups. The most important step of this technique, which allows complex vascular and biliary reconstruction in a blood-free field, is a preoperative assessment. During preoperative planning, computerized tomography is particularly effective in calculating the remnant liver volume and demonstrating the vascular anatomy in detail.[4] There are two important points to consider in EPOT technique. The first point is to ensure the patient is hemodynamically stable during the entire operation, and the second point is to protect the liver from ischemia-reperfusion damage. Majority of the researchers recommend venovenous bypass for hemodynamic stabilization, which can ensure minimal venous hypertension and prevent intestinal edema through decompression of the splenic area while maintaining a hemodynamic stability. However, venovenous bypass itself is associated with potential complications, such as air embolism, thrombotic events, and capillary leakage syndrome, secondary to mechanical damage which may lead to serious electrolyte and acid–base imbalances. To avoid the serious complications of the venovenous bypass, Wen recommended the use of a temporary portocaval shunt.[5] Zhang et al. previously described three cases of ex situ resection and autotransplantation with the use of portocaval shunt and underlined the safety of this technique.[6] Another important point to consider in EPOT is liver ischemia-reperfusion damage. The liver is more sensitive to warm than cold ischemia. Hannoun et al. demonstrated that liver resection could be performed using cold fluids in a blood-free field without causing serious liver damage.[7] There are two solutions which are frequently used for cold perfusion of the liver as follows: histidine-tryptophan-ketoglutarate solution and University of Wisconsin preservation solution. The histidine-tryptophan-ketoglutarate solution is more frequently preferred in ex situ cases as it is associated with fewer cardiovascular side effects compared to the other solution. Implantation of the remnant liver after resection is performed based on the same principles with liver transplantation from a live donor. Total vascular exclusion is a surgical method alternative to EPOT and preferred for surgical treatment of complex liver tumors that cannot be extracted by the conventional surgical techniques. Total vascular exclusion may potentially bring about blood congestion in the portal vein and the inferior vena cava.[8] When congested blood enters into systemic circulation, postoperative mortality rates may seriously increase up to 75% due to aggravated liver ischemia-reperfusion damage. The most important complication of total vascular exclusion is that it exposes the liver to warm ischemia. Previous experiences indicate that the liver may tolerate warm ischemia for 30–120 min. The time required by the surgeon is known to be longer in cases requiring complex vascular resection and reconstruction.[9] Ischemia tolerance during EPOT could be prolonged up to 4 h by hypothermic perfusion of the liver using preservation solutions. These procedures that involve complex vascular and reconstructive techniques are associated with higher mortality rates compared to conventional surgeries. Although 41 cases of ex situ resection and autotransplantation were defined between 1992 and 2007, only one of these cases is known to be alive.[10] In cases presented here, the use of temporary portocaval shunt was preferred instead of venovenous bypass. The portocaval shunt was preferred due to ease of implementation and also to ensure hemodynamic stability. The liver was cold-perfused in flash ice. We preferred this technique in our cases to protect the liver from vena cava injury and to ensure that aggressive surgery can be carried out in a blood-free field. While the first case was discharged without complication, the second case died in the early postoperative period due to acute respiratory distress syndrome. We believe that acute respiratory distress syndrome was a result of the end organ damage caused by liver ischemia-reperfusion damage.
CONCLUSION
As the survival outcomes of liver transplantation are rather poor in cholangiocarcinomas, after extensive researches, ex vivo liver resection and partial liver autotransplantation came to the forefront as alternative surgical treatment options. However, this technique is the only chance for surgical treatment of unresectable primary and secondary liver malignancies. The most important disadvantages of this technique include the risk of cancer recurrence and the high morbidity and mortality rates associated with surgery.
Informed consent
Written informed consent was obtained from the patient who participated in these cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given their consent for their images and other clinical information to be reported in the journal. The patient understand that names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chui AK, Rao AR, Wong J, Mann D, Leung KF, Lau WY, et al. Ex situ ex vivo liver resection, partial liver autotransplantation for advanced hilar cholangiocarcinoma: A case report. Transplant Proc. 2003;35:402–3. doi: 10.1016/s0041-1345(02)03774-0. [DOI] [PubMed] [Google Scholar]
- 2.Pichlmayr R, Grosse H, Hauss J, Gubernatis G, Lamesch P, Bretschneider HJ, et al. Technique and preliminary results of extracorporeal liver surgery (bench procedure) and of surgery on the in situ perfused liver. Br J Surg. 1990;77:21–6. doi: 10.1002/bjs.1800770107. [DOI] [PubMed] [Google Scholar]
- 3.Sauvanet A, Dousset B, Belghiti J. A simplified technique of ex situ hepatic surgical treatment. J Am Coll Surg. 1994;178:79–82. [PubMed] [Google Scholar]
- 4.Mönch J, Mühler K, Hansen C, Oldhafer KJ, Stavrou G, Hillert C, et al. The LiverSurgeryTrainer: Training of computer-based planning in liver resection surgery. Int J Comput Assist Radiol Surg. 2013;8:809–18. doi: 10.1007/s11548-013-0812-z. [DOI] [PubMed] [Google Scholar]
- 5.Wen PH, Lin KH, Chen YL, Hsieh CE, Ko CJ, Kuo SJ, et al. Extracorporeal hepatic resection and autotransplantation using temporary portocaval shunt provides an improved solution for conventionally unresectable HCC. Dig Dis Sci. 2013;58:3637–40. doi: 10.1007/s10620-013-2801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang KM, Hu XW, Dong JH, Hong ZX, Wang ZH, Li GH, et al. Ex situ liver surgery without veno-venous bypass. World J Gastroenterol. 2012;18:7290–5. doi: 10.3748/wjg.v18.i48.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannoun L, Delrivière L, Gibbs P, Borie D, Vaillant JC, Delva E, et al. Major extended hepatic resections in diseased livers using hypothermic protection: Preliminary results from the first 12 patients treated with this new technique. J Am Coll Surg. 1996;183:597–605. [PubMed] [Google Scholar]
- 8.Huguet C, Nordlinger B, Galopin JJ, Bloch P, Gallot D. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689–93. [PubMed] [Google Scholar]
- 9.Maeba T, Okano K, Mori S, Karasawa Y, Goda F, Wakabayashi H, et al. Retrohepatic vena cava replacement of hepatic malignancies without using total hepatic vascular exclusion or extracorporeal bypass. Hepatogastroenterology. 2001;48:1455–60. [PubMed] [Google Scholar]
- 10.Brekke IB, Mathisen Ø, Line PD, Hauss HJ. Hepatic autotransplantation with ex situ neoplasm extirpation and vena cava replacement. Hepatogastroenterology. 2003;50:2169–72. [PubMed] [Google Scholar]