Abstract
Aims:
The aim of this study was to analyze the tobacco-related genotoxic effects in individual with habit of smoking and chewing tobacco.
Materials and Methods:
The present study sample consisted of 120 individuals attending the outpatient department of D. J. College of Dental Sciences and Research, Modinagar, Uttar Pradesh (UP). The sample was divided into four groups as follows: Group I (individuals with habit of smoking tobacco), Group II (individuals with habit of chewing tobacco), Group III (individuals with habit of smoking and chewing tobacco), and Group IV control group (nontobacco-exposed individuals). Patients were asked to rinse their mouth gently with water. The exfoliated cells were obtained by scraping the buccal mucosa of individuals with a wooden spatula. The scraped cells were placed on the precleaned slides. The smears were then stained with RAPID-PAP™ and analyzed under the microscope. Data were analyzed using SPSS statistical software.
Results:
In the present study, an arbitrary unit was obtained using frequency/day multiplied by the duration of years (risk multiplication factor [RMF], a positive and significant correlation were observed between the RMF and the mean percentage of micronucleated cell count in smokers, chewers, and in individuals with both smoking and chewing habit, respectively. A weak positive and nonsignificant correlation were observed between age and mean percentage of micronucleated cells in smokers and smokers + chewers, respectively, while it was weak negative and nonsignificant in chewers. In control group, correlation between age and percentage of micronucleated cells was weak positive and nonsignificant at 5% level of significance.
Conclusion:
The micronuclei in exfoliated mucosal cells from buccal mucosa can be used as a biomarker of genotoxicity in predicting the effects of carcinogens.
KEYWORDS: Carcinogens, micronuclei, oral cancer, risk multiplication factor, tobacco
INTRODUCTION
Oral cancer is the sixth most common form of cancer in the world. It is a well-established fact that oral cancer is frequently preceded by visible lesions which are termed as potentially malignant disorders because a significant number of these lesions transform into oral cancer.[1,2]
Micronucleus (MN) is a recently upgraded topic, especially in the field of oral cancer. Micronuclei originate from chromosome fragments or whole chromosomes, which lag behind at anaphase during nuclear division. It could be argued that micronuclei in exfoliated oral epithelial cells represent a preferred target site for early genotoxic events induced by carcinogenic agents. Various studies have shown the correlation of frequency of micronuclei and severity of this genotoxic damage.[3] Micronuclei are characteristically seen in exfoliated epithelial cells such as buccal mucosa and urinary bladder wall during precancerous and cancerous conditions.[4]
The buccal cell MN is defined as the microscopically visible, round or oval cytoplasmic chromatin mass next to nucleus. Micronuclei originate from aberrant mitosis and consist of acentric chromosomes, chromatid fragments, or whole chromosomes that have failed to be incorporated in the daughter nuclei during mitosis.[5]
Micronuclei are induced in oral epithelium by a variety of substances including genotoxic agents and carcinogenic compounds in tobacco, betel nut, and alcohol. These are also seen in various conditions such as chronic tonsillitis,[6] chronic renal diseases,[7] and rheumatoid arthritis.[8]
The genotoxic and carcinogenic chemicals released from betel nut and tobacco and also the calcium hydroxide content of lime present in the betel quid are thought to be responsible for the promotion of reactive oxygen species from areca nut extracts. These reactive oxygen species can, in turn, cause damage to the DNA.[9]
The micronuclei count is increased in potentially malignant disorders such as oral submucous fibrosis, leukoplakia, erythroplakia, lichen planus, and squamous cell carcinoma.[10,11]
Thus, the quantitative estimation of micronuclei may serve as an indicator of genetic damage that has taken place. Oral carcinogenesis is a multi-step process of accumulated genetic damage leading to cell dysregulation with disruption in cell signaling. These events can be conveniently studied in the buccal mucosa, which is an easily accessible tissue for sampling cells in a minimally invasive manner.[12]
Buccal cells are the first barrier for the inhalation or ingestion route and are capable of metabolizing proximate carcinogens to reactive products.[13]
The buccal mucosa provides a barrier to potential carcinogens that can be metabolized to generate potential reactive products. Exfoliated buccal cells have been used noninvasively to successfully show the genotoxic effects of lifestyle factors such as tobacco smoking, chewing of betel nuts and/or quid, medical treatments such as radiotherapy as well as occupational exposure to potentially mutagenic and/or carcinogenic chemicals, and for studies of chemoprevention of cancer.[14]
The present study was carried out to assess the effect of tobacco on the cells of buccal mucosa by comparing the micronucleated cell count in patients with a habit of smoking tobacco (beedi or cigarette), chewing tobacco (gutkha), smoking and chewing tobacco, and in those not using tobacco in any form.
MATERIALS AND METHODS
The present study was conducted in D. J College of Dental Sciences and Research, Modinagar, UP, from 2010 to 2013. This study was approved by the institutional reviewer board (Reference no. DJ/2010/EC/OP), and an informed consent was obtained from all the participants. The individuals attending the outpatient Department and those who smoked four cigarettes/day for 2 years and chewed at least two packs of gutkha/day for 2 years were included in the study sample. Most of the individuals were occasional drinkers who consumed alcohol once a week.
The present study sample of 120 patients was divided into four groups with 30 patients in each group as follows:
Group I (individuals with habit of smoking tobacco)
Group II (individuals with habit of chewing tobacco)
Group III (individuals with habit of smoking and chewing tobacco)
Group IV Control group (nontobacco-exposed individuals).
Exclusion criterion
Individuals using anabolic androgenic steroids, chlorhexidine mouthwash and those suffering from rheumatoid arthritis, chronic renal disease, and chronic tonsillitis were excluded from the study sample.
Materials
Pair of gloves
Mouth mask
Cotton wool, probe, mirror, and tweezer
Wooden spatulas
Microscopic slides
Coplin jars
Rapid papanicolaou (PAP) kit
Diamond point glass marker
Light Microscope.
Reagents used
RAPID-PAP™ kit:-contents of RAPID-PAP™ kit which are as follows:
Nuclear stain (hematoxylin solution)
-
a) Cytoplasmic stain 2A (OG-6 solution)
b)Cytoplasmic stain 2B (Light green-Eosin)
Biofix spray (Micro-anatomy fixative)
Dibutyl Phthalate Xylol (Glass mounting medium)
Dehydrant (Propanolol)
Xylene
Wash buffer.
Working cytoplasmic stain was prepared by mixing an equal volume of cytoplasmic stain 2A (OG-6 solution) and 2B (Light green-Eosin). Working Scott's tap water buffer was prepared by adding 1 ml of RAPID-PAP™ wash buffer to 100 ml of tap water.
The patients were asked to rinse their mouth gently with water and the exfoliated cells were obtained by scraping the buccal mucosa of individuals with a wooden spatula [Figure 1]. The scraped cells were placed on the precleaned slides [Figure 2], and then, fixed and stained with Biofix spray and RAPID-PAP, respectively.
Figure 1.
Sample collection
Figure 2.
Smear preparation
Scoring procedure
The most commonly used method, i.e., the zigzag method, was followed for the screening of slides. The counting was started from the lower right corner of the slide going across the shorter dimension of the slides, then the field of vision was moved slightly laterally and screening was done vertically downward along the slide. In this way, screening was done along vertical columns until 100 intact exfoliated cells were counted [Figure 3].
Figure 3.
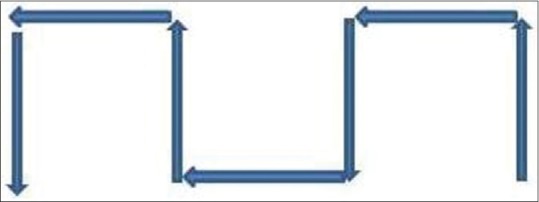
Scoring procedure (zigzag method)
Scoring criteria
Criteria for the inclusion of total cell count which are as follows:[15]
Cytoplasm intact and lying relatively flat
Little or no overlap with adjacent cells
Little or no debris/stain residue
Nucleus normal and intact, nuclear perimeter smooth, and distinct.
In order for a cell to be considered a micronucleated cell should satisfy the above criteria regarding inclusion in total cell count and the suggested criteria for identifying micronuclei which are as follows:
Rounded, smooth perimeter suggestive of membrane
Less than a third the diameter of the associated nucleus but large enough to discern shape and color
Feulgen positive (i.e., pink in bright field illumination)
Staining intensity similar to that of nucleus
Texture similar to that of nucleus
Same focal plane as nucleus
The absence of overlap or bridge to nucleus.
RESULTS
The present study was carried out to assess the effect of tobacco on the cells of the buccal mucosa by comparing the micronucleated cell count in patients with a habit of smoking tobacco (beedi or cigarette), chewing tobacco (gutkha), smoking and chewing tobacco both, and in those not using tobacco in any form. This study sample of 120 individuals was divided into four groups with 30 patients in each group as follows: Group I (individuals with a habit of smoking tobacco), Group II (individuals with a habit of chewing tobacco), Group III (individuals with a habit of smoking and chewing tobacco), and Group IV (nontobacco-exposed individuals).
Smears were taken, stained with Rapid Pap stain and analyzed with the help of binocular light microscope and manual cell counter. A total of 300 cells were examined from each slide for the presence of micronuclei.
Data were subjected to statistical analysis using unpaired t-test and Karl Pearson's correlation coefficient. Comparison of mean percentage of micronucleated cells in different groups was made using unpaired t-test and using the statistics package (Statistical Package for Scientific Studies) for Windows SPSS 20, IBM, Armonk, NY, USA. A value of P = 0.05 or less was considered for statistical significance. Karl Pearson's correlation coefficient was used to compare age, risk multiplication factor (RMF), and percentage of micronucleated cells in different groups.
All the values of age, RMF, and mean percentage of micro nucleated cells in smokers, chewers, smokers + chewers, and control groups were expressed as mean and standard deviation, respectively [Table 1]. The unpaired “t” test revealed a significant difference between different pairs of groups for mean percentage of micronucleated cells [Table 2]. However, Karl Pearson's correlation coefficient revealed a positive and significant correlation between RMF and mean percentage of micronucleated cells in each experimental group, respectively [Tables 3–5]. Further, a weak positive and nonsignificant correlation were observed between age and mean percentage of micronucleated cells in smokers and smokers + chewers, respectively, while it was weak negative and nonsignificant in chewers. In control group, correlation between age and percentage of micronucleated cells was weak positive and nonsignificant at 5% level of significance [Table 6].
Table 1.
Mean and standard deviation of age, risk multiplication factor and percentage micronucleated cells in the different groups
| Groups | Mean±SD | ||
|---|---|---|---|
| Age | RMF | Percentage of micro nucleated cells | |
| Smokers | 48.166±13.901 | 169.966±130.593 | 7.589±5.672 |
| Chewers | 33.566±11.542 | 54.033±45.094 | 10.413±3.865 |
| Smokers + chewers | 33.33±11.7 | 171.56±138.275 | 21.996±9.916 |
| Control | 34.9±10.456 | 0±0 | 1.033±1.265 |
RMF: Risk multiplication factor, SD: Standard deviation
Table 2.
Comparison of mean percentage of micronucleatead cell count in different groups by unpaired t-test
| Groups | Probability of unpaired t-test | P/significance |
|---|---|---|
| Smokers and chewers | 0.0281 | <0.05 significant |
| Smokers and smokers + chewers | 0.0000 | <0.05 significant |
| Smokers and control | 0.0000 | <0.05 significant |
| Chewers and smokers + chewers | 0.0000 | <0.05 significant |
| Chewers and control | 0.0000 | <0.05 significant |
| Smokers + chewers and control | 0.0000 | <0.05 significant |
Table 3.
Correlation of age, risk multiplication factor and percentage of micronucleated cells in smokers (Karl-Pearsons correlation)
| Age | RMF | Percentage of micronucleated cells | |
|---|---|---|---|
| Age | 1 | ||
| RMF | 0.347503766 | 1 | |
| Percentage of micronucleated cells | 0.258221645 | 0.64874403 (significant) | 1 |
RMF: Risk multiplication factor
Table 5.
Correlation of age, risk multiplication factor and percentage of micronucleated cells in (smokers + chewers (Karl-Pearsons correlation)
| Age | RMF | Percentage of micronucleated cells | |
|---|---|---|---|
| Age | 1 | ||
| RMF | 0.09197654 | 1 | |
| Percentage of micronucleated cells | 0.000969449 | 0.945532725 (significant) | 1 |
RMF: Risk multiplication factor
Table 6.
Karl-Pearson correlation coefficient between age and percentage of micronucleated cells in control group
| Group | Correlation cofficient | P/significance |
|---|---|---|
| Control (age and percentage of micronucleatead cells) | 0.1704 | 0.0987 >0.05 (NS) |
NS: Not significant
Table 4.
Correlation of age, risk multiplication factor and percentage of micronucleated cells in chewers (Karl-Pearsons correlation)
| Age | RMF | Percentage of micronucleated cells | |
|---|---|---|---|
| Age | 1 | ||
| RMF | −0.031237507 | 1 | |
| Percentage of micronucleated cells | −0.068809698 | 0.831414245 (significant) | 1 |
RMF: Risk multiplication factor
DISCUSSION
Oral cancer is considered as the sixth most common malignancy, and is a major cause of cancer morbidity and mortality worldwide. Early detection of precancerous or premalignant oral lesions would improve the survival to a great extent.[12] Oral cancer arises as a result of gradual accumulations of mutations over time, but there is a more profound event termed “chromothripsis” that represents a catastrophic change of genome structure, with widespread damage and simultaneous acquisition of multiple mutations. This latter process involves a cluster of chromosomal rearrangements.[16]
This study aimed to explore a link between the tobacco-related genotoxic effects in individuals and increased frequency of micronuclei.
Cytology has now been widely accepted as a tool in the early diagnosis of cancer. Oral exfoliative cytology can reveal various cellular alterations that include karyorrhexis, karyolysis, MN formation, pyknosis, and binucleation.[15] Micronucleated cells in buccal mucosa seem to reveal the occurrence of genotoxic, and by implication, carcinogenic agents within the oral cavity. They seem to represent an internal dosimeter in tissue exposed to genotoxic and/or carcinogenic agents.[17] MN is formed in the cell by numerous genotoxic agents that damage the chromosomes during the metaphase/anaphase transition of mitosis cell division. Micronuclei form from lagging whole chromosomes that are not incorporated into the main nucleus following anaphase.[18]
Cigarette smoking and the use of smokeless chewing tobacco have been closely related to the development of micronuclei in cells of oral mucosa, The most well-characterized chemicals found in tobacco and tobacco smoke are polycyclic aromatic hydrocarbons, such as benzopyrene, and the highly addictive alkaloid, nicotine, and its metabolites that result in DNA damage/mutation.[19]
The cytological smears were obtained from the buccal mucosa of thirty individuals from each group and were stained with RAPID PAP method [Figures 4–7].
Figure 4.
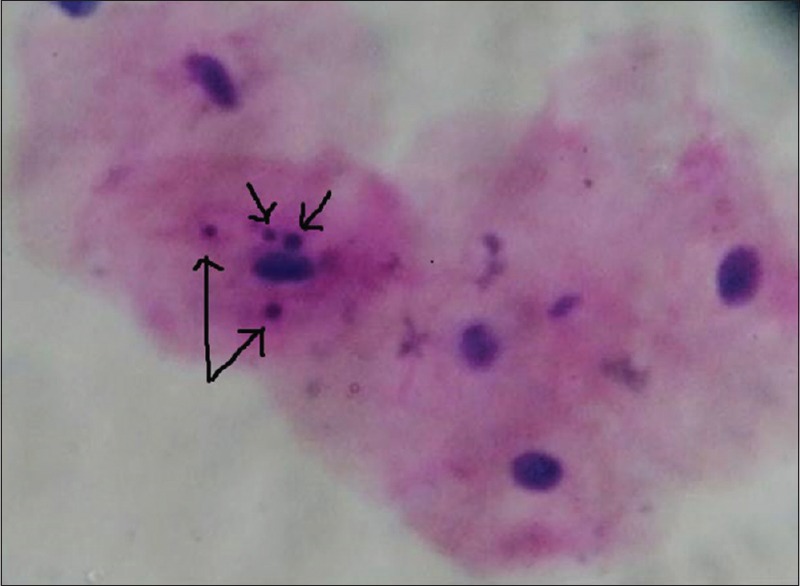
Oral exfoliated cells showing micronuclei in oral mucosa of smokers. RAPID-PAP™ Stain (×40)
Figure 7.
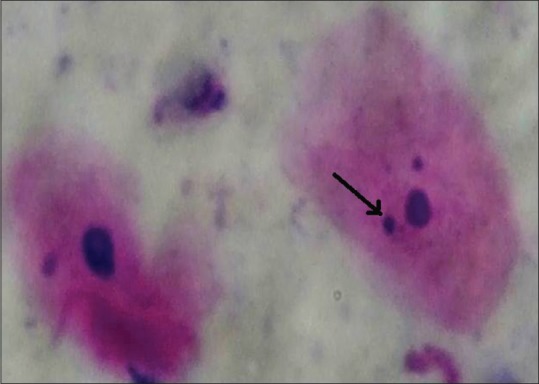
Oral exfoliated cell showing micronuclei in non-tobacco exposed subjects. RAPID-PAP™ Stain (×40)
Figure 5.
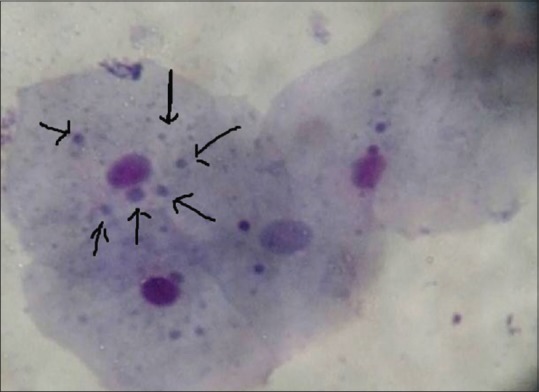
Oral exfoliated cell showing micronuclei in oral mucosa of chewers. RAPID-PAP™ Stain (×40)
Figure 6.

Oral exfoliated cell showing micronuclei in oral mucosa of individuals with smoking and chewing habit. RAPID-PAP™ Stain (×40)
In the present study, the mean percentage of micronucleated cells was 7.589 ± 5.672 in smokers, 10.413 ± 3.865 in chewers, 21.996 ± 9.916 in smokers and chewers and 1.033 ± 1.2658 in control group. Decreased percentage of micronucleated cells was observed in the buccal mucosa of the control group; however, the highest mean percentage was observed in group III individuals, which was similar to the findings reported by Sudha Sellappa et al.[20] who observed a gradual increase in the mean percentage of micronucleated cells from control, chewers and in chewers with smoking habits. In a similar study conducted by Kohli et al. concluded that there was a significant increase in the micronuclei count during the transition of normal mucosa to Oral premalignant disorders.[21]
de Geus et al. conducted a systematic review of clinical studies to evaluate the frequency of micronuclei in the oral mucosa of smokers and non-smokers in adult patients and concluded that there was a higher frequency of micronuclei exfoliated cells in smokers compared to non-smokers.[22]
Derici Eker et al. conducted a study to determine genotoxic effects of hookah smoking by using MN test and chromosome aberration methods and concluded that a significant statistical difference was observed in individuals who smoked hookah and those who did not in terms of fragment, gap, MN, and binucleus parameters, suggesting that smoking a hookah may cause genotoxic effects.[23]
A significant (P < 0.05) increase in frequency of micronucleated cells was observed in three groups (Group I, II, III) when compared to control (Group IV) which was in accordance to the findings of Kamel Ahmad Jaber Saleh[24] who also observed significant increase of micronucleated frequency in three groups (smokers but not khat chewers, khat chewers but not smokers, both smokers, and khat chewers) when compared to control (nonsmokers and non khat chewers) and concluded that the combination of chewing and smoking almost duplicate the genotoxicity effect. Kayal et al.[25] observed significantly higher frequencies of micronucleated cells in those with “no habit” healthy individuals compared to chewers using either areca nut alone, mava, tamol, tobacco with lime, dry snuff, or masher.
The mean percentage of micronucleated cells in our study was found to be significantly higher in chewers (10.4133 ± 3.8657) as compared to smokers (7.5897 ± 5.6722) and this was in accordance with the findings of Palaskar and Jindal[26] who observed significant increase in mean percentage of micronucleated cells in smokeless tobacco users (4.5 ± 0.61) as compared to smokers (2.20 ± 0.5).
The reason for increased micronucleated cell count in chewers when compared to smokers was because chewing tobacco remains in contact with the oral mucosa for longer period of time. According to the Centre for Disease Control, chewing tobacco used 7–8 times a day may be equivalent to smoking 30–40 cigarettes per day. Other factors such as the use of slaked lime and continuous contact with the oral mucosa, led to more absorption of nicotine through smokeless tobacco use. In addition, in contrast to the smokers who absorbed nicotine primarily through the pulmonary vasculature, chewing tobacco users were found to absorb nicotine through the buccal mucosa and the gastrointestinal tract mucosa.[27]
There was a significant increase in the mean percentage of micronucleated cells in individuals with both smoking and chewing habit (21.9967 ± 9.9164) when compared to smokers (7.5897 ± 5.6722) and chewers (10.4133 ± 3.8657), respectively, which was in contrast to the study conducted by Sudha Sellapa et al.[25] in which nonsignificant results were observed between the mean percentage of micronucleated cells in chewers (1.90 ± 1.03) and in chewers with smoking habit (2.00 ± 1.12).
The increased mean percentage of micronucleated cells in individuals with both smoking and chewing habit when compared to individuals with smoking and chewing habit alone in our study was due to the synergistic effect of more than one habit.
In our study, an arbitrary unit was obtained using frequency/day multiplied by duration of years (RMF), a positive and significant correlation was observed between the RMF and the mean percentage of micronucleated cell count in smokers, chewers and in individuals with both smoking and chewing habit respectively. This indicates that with increase in tobacco exposure the mean percentage of micronucleated cells also increases. These findings were by the study conducted by Patel et al.[28] in which there was a significant increase in micronuclei count with an increase in lifetime tobacco exposure in tobacco chewers.
The significant positive correlation was observed between the duration and frequency of tobacco consumption and the frequency of micronuclei in buccal mucosa as reported in earlier studies by Sarto et al.[29] and Chadha and Yadav[30] that is mainly due to increased exposure to the carcinogens to buccal mucosa present in tobacco products.
In the present study, a weak positive and nonsignificant correlation was observed between the age of individuals and mean percentage of micronucleated cell count in three groups (Group I, Group III and Group IV), while in Group II, a weak negative and nonsignificant correlation was observed. These results were in accordance with the study conducted by Tolbert et al.,[31] Armen Nersesyan et al.[32] that showed a weak positive nonsignificant correlation between these two parameters, however some earlier studies conducted by Kayal et al.,[25] Chadha and Yadav[30] showed a significant positive correlation between the age of individuals and mean percentage of micronucleated cell count.
Limitations
The weak positive correlation was observed in our study in Group I, III and IV probably due to longer period of exposure in older individuals, but weak negative correlation in Group II were due to the negative correlation of RMF with mean percentage of micronucleated cell count in these individuals. The nonsignificant association between the age and the micronucleated cell count in our study was because the number of the individuals in the specific strata was unstable and could be due to narrow age range that is 48.16 ± 13.90 years in smokers, 33.56 ± 11.54 years in chewers, 33.33 ± 11.7 years in smokers and chewers and 34.9 ± 10.45 years in control.
CONCLUSION
The following conclusions were drawn from the present study:
There is a significant increase in the frequency of micronucleated cell count in individuals with habit of smoking, chewing and both smoking as well as chewing tobacco when compared to the nontobacco exposed patients normal counterparts, indicating strong genetic damage secondary to genotoxic and carcinogenic agents released by tobacco
The significant increase in micronucleated cell count in chewers as compared to smokers indicated the greater genotoxic effect of chewing tobacco
The synergistic effect of smoking and chewing tobacco was greater than the smoking or chewing tobacco alone indicated by a significant increase of micronucleated cell count in former
A positive and significant correlation was observed for the risk of tobacco exposure and the frequency of micronucleated cell count
No significant correlation was observed between the age of the individuals and the frequency of micronucleated cell count.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta L, Naik SK, Balakrishnan S. A new feature selection and classification scheme for screening of oral cancer using laser induced fluorescence. Med Biom LNCS. 2007;4901:1–8. [Google Scholar]
- 2.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivasankari NP, Reddy KS, Kaur S, Vivekanandam S, Rao KR. Micronucleus index: An early diagnosis in oral carcinoma. J Anat Soc India. 2008;57:8–13. [Google Scholar]
- 4.Jadhav K, Gupta N, Ahmed MB. Micronuclei: An essential biomarker in oral exfoliated cells for grading of oral squamous cell carcinoma. J Cytol. 2011;28:7–12. doi: 10.4103/0970-9371.76941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellappa S, Balakrishnan M, Raman S, Palanisamy S. Induction of micronuclei in buccal mucosa on chewing a mixture of betel leaf, areca nut and tobacco. J Oral Sci. 2009;51:289–92. doi: 10.2334/josnusd.51.289. [DOI] [PubMed] [Google Scholar]
- 6.Unal M, Celik A, Ateş NA, Micozkadioǧlu D, Derici E, Pata YS, et al. Cytogenetic biomonitoring in children with chronic tonsillitis: Micronucleus frequency in exfoliated buccal epithelium cells. Int J Pediatr Otorhinolaryngol. 2005;69:1483–8. doi: 10.1016/j.ijporl.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Roth JM, Restani RG, Gonçalves TT, Sphor SL, Ness AB, Martino-Roth MG, et al. Genotoxicity evaluation in chronic renal patients undergoing hemodialysis and peritoneal dialysis, using the micronucleus test. Genet Mol Res. 2008;7:433–43. doi: 10.4238/vol7-2gmr441. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Remus C, Dorazco-Barragan G, Aceves-Avila FJ, Alcaraz-Lopez F, Fuentes-Ramirez F, Michel-Diaz J, et al. Genotoxicity assessment using micronuclei assay in rheumatoid arthritis patients. Clin Exp Rheumatol. 2002;20:208–12. [PubMed] [Google Scholar]
- 9.Kumar V, Rao NN, Nair NS. Micronuclei in oral squamous cell carcinoma. A marker of genotoxic damage. Indian J Dent Res. 2000;11:101–6. [PubMed] [Google Scholar]
- 10.Kamboj M, Mahajan S. Micronucleus – An upcoming marker of genotoxic damage. Clin Oral Investig. 2007;11:121–6. doi: 10.1007/s00784-006-0075-y. [DOI] [PubMed] [Google Scholar]
- 11.Buajeeb W, Kraivaphan P, Amornchat C, Suthamajariya K. Reduction of micronuclei in oral lichen planus supplemented with beta-carotene. J Oral Sci. 2008;50:461–7. doi: 10.2334/josnusd.50.461. [DOI] [PubMed] [Google Scholar]
- 12.Jois HS, Kale AD, Mohan Kumar KP. Micronucleus as potential biomarker of oral carcinogenesis. IJDA. 2010;2:197–202. [Google Scholar]
- 13.Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659:93–108. doi: 10.1016/j.mrrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, et al. Buccal micronucleus cytome assay. Nat Protoc. 2009;4:825–37. doi: 10.1038/nprot.2009.53. [DOI] [PubMed] [Google Scholar]
- 15.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat Res. 1992;271:69–77. doi: 10.1016/0165-1161(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 16.Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, et al. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–84. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SC, Soberman A, Stahl SS. A study of the cornification of the oral mucosa of young male adults. J Dent Res. 1951;30:4–11. doi: 10.1177/00220345510300011301. [DOI] [PubMed] [Google Scholar]
- 18.Lewis CW, Golsteyn RM. Cancer cells that survive checkpoint adaptation contain micronuclei that harbor damaged DNA. Cell Cycle. 2016;15:3131–45. doi: 10.1080/15384101.2016.1231287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campain JA. Nicotine: Potentially a multifunctional carcinogen? Toxicol Sci. 2004;79:1–3. doi: 10.1093/toxsci/kfh106. [DOI] [PubMed] [Google Scholar]
- 20.Sudha S, Kripa SK, Shibily P, Shyn J. Elevated frequencies of micronuclei and other nuclear abnormalities of chrome plating workers occupationally exposed to hexavalent chromium. Iran J Cancer Prev. 2011;4:119–24. [PMC free article] [PubMed] [Google Scholar]
- 21.Kohli M, Ahuja P, Mehendiratta M, Sharma M, Dutta J. Micronucleus assay: An early diagnostic tool to assess genotoxic changes in patients with tobacco use, oral leukoplakia and oral submucous fibrosis. J Clin Diagn Res. 2017;11:ZC28–32. doi: 10.7860/JCDR/2017/27711.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Geus JL, Wambier LM, Bortoluzzi MC, Loguercio AD, Kossatz S, Reis A, et al. Does smoking habit increase the micronuclei frequency in the oral mucosa of adults compared to non-smokers? A systematic review and meta-analysis. Clin Oral Investig. 2018;22:81–91. doi: 10.1007/s00784-017-2246-4. [DOI] [PubMed] [Google Scholar]
- 23.Derici Eker E, Koyuncu H, Şahin NÖ, Yüksel A, Berköz M, Budak Diler S, et al. Determination of genotoxic effects of hookah smoking by micronucleus and chromosome aberration methods. Med Sci Monit. 2016;22:4490–4. doi: 10.12659/MSM.898593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad K, Saleh J. Synergistic effect of Catha edulis and smoking on exfoliated buccal cells in south west region of Saudi Arabia (Asser) Acta Pharm Sci. 2010;52:453–60. [Google Scholar]
- 25.Kayal JJ, Trivedi AH, Dave BJ, Nair J, Nair UJ, Bhide SV, et al. Incidence of micronuclei in oral mucosa of users of tobacco products singly or in various combinations. Mutagenesis. 1993;8:31–3. doi: 10.1093/mutage/8.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Palaskar S, Jindal C. Evaluation of micronuclei using Papanicolaou and May Grunwald Giemsa stain in individuals with different tobacco habits – A comparative study. J Clin Diagn Res. 2010;4:3607–13. [Google Scholar]
- 27.Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control. 1999;8:387–92. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel BP, Trivedi PJ, Brahmbhatt MM, Shukla SN, Shah PM, Bakshi SR, et al. Micronuclei and chromosomal aberrations in healthy tobacco chewers and controls: A study from Gujarat, India. Arch Oncol. 2009;17:7–10. [Google Scholar]
- 29.Sarto F, Finotto S, Giacomelli L, Mazzotti D, Tomanin R, Levis AG, et al. The micronucleus assay in exfoliated cells of the human buccal mucosa. Mutagenesis. 1987;2:11–7. doi: 10.1093/mutage/2.1.11. [DOI] [PubMed] [Google Scholar]
- 30.Chadha P, Yadav JS. Studies on the genotoxicity of Gutkha. Int J Hum Genet. 2011;11:277–82. [Google Scholar]
- 31.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: A field test in snuff users. Am J Epidemiol. 1991;134:840–50. doi: 10.1093/oxfordjournals.aje.a116159. [DOI] [PubMed] [Google Scholar]
- 32.Nersesyan A, Muradyan R, Kundi M, Knasmueller S. Impact of smoking on the frequencies of micronuclei and other nuclear abnormalities in exfoliated oral cells: A comparative study with different cigarette types. Mutagenesis. 2011;26:295–301. doi: 10.1093/mutage/geq092. [DOI] [PubMed] [Google Scholar]


