Abstract
Purpose
Although both anti-PD-1 antibody and treatments using anti-PD-L1 antibody are currently in clinical use, their therapeutic effects vary according to cancer type. One of the factors accounting for this variability is the expression level of the immune checkpoint molecule that differs between cancer types; thus, it is important to clarify the relationship between clinical outcomes and immune checkpoint molecules for all types of human cancer. The purpose of this study is to evaluate the clinical outcome of osteosarcoma in relation to PD-L1, PRF, GZMB, and IFNγ expression.
Methods
Using 19 clinical specimens of osteosarcoma, we examined the expression of PD-L1, PRF, GZMB, and IFNγ in relation to their clinical outcomes.
Results
PD-L1 expression correlated with early metastatic formation in clinical specimens of osteosarcoma, and the group with highly expressed functional markers for T cells such as PRF and GZMB resulted in a long overall survival time.
Conclusion
This is the first study to elucidate the clinical outcomes of osteosarcoma in relation to PD-L1, PRF, GZMB, and IFNγ expression. This study provides valuable information regarding the clinical indication and prediction of effect for anti-PD-1 antibody in osteosarcoma.
Keywords: anti-PD-1 antibody, perforin, granzyme B, IFNγ, osteosarcoma, clinical outcome
Introduction
Osteosarcoma is the most common tumor among primary malignant bone tumors, and patients are treated by a combination of pre- and post-operative chemotherapy and surgery.1 The current standard chemotherapy for osteosarcoma is a triple-combination therapy consisting of methotrexate, adriamycin, and cisplatin. The 5-year survival rate for the triple-combination therapy regimen has demonstrated an improvement of 50%–70% or more when the tumor is localized.1–7 Despite these improvements, existing treatments remain ineffective for select patients with a 5-year survival rate of 20%–30% for patients with metastasis at first visit and 20% for recurrent cases.1,4,7,8 The poor efficacy of non-localized cases could be partially attributed to the lack of progress in the development of novel drugs such as molecular targeted drugs. Therefore, a better understanding of the pathology of osteosarcoma and the development of novel therapeutic agents with new mechanism of action may potentially improve the survival outcome of patients with metastatic disease.
On the other hand, in the microenvironment of cancer, the role of innate immunity is inhibited in a process known as immune tolerance. One of the mechanisms of immune tolerance is the immune checkpoint mechanism, whereby T cells are suppressed to prevent excessive immune responses. Several types of immune checkpoint molecules are known, namely the cytotoxic T-lymphocyte antigen 4 (CTLA-4) and lymphocyte activation gene 3 (LAG-3), in addition to programmed cell death 1 (PD-1) and its ligand (PD-L1).8,9 PD-1 is expressed on the surface of cytotoxic T cells and transmits suppressive signals to T cells by binding to PD-L1. Normal cells are believed to express PD-L1 in an inflammatory environment, suppress T cells, and prevent excessive tissue damage from long-term persistence and spread of inflammation.10 However, in some types of cancers, PD-L1 is reported to be expressed on the surface of cancer cells by means of stimulation by IFNγ, a proinflammatory cytokine.8,11–13 Cancer has been known to prevent attacks from the immune system by suppressing T cell activation by binding the PD-L1 that are expressed on cancer cells to the PD-1 on cytotoxic T cells.14
Anti-PD-1 antibody and treatments using anti-PD-L1 antibody are already in clinical use; however, the efficacy of these treatments differs between cancer types. One of the factors contributing to this variability is different expression levels of PD-L1 that depend on cancer type,15 and there is a need for further clarification in terms of understanding the relationship between clinical outcomes and immune check point molecules for all types of human cancer. Although osteosarcoma is considered a cancer that is especially responsive to immunotherapy,16 the efficacy of treatments that utilizes anti-PD-1 and anti-PD-L1 antibodies remains relatively unexplored. We analyzed gene expression in clinical specimens of osteosarcoma, and our study for the first time has clarified the relationship of PD-L1 and T cell activation markers with clinical outcomes of osteosarcoma.
Material and methods
Clinical specimens and analysis
Since 1995, clinical specimens from cases diagnosed and treated by the Department of Orthopaedic Surgery at Shinshu University Hospital were collected, frozen, and stored. The subjects were diagnosed with conventional osteosarcoma and biopsy specimens analyzed only when a subject had not received chemotherapy prior to sample collection.
Expressions of PD-L1, GZMB, PRF, and IFNγ were evaluated using real-time PCR. Iliac cancellous bone of healthy control subject (one sample) was adjusted as baseline (set to 1) and subsequently evaluated. Survival and metastasis were evaluated using the Kaplan–Meier method according to high and low expression groups that indicated either a higher or lower expression than the control group, respectively.
All patients provided written informed consent regarding the use of their specimens for basic research. Permission for using human specimens was acquired from the Genetic Analysis Ethics Committee of Shinshu University Hospital (approval number 470). Patient information was made anonymous to ensure their privacy. This study was conducted in accordance with the Declaration of Helsinki.
RNA-based expression analysis
For quantitative reverse-transcriptase PCR (RT-PCR) analysis of immune related genes in human osteosarcoma clinical samples, RNA extracted with Trizol (Invitrogen, Carlsbad, CA, USA) was transcribed into cDNA using ReverTra Ace reverse transcriptase (Toyobo, Tokyo, Japan). The genes consisting of human PD-L1, IFNγ, PRF and GZMB were analyzed by qPCR. RT-PCR was performed using SYBR Green Master Mixes (Life Technologies, Carlsbad, CA, USA) with the StepOnePlus system (Life Technologies, Carlsbad, CA, United States). mRNA levels were calculated by normalizing to the house keeping gene GAPDH using the ΔCT method. Changes in gene expression of samples were expressed relative to that of untreated samples. Sequences of the primers used for PCR and RT-PCR analyses are described in Table 1.
Table 1.
Primers used in analysis of immune related genes in human osteosarcoma
| Species | Target gene | Forward | Reverse |
|---|---|---|---|
| Human | GAPDH | GCGCCGTCAGGCTGAGAAC | TGGTGAAGACGGCCGTGGA |
| Human | PRF1 | ATTCTCAAGCCCTGCAGTCACA | AATGAAGGCTTTGCCACACCA |
| Human | GZMB | GCTGACAGCTGCTCACTGTTG | GCAGTAGCATGATGTCGTTGGA |
| Human | PDL1 | CAATGTGACCAGCACACTGAGAA | GGCATAATAAGATGGCTCCCAGAA |
| Human | IFNγ | CTTTAAAGATGACCAGAGCATCCAA | GGCGACAGTTCAGCCATCAC |
Statistical analysis
Statistical analysis was performed with SPSS software version 25 (IBM, Armonk, NY, USA) using Pearson’s correlation analysis. Kaplan–Meier curves were created by the same software and log rank analysis was used for comparison between groups. P-values less than 0.05 were considered statistically significant.
Results
Since 1995, 28 cases of osteosarcoma were treated at our institution, of which 22 cases were conventional osteosarcomas.29,30 Because the biopsies for two of these cases were conducted at another institution, frozen specimens prior to the initial treatment were not stored at our hospital. In addition, another frozen specimen was lost. Thus, the 19 remaining cases were included in this study. Demographic data are shown in Table 2. The effect of preoperative chemotherapy was evaluated by pathological examination, and cases were classified into effective and ineffective groups. Because one of the 19 cases was contraindicated for surgery of the primary lesion, 18 cases were evaluated for response to chemotherapy and degree of tumor resection.
Table 2.
Background and gene expression in clinical specimens of osteosarcoma
| Age (years) | 6–77 | Range (average ± SD) (24.7±19.8) |
|---|---|---|
| Gender | Female 6 | Male 13 |
| Location | Femur | Distal 11 Mid shaft 1 Proximal 1 |
| Tibia | Proximal 2 | |
| Fibula | Proximal 1 Mid shaft 1 |
|
| Humerus | Proximal 2 | |
| Metastasis at first visit | Yes | 10 |
| No | 9 | |
| Response to chemotherapy | Good | 12 |
| Bad | 6 | |
| Degree of tumor resection | R0 | 18 |
| Follow up (days) | 245–7,123 | (1,921±1,906) |
| Outcome | CDF | 6 |
| NED | 1 | |
| AWD | 3 | |
| DOD | 9 | |
| GZMB | 0.0073–3.4 | Range (average ± SD) (0.87±1.1) |
| High 4 | Low 15 | |
| PRF | 0.0011–4.3 | Range (average ± SD) (0.79±1.3) |
| High 4 | Low 15 | |
| IFNγ | 0.0048–5.7 | Range (average ± SD) (0.73±1.5) |
| High 2 | Low 17 | |
| PD-L1 | 0.062–9.3 | Range (average ± SD) (2.0±2.8) |
| High 8 | Low 11 |
Abbreviations: AWD, alive with disease; CDF, continuously disease-free; DOD, dead of disease; NED, no evidence of disease.
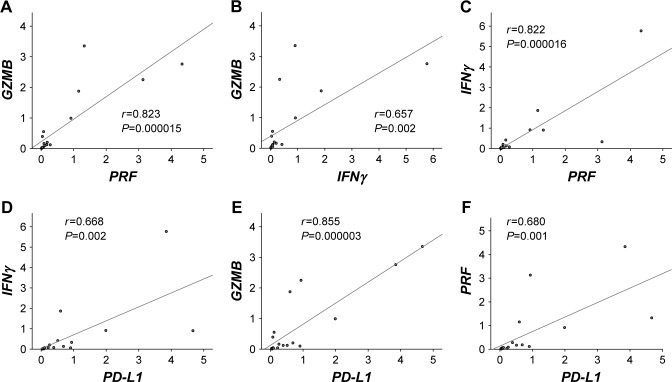
We first evaluated the correlation between PD-L1 and T cell activation markers in osteosarcoma. There was a considerably strong positive correlation between the expression of PRF and GZMB (Figure 1A, r=0.823, P<0.0001). Expression of IFNγ also showed a significant positive correlation with the expression of GZMB and PRF (Figure 1B, r=0.657, P=0.0022; Figure 1C, r=0.822, P<0.0001, respectively). Expression of PD-L1 exhibited a significant positive correlation with the expression of IFNγ, GZMB, and PRF (Figure 1D, r=0.668, P=0.0018; Figure 1E, r=0.855, P<0.0001; Figure 1F, r=0.680, P=0.0013, respectively).
Figure 1.
Correlation between each T cell activation marker and PD-L1.
Notes: The relationship between gene expressions and clinical outcomes of human osteosarcoma clinical specimen was evaluated. (A) Scatter plot and approximate curve of GZMB and PRF expression (correlation coefficient, r=0.823; P<0.0001). (B) Scatter plot and approximate curve of GZMB and IFNγ expression (correlation coefficient, r=0.657; P=0.0022. (C) Scatter plot and approximate curve of IFNγ and PRF expression (correlation coefficient r=0.822, P<0.0001). (D) Scatter plot and approximate curve of expression of IFNγ and PD-L1 (correlation coefficient, r=0.668; P=0.0018). (E) Scatter plot and approximate curve of expression of GZMB and PD-L1 (correlation coefficient, r=0.855; P<0.0001). (F) Scatter plot and approximate curve of expression of PRF and PD-L1 (correlation coefficient r=0.680; P=0.0013).
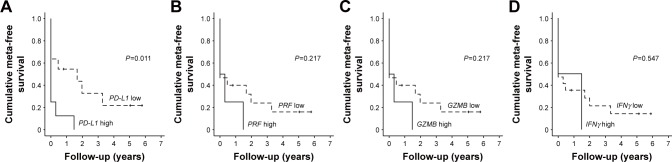
Two groups were divided into high and low PD-L1 expression groups, and a survival curve was created with metastasis as an endpoint. Metastases were detected significantly earlier in the high PD-L1 expression group (Figure 2A, P=0.011). The same deductions were made in terms of PRF, GZMB and IFNγ. There was also no significant relationship between the time of onset of lung metastases and the expression levels of PRF, GZMB, and IFNγ (P=0.217, P=0.217, P=0.547 in Figure 2B–D; respectively).
Figure 2.
Relationship between PD-L1, each T cell activation marker, and metastasis.
Notes: (A) Kaplan–Meier curves when metastasis is defined as endpoint. We classified groups into high- and low-expression of PD-L1 (P=0.011, log rank analysis). (B–D) From the left, grouping was done with high and low PRF, GZMB, IFNγ expression, P=0.217, P=0.217, P=0.547 in log rank analysis.
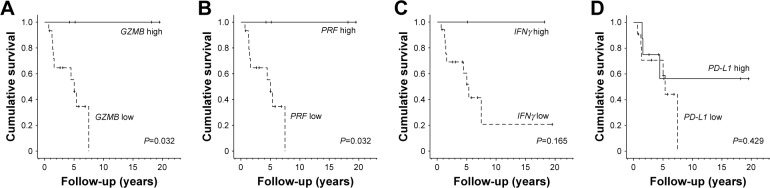
A comparison of survival curves showed that the overall survival rate of the high GZMB or PRF expression group was significantly higher (both P=0.032 in Figure 3A and B, respectively). On the other hand, the high and low levels of IFNγ and PD-L1 did not affect the overall survival rate (Figure 3C and D, respectively).
Figure 3.
Relationship between PD-L1, each T cell activation marker, and overall survival.
Notes: (A) Kaplan–Meier curve with death as endpoint. We classified groups into high- and low-expression of GZMB (P=0.032, log rank analysis). (B–D) From the left, grouping was done by high and low expression of PRF, IFNγ, PD-L1 expression, and in log rank analysis P=0.032, P=0.165, P=0.429, respectively.
Discussion
The current understanding in regard to the efficacy of anti-PD-1 antibody against osteosarcoma is unclear. In basic research, a report has demonstrated the suppression of lung metastasis when human osteosarcoma is subcutaneously transplanted into humanized mice.17 On the other hand, multiple clinical trials have been conducted for human osteosarcoma, in addition to those currently in progress (NCT 02301039, NCT 02304458, NCT 03282344, and others). However, there are no reports that place their focus on clinical trials of osteosarcoma alone, and existing reports only describe osteosarcoma as a segment of broader research on multiple types of sarcomas.
Paoluzzi et al examined the therapeutic effect of nivolumab in advanced sarcoma and reported that one case was partial response (PR), one case was stable disease (SD), and two cases were progressive disease (PD) for four cases of osteosarcoma.18 In addition, Tawbi et al reported the interim progress of pembrolizumab’s Phase II study on advanced sarcoma. Among 22 cases of osteosarcoma that were reported, one case was PR, six cases were SD, and 15 cases were PD.19,20 Normally, the response rate of the drug is proportional to cases with complete response (CR) and PR. However, since osteosarcomas produce an extracellular matrix of osseous tissues, many cases do not result in the reduction of tumor size despite effective chemotherapy. Therefore, in the treatment of osteosarcoma, the effectiveness of clinical chemotherapy is not only determined by changes to the size of the tumor, but also by the results of pathological examination.2,6
Because currently reported clinical trials include subjects with soft tissue sarcoma, only Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1), which is based on changes in tumor size, is used to determine its efficacy. Pathological evaluation has not been reported in these current clinical trials, and the effectiveness of anti-PD-1 antibody against osteosarcoma remains difficult with the limited results of these trials alone; thus, it is important to analyze gene expressions using clinical specimens and to evaluate the correlation of clinical outcomes with PD-L1 and T cell activation markers.
In gene expression analysis using clinical specimens, it has been reported that the prognosis is poor when the tumor PD-L1 is highly expressed in melanoma and lung cancer, which have already been confirmed to have anti-PD-1 antibody efficacy.21–23 In osteosarcoma, a high expression of PD-L1 has been reported to decrease survival time.24 There have been no standards for classifying the degree of PD-L1 expression, and as a status quo, such classifications have varied according to each individual study.25 One study used normal bone as a control for osteosarcoma to analyze PD-L1 expression using tissue microarray,26 and we classified our high- and low-expression groups by referencing the normal bone from this study.
In this study, metastasis occurred at an early stage when the PD-L1 is highly expressed in clinical specimens of osteosarcoma, which is consistent with previous findings. This result indicates that the prognosis of osteosarcoma patients may be deteriorated due to the interaction between PD-1 and PD-L1.
On the other hand, it has been reported that GZMB and PRF produced by T cells are important for tumor immunity.27 For osteosarcoma, GZMB expression has been reported to increase with the administration of anti-PD-1 antibody in mouse models of osteosarcoma and humanized mice.17,28 In this study, we evaluated T cell activation markers for clinical specimens of osteosarcoma and showed that good overall survival is obtained with high expression of GZMB and PRF. This suggests that good survival can be attained when T cell activity is high in human osteosarcoma and that the reactivation of T cells by anti-PD-1 antibody can improve the life prognosis of human osteosarcoma.
In this study, the relationship between T cell activity markers and PD-L1 was also evaluated, indicating that IFNγ, GZMB, and PRF show significant positive correlation with PD-L1. Previous reports have shown that IFNγ induces PD-L1 expression in tumors such as angiosarcoma.11–13,29 There is also a report that IFNγ production of CD8+ T cells is elevated by the administration of anti-PD-L1 antibody,30 and another report states that the administration of anti-PD-1 antibody increases the expression of GZMB within the tumor.28 These reports indicate that PD-1/PD-L1 interactions demonstrate the suppression of T cell activity markers. A positive correlation between T cell activity markers and PD-L1 expression is thought to be due to the increased expression of PD-L1 by T cell activation. In addition, the suppression of T cells due to increased PD-L1 expression may also suppress PD-L1 expression, resulting in a potential decrease in PD-L1 expression; however, further examination is recommended for future studies.
Limitations
A limitation of this study was the small sample size; thus, we were unable to use multivariate analysis to adjust for confounding factors. However, osteosarcoma is a rare type of cancer. As a point of comparison, colorectal cancer is the most common type of cancer in Japan and constitute 130,000 new cases per year, whereas the incidence of osteosarcoma is only 300 new cases per year, which is only 0.23% of colorectal cancer. Although the sample size was relatively small in this study, we believe that the fact that we were able to obtain results with statistical significance is of valuable importance.
Conclusion
For the first time, to the best of our knowledge, we clarified the relationship between the clinical outcomes of osteosarcoma in relation to PD-L1, perforin (PRF), granzyme B (GZMB), and IFNγ expression. This study provides important information regarding the clinical indication and prediction of effect for anti-PD-1 and anti-PD-L1 antibodies in osteosarcoma.
Acknowledgments
We would like to thank our laboratory assistant, Mukuno Midori, for her indispensable support in our research. We would also like to thank Sho Steven Sugita at OrthoTranslations (Matsumoto, Japan) for English language editing services. This research was supported by the Japan Society for the Promotion of Science KAKENHI (grant nos. 16K20045 and 18K16652).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- 2.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma Study Group protocols. J Clin Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative map: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33(20):2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3(2):221–243. doi: 10.1007/s40744-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto A, Iwamoto Y. Current status and perspectives regarding the treatment of osteo-sarcoma: chemotherapy. Rev Recent Clin Trials. 2008;3(3):228–231. doi: 10.2174/157488708785700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taran SJ, Taran R, Malipatil NB. Pediatric osteosarcoma: an updated review. Indian J Med Paediatr Oncol. 2017;38(1):33–43. doi: 10.4103/0971-5851.203513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209(2):201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abiko K, Mandai M, Hamanishi J, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19(6):1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 12.Honda Y, Otsuka A, Ono S, et al. Infiltration of PD-1-positive cells in combination with tumor site PD-L1 expression is a positive prognostic factor in cutaneous angiosarcoma. Oncoimmunology. 2017;6(1):e1253657. doi: 10.1080/2162402X.2016.1253657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of IFNγ in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res. 2016;22(10):2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Li B, Ren Y, Ye Z. T-cell-based immunotherapy for osteosarcoma: challenges and opportunities. Front Immunol. 2016;7:353–353. doi: 10.3389/fimmu.2016.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer. 2018;65(9):e27227. doi: 10.1002/pbc.27227. [DOI] [PubMed] [Google Scholar]
- 17.Zheng B, Ren T, Huang Y, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J Hematol Oncol. 2018;11(1):16. doi: 10.1186/s13045-018-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoluzzi L, Cacavio A, Ghesani M, et al. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6(1):24. doi: 10.1186/s13569-016-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess MA, Crowley J, Reinke DK, et al. Sarc 028: a phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (PTS) with advanced sarcomas. J Clin Oncol. 2015;33(15 Suppl):TPS10578. [Google Scholar]
- 20.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and Bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 22.Ito S, Okano S, Morita M, et al. Expression of PD-L1 and HLA class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann Surg Oncol. 2016;23(Suppl 4):508–515. doi: 10.1245/s10434-016-5376-z. [DOI] [PubMed] [Google Scholar]
- 23.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koirala P, Roth ME, Gill J, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep. 2016;6:30093. doi: 10.1038/srep30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–5039. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torabi A, Amaya CN, Wians FH, Jr, Bryan BA. PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology. 2017;49(5):506–513. doi: 10.1016/j.pathol.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 28.Ratti C, Botti L, Cancila V, et al. Trabectedin overrides osteosarcoma differentiative block and reprograms the tumor immune environment enabling effective combination with immune checkpoint inhibitors. Clin Cancer Res. 2017;23(17):5149–5161. doi: 10.1158/1078-0432.CCR-16-3186. [DOI] [PubMed] [Google Scholar]
- 29.Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 30.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]