Abstract
Background
Antiretroviral therapy (ART), when taken consistently, reduces morbidity and mortality associated with human immunodeficiency virus and viral transmission. Suboptimal treatment adherence is associated with regimen complexity and high tablet burden. Single-tablet regimens (STRs) provide a complete treatment regimen in a single tablet. This study examined the relationship between STRs (vs multiple-tablet regimens [MTRs]), treatment adherence, and viral suppression.
Methods
A systematic review was conducted to identify studies investigating at least one of the following: (1) STR/MTR use and adherence; (2) levels of adherence and viral suppression; and (3) STR/MTR use and viral suppression. Meta-analysis was performed to assess the relationship between STR vs MTR use and adherence in observational settings at ≥95% and ≥90% adherence thresholds.
Results
In total, 29 studies were identified across the three objectives; two studies were relevant for all objectives. STRs were associated with higher treatment adherence than MTRs in 10/11 observational studies: a 63% greater likelihood of achieving ≥95% adherence (95% CI=1.52–1.74; P<0.001) and a 43% increase in the likelihood of achieving ≥90% adherence (95% CI=1.21–1.69; P<0.001). Higher adherence rates were associated with higher levels of viral suppression in 13/18 studies. Results were mixed in five studies investigating the association between STR or MTR use and viral suppression.
Conclusion
Although the direct effect of STRs vs MTRs on viral suppression remains unclear, this study provided a quantitative estimate of the relationship between STRs and ART adherence, demonstrating that STRs are associated with significantly higher ART adherence levels at 95% and 90% thresholds. Findings from the systematic review showed that improved adherence results in an increased likelihood of achieving viral suppression in observational settings. Future research should utilize similar measures for adherence and evaluate viral suppression to improve assessment of the relationship between pill burden, adherence, and viral suppression.
Keywords: human immunodeficiency virus, antiretroviral therapy, treatment adherence, systematic review, meta-analysis
Introduction
The introduction of highly effective antiretroviral therapy (ART) has transformed the treatment of people living with human immunodeficiency virus (PLWH). When patients are optimally adherent to potent combination ART, human immunodeficiency virus (HIV) is transformed from a potentially fatal condition to a manageable chronic disease. Current national guidelines in the USA recommend a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with an integrase strand transfer inhibitor (INSTI) for initial treatment in ART-naïve individuals.1
Combination ART has multiple individual and societal benefits mediated by achieving viral suppression, which reduces HIV-associated morbidity and mortality, increases health-related quality of life (HRQoL), and prevents HIV transmission. However, ART regimens differ in dosing complexity, toxicity, and tolerability – factors that influence adherence to treatment and outcomes.2–6 Suboptimal adherence reduces the likelihood of viral suppression,4,7 which in turn increases the risk of transmission8 and the development of drug resistance, thereby limiting future treatment options.7 Suboptimal adherence has both clinical and economic consequences, including accelerated disease progression and mortality,9–11 decreased HRQoL,12 and higher healthcare costs.13–15
Single-tablet regimens (STRs), which combine a complete treatment regimen into a single fixed-dose tablet, have the potential to address regimen complexity and high pill burden. STRs have been shown to improve adherence to antihypertensive agents.16 Generally, studies assessing real-world medication-taking behaviors in PLWH show greater adherence to STRs than to multiple-tablet regimens (MTRs).6,17–19 In a study published in 2000, high adherence (taking ≥95% of prescribed doses) was associated with greater viral suppression, and avoidance of drug resistance and HIV-associated complications.20 More recently, in 2015, a meta-analysis looked at the impact of pill burden on viral suppression.21 While the authors found that STRs were associated with greater viral suppression than MTRs, the results were based on only three studies and did not include sensitivity analyses. The development of new treatment options, including several STRs with improved tolerability and efficacy, warrants a stepwise appraisal of the literature on adherence and outcomes with emphasis on the impact of STRs vs MTRs. Specifically, these new regimens may improve the relationship between adherence and viral suppression, potentially reducing the adherence thresholds needed for treatment success. Results of a recent meta-analysis suggest that adherence of 80%–90% may be adequate to achieve viral suppression.22 In the current era of newer, more potent, treatment options, it is important to understand whether the older paradigms associating regimen simplicity (STR vs MTR), adherence, and patient outcomes remain valid. Furthermore, given demographic changes, with more than half of PLWH in the USA being over the age of 50 years,23 the high prevalence of age-related comorbidities, such as cardiovascular disease,24 may complicate HIV management or adherence due to higher pill burden and polypharmacy.25 The results of the current study extend findings from earlier research,20,21 taking into account newer regimens and emerging data on the level of adherence required for successful viral suppression.
Adherence is most likely best assessed in observational studies in which patients’ medication usage may more closely reflect actual clinical practice, because real-world studies often find poor long-term adherence.26 Adherence can be higher in randomized controlled trials (RCTs) due to factors such as careful selection of participants or more intensive follow-up;27 however, RCTs may provide useful information about the effect of adherence on virologic outcomes. We therefore conducted a systematic review to appraise the published literature reporting associations between STR vs MTR use and adherence to ART in observational studies alone, and that reporting the effects of adherence or STR vs MTR use on virologic outcomes in either observational studies or RCTs.
Methods
Search strategy
A systematic literature review was performed to identify studies assessing at least one of the following objectives: 1) the association between STR vs MTR use and adherence in observational settings; 2) the association between adherence (in either observational studies or RCTs) and viral suppression; and 3) the association between STR vs MTR use and viral suppression (in either observational studies or RCTs).
The systematic literature review was performed in two parts. An initial search of MEDLINE In-Process was completed on September 6, 2013, to identify any studies that were relevant to the three objectives that were published between 2006 and 2013. A follow-up search using the same search criteria and database was conducted on September 14, 2016, to identify relevant literature published since the initial search (2013–2016) to account for more contemporary regimens. Search terms and categories were consistent across both elements of the systematic review (Table S1).
Supplemental searches were conducted to identify recent relevant studies published in the proceedings of the following conferences (2013–2016): International AIDS Society Conference, International AIDS Conference, Conference on Retroviruses and Opportunistic Infections, American Society for Microbiology Interscience Conference on Antimicrobial Agents and Chemotherapy (2015), European AIDS Clinical Society Conference, and Infectious Disease Week. The 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines28 were followed for both initial and current searches.
Eligibility criteria
The titles and abstracts of identified publications were screened manually by one reviewer (OE) against pre-specified eligibility criteria for each objective (Table S2). Literature eligible for inclusion included full-text reports of original research published in English, conducted in North America or the European Union (EU), and involving adults diagnosed with HIV. Studies finding that patients received once-daily ART and those with a one-pill regimen arm with no confirmation that treatment was administered once daily were included; studies in which all patients received more-than-once-daily regimens were excluded. For objective 1, publications from observational studies (including prospective or retrospective non-randomized studies, disease registries or databases, electronic medical records, and claims data) reporting the effects of STRs vs MTRs on adherence were included. Observational studies and RCTs investigating the association between adherence and treatment efficacy or effectiveness were included for objective 2. Studies researching the effect of treatment regimen (STR vs MTR) on treatment efficacy or effectiveness in the observational setting were included for objective 3.
Full-text versions of all publications meeting the eligibility criteria at initial screening were reviewed. Once eligibility was confirmed, data were extracted manually into pre-defined summary tables for each objective.
Information extracted from relevant studies included details of the study (enrollment start year, study end year, study location [EU or North America], sample size, and period of follow-up), patient characteristics (age, sex, CD4+ count, baseline HIV RNA level, and previous treatment), and adherence and efficacy results (eg, method of assessing adherence, viral suppression measures, and definitions).
Meta-analysis
A meta-analysis was performed to assess the relationship between STR vs MTR use and adherence at the ≥95% and ≥90% thresholds (objective 1) using random- and fixed-effects models. Heterogeneity between studies was examined using Q and I2 statistics.29,30 A random-effects model was used in each analysis, and an additional fixed-effects model was used when heterogeneity among studies was not significant at the P,0.05 level. Publication bias for the ≥95% adherence outcome was determined by plotting the odds ratio (OR) against the inverse standard error and visually assessing the symmetry of funnel plots and statistical significance confirmed using Egger’s and Begg’s tests.31 Data management and statistical analyses were undertaken using Stata (StataCorp LLC, College Station, TX, USA).
Sensitivity analyses were performed to examine the following potential treatment effect modifiers on the influence of STR vs MTR use on adherence (≥95%): a) studies in which once-daily dosing was confirmed in the publication compared with those studies in which the daily dose could not be confirmed; b) studies in which adherence was measured using the medication possession ratio (MPR) vs the proportion of days covered (PDC); and c) studies conducted in North America compared with those in the EU.
A meta-regression was performed to assess major moderators of between-study heterogeneity (age, sex, race, and study period) in data from studies reporting ≥95% adherence to treatment.
Results
Search results
Overall, 2,117 relevant publications were initially identified, of which 119 were duplicates. Of the remaining 1,998 titles and abstracts screened, 1,731 were excluded, leaving 267 that qualified for full-text review (Figure 1). Ultimately, 29 studies met the inclusion criteria for one or more of the review objectives: 11 for objective 1, 18 for objective 2, and four for objective 3 (two studies were relevant for all three objectives).5,32
Figure 1.
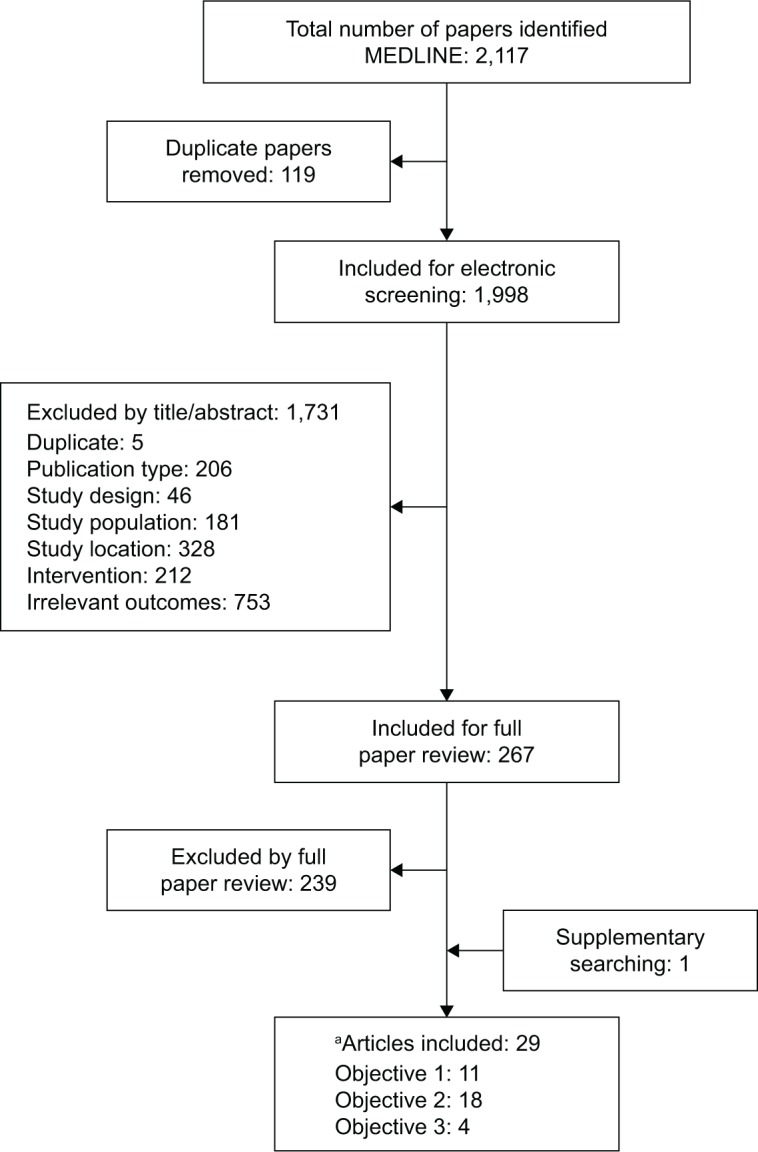
PRISMA diagram.
Note: aTwo identified studies were relevant for all three objectives.5,32
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Objective 1 – comparison of STR vs MTR use and adherence
Eleven studies (10 full papers5,32–41 and one conference abstract37) described the association between STR or MTR use and treatment adherence. Of these studies, nine were conducted in the USA5,32–36,39–41 and two were conducted in the EU (Table 1).37,38 Six studies used the MPR method to assess adherence,32–35,38,39 four used the PDC method,36,37,40,41 and, in one study, patients self-reported their adherence (Table 1).5
Table 1.
Characteristics of observational studies identified by the systematic literature review that assess the relationship between adherence and use of STR vs MTR (objective 1)
| Study, year(country or region) | Study population (study period) | Sample size | Adherence measurement | Results | Did STR improve Adherence (yes/no)? |
|---|---|---|---|---|---|
| Cohen et al,33 2013 (USA) | Medicaid patients with an HIV diagnosis from 2005 to 2009 receiving complete ART (ie, two nucleoside/nucleotide reverse transcriptase inhibitors plus a third agent) for ≥60 days as STR or 2+PPD (2005–2009) | STR: 1,797 MTR: 5,584 | MPR | Patients who received ART as a single pill per day were significantly more likely to be highly adherent to therapy than those who received MTR | Yes |
| Cooke et al,34 2014 (USA) | Managed care members with HIV and ART regimen recommended by US Department of Health and Human Services 2011 Antiretroviral Guidelines (2010–2011) | STR: 1,136 MTR: 1,241 | MPR | Patients who received STRs were about 1.3-times more likely to be adherent to therapy (ie, MPR ≥90%) than those receiving more than one ART | Yes |
| Hanna et al,5 2014 (USA) | Any person-visit in the Women’s Interagency HIV Study during which an HIV-infected woman self-reported ART use in the previous 6 months and had a valid HIV-1 viral load measurement (2006–2013) | STR: 1,846a MTR: 5,584b | Self-reported | STR use was associated with increased adherence and virologic suppression | Yes |
| Kauf et al,35 2012 (USA) | Patients with ≥1 pharmacy claim for ABC-3TC or for ≥2 components of an NRTI backbone (1997–2005) | STR: 650 MTR: 1,947 | MPR | Use of an STR improved adherence to a third regimen component and, thus, the likelihood of achieving the accepted standard for adherence to HIV therapy of 95% | Yes |
| Langness et al,36 2014 (USA) | Patients (aged ≥18 years) on any prescribed HIV ART, scheduled prescription blood pressure medication, or scheduled prescription mental health medication (2012–2013) | STR: 282 MTR: 295 | PDC | People with HIV were more adherent to a once-daily STR than to a once-daily MTR | Yes |
| Rogato et al,37 2016 (Europe) | HIV-infected patients on an ART regimen recommended by European AIDS Clinical Society guidelines, version 7.0 (2009–2013) | STR: 404c MTR: 404c | PDC | STRs were associated with improved adherence when compared with a matched cohort of patient regimens consisting of recommended once-daily MTRs | Yes |
| Raffi et al,38 2015 (France) | Treatment-naïve HIV-positive patients (aged ≥18 years) (2006–2011) | STR: 76 MTR: 242 | MPR | Significant benefit in terms of adherence was observed with the STR in comparison with regimens with one daily intake but no difference was observed when compared with regimens involving >1 pill once daily | Yes |
| Sax et al,39 2012 (USA) | Commercially insured patients in the LifeLink database with an HIV diagnosis between June 1, 2006 and December 31, 2008, and receipt of a complete ART regimen (2006–2008) | STR: 2,365 MTR: 4,708 | MPR | ART consisting of a single pill per day was associated with significantly better adherence and lower risk of hospitalization in patients with HIV than in those receiving ≥3 pills per day | Yes |
| Sutton et al,40 2016 (USA) | Patients with HIV covered by South Carolina Medicaid (2006–2013) | STR: 580 MTR: 1,594 | PDC | The STR was associated with higher adherence rates and a lower risk of hospitalization (both in the adjusted and unadjusted analyses) | Yes |
| Taneja et al,41 2012 (USA) | Patients (aged ≥18 years) with HIV, who began EFV/FTC/TDF or other EFV+≥2 NRTIs regimens or NVP+≥2 NRTI regimens between January 1, 2003 and September 30, 2009 (2003–2009) | STR: 1,874 MTR: 1,100 | PDC | Adherence was lower for both EFV+≥2 NRTIs (MTR) and NVP+≥2 NRTIs (MTR) than for EFV/FTC/TDF (STR) | Yes |
| Tennant et al,32 2015 (USA) | Treatment-naïve HIV-positive patients (aged 18 years) (2007–2010) | STR: 165 MTR: 224 | MPR | Treatment regimen was not predictive of adherence. A once- daily protease inhibitor-based MTR may result in comparable adherence to an STR in a rural HIV-infected population | No |
Notes:
Person visits.
Person visits (after propensity score matching).
After propensity score matching.
Abbreviations: ABC-3TC, abacavir and lamivudine; AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; MPR, medication possession ratio; MTR, multiple-tablet regimen; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine-based regimen; PDC, proportion of days covered; PPD, pills per day; STR, single-tablet regimen; TDF, tenofovir disoproxil fumarate.
In all studies, the proportion of patients reaching a defined minimum threshold of adherence was determined. Eight studies reported the proportion of patients with ≥95% adherence.5,33,35–37,39–41 Some publications reported the proportion of patients with ≥90%,32,34,35,40 >90%,38 ≥80%,40 and >80%38 adherence throughout the study. Ten of 11 eligible studies found that STRs were associated with higher adherence than MTRs.5,33–41 The differences were statistically significant in nine studies.5,33–37,39–41 Of the remaining two studies, one investigated adherence to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based (STR – consisting of efavirenz, emtricitabine, and tenofovir) and protease inhibitor (PI)-based (MTR) regimens, and found that patients using MTRs had significantly higher rates of adherence than those using STRs.32 In this study, however, multivariate analysis showed that treatment-experienced patients were 52% less likely than treatment-naïve patients to achieve 95% adherence, with the STR arm having a higher proportion of treatment-experienced patients than the MTR arm (75.2% vs 61.5%).32 The other study found no significant difference in >90% adherence between patients receiving STRs and those receiving MTRs.38
The meta-analysis, involving eight studies describing nine comparisons, compared the associations between STR vs MTR use and adherence at the ≥95% adherence threshold.5,33,35–37,39–41 In the random-effects model, STR use was 1.72-fold more likely to be associated with ≥95% treatment adherence than MTR use (95% CI=1.54–1.93; P<0.001).
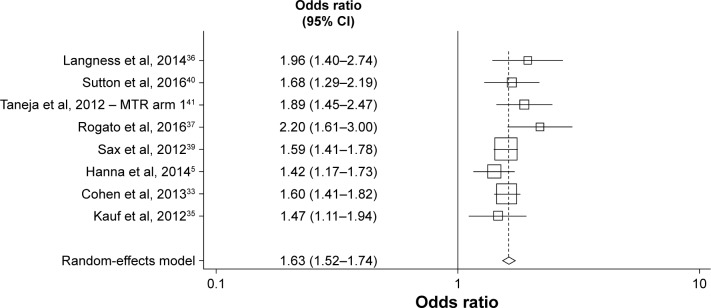
A funnel plot using estimates of ≥95% adherence from all studies included in the meta-analysis found that the nevi-rapine (NVP)-based regimen plus at least two NRTIs arm in the 2012 study of Taneja et al41 was an outlier (Egger’s test, P=0.050, Begg’s test, P=0.048). Excluding this arm, an updated funnel plot showed no evidence of publication bias using Egger’s (P=0.185) or Begg’s (P=0.174) tests (Figure 2). The revised fixed-effects meta-analysis excluding the NVP arm resulted in similar findings, with STRs remaining significantly associated with ≥95% adherence than MTRs (OR=1.63; 95% CI=1.52–1.74; P,0.001).
Figure 2.
Objective 1 – meta-analysis comparing the effects of STRs and MTRs on optimal (≥95%) adherence levels. Excluding nevirapine-based regimen plus at least two nucleoside reverse transcriptase inhibitors arm in Taneja et al.41
Abbreviations: MTR, multiple-tablet regimen; STR, single-tablet regimen.
Sensitivity analyses using meta-regression (Table 2) confirmed no significant differences in OR for ≥95% adherence in studies in which once-daily dosing was confirmed in the publications compared with those in which once-daily dosing could not be confirmed (P=0.368), in studies in which adherence was measured by MPR or PDC (P=0.056), and in those conducted in the USA vs the EU (P=0.098).
Table 2.
Sensitivity analysis of moderators for the association between STR vs MTR use and adherence (objective 1)
| Moderator | Category 1 | Category 2 | Regression coefficient (95% CI) | P-value |
|---|---|---|---|---|
| Daily regimen | Confirmed once daily | Unconfirmed once daily | 0.09 (−0.14–0.32) | 0.368 |
| Adherence measurement | PDC | MPR | 0.20 (−0.0120130.35) | 0.056 |
| Region | USA | EU | −0.32 (−0.71–0.40) | 0.098 |
Note: The effect of study period had to be considered at the study level because this variable does not differ between STR and MTR groups in any one study.
Abbreviations: EU, European Union; MPR, medication possession ratio; MTR, multiple-tablet regimen; PDC, proportion of days covered; STR, single-tablet regimen.
Meta-regression analyses did not find a significant effect of sex (P=0.914), age (P=0.898), or race (P=0.412) on the association between STR or MTR use and treatment adherence. The effect of the study period had to be considered at the study-level because this variable did not differ between STR and MTR groups in any one study. Meta-regression did not demonstrate any significant effect of study start or end date on the results of the meta-analysis (P=0.419).
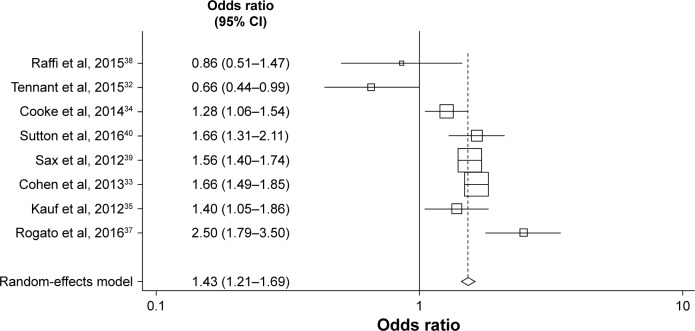
A meta-analysis also compared the effects of STRs and MTRs on lower adherence thresholds (≥90%), which were reported in eight studies (Figure 3).32–35,37–40 Findings were similar to those for ≥95% adherence, with an OR in favor of STR of 1.43 (random-effects model; 95% CI=1.21–1.69; P<0.001).
Figure 3.
Objective 1 – meta-analysis comparing the effects of STRs and MTRs on adherence levels (≥90% threshold).
Abbreviations: MTR, multiple-tablet regimen; STR, single-tablet regimen.
Objective 2 – association between adherence and viral suppression
Overall, 18 studies (five RCTs42–46 and 13 observational studies)5,32,47–57 investigated the association between adherence thresholds and viral suppression (Table 3), of which 13 (72%) found that higher levels of adherence were associated with greater viral suppression.5,32,42,44,47,50–57 Owing to high variation in the adherence thresholds and viral outcomes reported in the identified studies, a meta-analysis addressing this objective was not performed. One study using MPR to measure adherence, however, found that patients optimally adherent to ART were three times more likely than non-adherent patients to be virology-cally suppressed.32
Table 3.
Characteristics of studies identified by the systematic literature review that assess the effect of treatment adherence on viral suppression (objective 2)
| Study, year (country or region) | Study population (study design) | Adherence measurement | Result | Did adherence improve viral outcomes (yes/no)? |
|---|---|---|---|---|
| Baxi et al,47 2015 (USA) | HIV-infected women reporting ART use for >1 month between April 2003 and April 2008 who provided at least one hair sample for analysis (Observational study) | Self-reported (VAS) | Higher self-reported adherence was associated with higher odds of suppression | Yes |
| Bonora et al,50 2014 (Italy) | Treatment-experienced HIV-infected patients (Observational study) | Self-reported (VAS) | Suboptimal adherence was associated with virological failure | Yes |
| Cohen et al,42 2013 (Multinational) | Treatment-naïve, HIV-infected adults (RCT) | M-MASRI | Suboptimal adherence (≤95%) was associated with lower treatment responses and higher rates of virologic failure | Yes |
| Dragovic et al,51 2014 (Serbia) | HIV-infected women initiating HAART (Observational study) | Compliant vs non-compliant | Multivariate analyses revealed that the single factor independently related to a favorable response to HAART was good compliance | Yes |
| Hanna et al,5 2014 (USA) | Any person-visit in the WIHS during which an HIV- infected woman self-reported ART use in the previous 6 months and had a valid HIV-1 viral load measurement (Observational study) | Self-reported (WIHS) | Single-tablet regimen use was associated with increased adherence and virologic suppression | Yes |
| Hernandez Arroyo et al,52 2013 (Spain) | Patients (either naïve or pretreated) with confirmed HIV infection who had been receiving active ART for >6 months (Observational study) | TDM/SMAQ | Improved adherence increased permanence time of the patient with undetectable plasma viral loads and improved patient lymphocyte counts | Yes |
| Jayaweera et al,53 2009 (USA) | Adult patients weighing 40 kg or more with no more than 7 days of prior antiretroviral therapy (Observational study) | Pill counts and AMAF | Adherence levels of ≥80% were associated with a high proportion of patients with a virological response (<400 copies/mL) | Yes |
| Josephson et al,43 2010 (Sweden) | Treatment-naïve HIV patients (RCT) | ACTG questionnaire | The number of missed doses (self-reported adherence) reported was not predictive of virologic failure, treatment failure, or plasma concentration of lopinavir, nor was it predictive of the viral load decline from baseline to week 4, in any of the treatment groups | No |
| Julian et al,54 2010 (USA) | Adult patients actively enrolled in HIV/AIDS clinic and HAART for at least 6 months and had provided at least two completed surveys (Observational study) | SPNS score | Higher adherence (SPNS score ≥10) was significantly correlated with lower viral load | Yes |
| Lathouwers et al,44 2011 (Multinational) | Treatment-naïve HIV-infected patients (RCT) | M-MASRI | A higher frequency of suboptimal adherence was reported in patients with virologic failure compared with patients without virologic failure | Yes |
| Martin et al,55 2008 (Spain) | Patients taking first-line therapy and antiretroviral- experienced patients (Observational study) | Pill count (70%), Pharmacy records (30%) | Risk of virologic failure was lowest in patients 80%–89.9% adherence and higher in patients with lower adherence (≤70%–79.9%) | Yes |
| Nelson et al,45 2010 (Multinational) | Treatment-naïve HIV-infected patients (RCT) | M-MASRI | In one MTR (darunavir/ritonavir), suboptimal adherence had no impact on virologic response, whereas a significantly higher virological response rate was achieved in patients optimally adherent to lopinavir/ritanovir | No |
| Podzamczer et al,56 2014 | Adult HIV-infected patients with viral load under 1,000 copies/mL while receiving a stable ART for at least the last 3 months and switched to RPV/FTC/TDF due to intolerance of previous regimen (Observational study) | SMAQ | Switching to RPV/FTC/TDF improved adherence while maintaining a good immune and virological response | Yes |
| Sax et al,46 2012 (North America) | Adults (≥18 years) infected with HIV with plasma HIV-1 RNA concentrations of 5,000 copies/mL or more and no previous use of antiretroviral drugs (RCT) | Pill count | There was no difference in virologic response between an STR and standard of care, regardless of adherence levels (<95% or ≥95%) | No |
| Tennant et al,32 2015 (USA) | Adults (≥18 years) with HIV (Observational study) | MPR | Patients adherent to ART were 3-fold more likely than non-adherent patients to be virologically suppressed | Yes |
| Torres-Cornejo et al,57 2014 (Spain) | Adult HIV-infected patients with a plasma HIV-RNA concentration of less than 50 copies/mL for at least 6 months (Observational study) | Self-reported | Higher adherence and an HIV-DNA level less than 2 log10 copies/106 PBMCs at baseline were associated with a lower risk of virological failure | Yes |
| Viswanathan et al,48 2015 (USA) | HIV-positive persons on ART and followed in the Veterans Aging Cohort Study Virtual Cohort (VACS VC) (Observational study) | PDC | Adherence improved viral suppression in patients using PI-based regimens but not in patients on NNRTI regimens | No |
| Wilkins et al,49 2016 (Multinational) | Treatment naïve HIV-infected adults (Randomized open- label study) | M-MASRI/self- reports (VAS) | There was no significant difference between two STRs in terms of virological success, regardless of adherence levels (<95% or ≥95%) | No |
Abbreviations: ACTG, AIDS Clinical Trials Group; AIDS, acquired immune deficiency syndrome; AMAF, antiviral medication adherence form; ART, antiretroviral therapy; FTC, emtricitabine; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; M-MASRI, Modified Medication Adherence Self-Report Inventory; MPR, medication possession ratio; MTR, multiple-tablet regimen; NNRTI, non-nucleoside reverse transcriptase inhibitor; PBMC, peripheral blood mononuclear cell; PDC, proportion of days covered; RCT, randomized controlled trial; RPV, rilpivirine; SMAQ, simplified medication adherence questionnaire; SPNS, Special Project of National Significance; STR, single-tablet regimen; TDF, tenofovir disoproxil fumarate; TDM, therapeutic drug monitoring; VAS, visual analog scale; WIHS, Women’s Interagency HIV Study.
Of the 11 studies that used 95% as the adherence threshold, seven (64%) found that adherence was strongly associated with viral suppression.5,42,44,47,50,52,57 All three studies using 90% as a threshold showed improved virologic outcomes with adherence,32,53,55 and two studies found high levels of viral suppression when adherence was ≥80%40 and >75%.35 Martin et al55 reported mixed virologic results with <70% adherence, resulting in virologic failure in 100% of patients prescribed unboosted PI-based regimens, 50% virologic failure in those receiving boosted PI-based regimens, and 34.5% virologic failure in individuals on NNRTI-based regimens.
Five studies demonstrated a positive effect of self-reported adherence on viral outcome. The results of two suggested that higher (≥95%) adherence improved virologic outcomes,5,47 while three found that suboptimal adherence (self-reported non-adherence50 or <95% adherence)42,44 was associated with virologic failure. The remaining studies used a variety of other methods to assess adherence, including therapeutic drug monitoring,52 a simplified medication adherence questionnaire,52,56 pill counts,53,55 pharmacy refills,55 and the antiviral medication adherence form.53 All studies demonstrated a positive association between treatment adherence and virologic outcomes.
Of the 18 identified studies reporting the association between adherence and viral suppression, five (28%) had mixed outcomes or did not find an effect of adherence on virologic outcomes.43,45,46,48,49 In two studies, adherence improved treatment outcomes with some regimens but not with others. For example, Nelson et al45 found that suboptimal adherence (<95%) to darunavir/ritonavir was not associated with virologic response, whereas virologic response to lopinavir/ritanovir improved with better adherence (≥95%). Similarly, Viswanathan et al48 demonstrated that adherence was associated with greater viral suppression in patients using PI-based regimens, but not in those receiving NNRTI-based regimens (≥95%). In the remaining three studies, adherence was not predictive of virologic outcomes.43,46,49
Objective 3 – association between STR vs MTR use and viral suppression
Overall, five studies investigated the association between STR vs MTR use and viral suppression (Table 4).3,5,32,58,59 Chakraborty et al58 examined viral load trends in population subgroups by assessing a state-wide surveillance dataset, and showed that there was a more rapid decrease in viral load among people using STRs than in those using MTRs. Hanna et al5 reported that STR use was significantly associated with virologic suppression. The remaining studies found no significant difference between the effect of STRs and that of MTRs on virologic outcomes.3,32,59 Of note, one of these studies found no significant effect of STRs compared with MTRs on achievement of >90% adherence.32
Table 4.
Characteristics of studies identified by the systematic literature review that assessed the effect of use of STR vs MTR on viral suppression (objective 3)
| Study, year (country or region) | Study population (study period) | Adherence measurement | Results | Did STR improve viral outcomes (yes/no)? |
|---|---|---|---|---|
| Chakraborty et al,58 2016 (USA) | South Carolina residents, aged ≥13 years, living with HIV who have at least one viral load reported (2004–2013) | NA | Significant association between STR use and faster decline in community viral load | Yes |
| Dejesus et al,3 2009 (USA, Puerto Rico) | ART-experienced HIV-infected patients who are on their first ART regimen or who had documented viral suppression on a previous protease inhibitor-based regimen at the time of prior change in therapy (2006–2007) | Pill counts and self-reported VAS | Both STR and MTR resulted in comparable virologic suppression | No |
| DeJesus et al,59 2012 (Multinational) | Treatment-naïve patients with plasma HIV-1 RNA concentrations of ≥5,000 copies/mL and susceptibility to atazanavir, emtricitabine, and tenofovir (2011–2014) | NA | Proportion of patients with virologic success (HIV-1 RNA concentrations of ≤50 copies/mL) at week 48 did not differ significantly between regimens | No |
| Hanna et al,5 2014 (USA) | Any person-visit in the WIHS during which an HIV-infected woman self-reported ART use in the previous 6 months and had a valid HIV-1 viral load measurement (2006–2013) | Self-reported (WIHS) | STR use was significantly associated with virologic suppression | Yes |
| Tennant et al,32 2015 (USA) | Patients with HIV (aged ≥18 years) (2007–2010) | MPR | MTR was similarly likely to result in virologic suppression (OR=1.11; 95% CI=0.62–1.99) | No |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MPR, medication possession ratio; MTR, multiple-tablet regimen; NA, not applicable; OR, odds ratio; STR, single-tablet regimen; VAS, visual analog scale; WIHS, Women’s Interagency HIV Study.
Discussion
To our knowledge, this systematic literature review provides the most current summary of published literature assessing associations between STR vs MTR use and ART adherence and the impact of adherence on virologic outcome. The results of this study suggest that STR regimens are related to better adherence in real-world, observational settings. We identified many observational studies investigating the association between STR vs MTR use and adherence in observational settings (objective 1) and between adherence and viral suppression in observational settings and RCTs (objective 2); however, few studies assessing the association between observational STR vs MTR use and viral suppression were found (objective 3). Although the studies identified included patients on a variety of ART regimens, there was a paucity of available data on INSTI regimens, which are now recommended. Only one study specifically included patients receiving an INSTI (elvitegravir).5 The remaining studies that were included consisted of combinations of patients receiving NRTIs, NNRTIs, and PIs.
A meta-analysis of objective 1 using data from eight observational studies demonstrated that STRs are significantly associated with a 63%–72% higher level of adherence (at the ≥95% adherence threshold) than MTRs. The results of this systematic literature review and meta-analysis assessing the association between STR vs MTR use and adherence in observational settings (objective 1) are consistent with those of previous studies that demonstrated significant benefits of STRs compared with MTRs. These findings, together with those from a meta-analysis by Clay et al21 that assessed adherence and clinical and cost outcomes (reporting a 2.37-fold adherence advantage of STRs over MTRs) confirm the importance of STRs in optimizing clinical outcomes. Our study extends these earlier findings by using specific thresholds of adherence rather than the relative adherence in patients on STRs vs MTRs.21 The current study also included a greater number of publications and patients (eight studies involving 30,470 patients compared with four studies involving 1,224 patients), leading to more robust meta-analyses. Furthermore, the larger sample size allowed for in-depth sensitivity analyses to account for factors that could have confounded the observed associations. Studies from the EU and USA were assessed in a sensitivity analysis owing to the differences in healthcare provision in these regions.60 Additionally, because we were unable to confirm whether once-daily dosing was used in all the included studies, we performed a sensitivity analysis to clarify the impact of confirmation of once-daily dosing, since reducing dosage frequency to once-daily has been shown to be related to higher treatment adherence.61 Sensitivity analysis was also used to investigate the impact of different methods of adherence measurement (MPR vs PDC). The results were robust to these sensitivity analyses, and to meta-regression analyses assessing the effect of sex, age, race, and study period.
This study was also further able to incorporate RCT data and to examine different levels of adherence thresholds (objective 2). Importantly, thresholds of >90% and >95% were consistently associated with viral suppression, but findings did not remain consistent when lower thresholds were investigated (<90%). Of the identified studies, results of two suggested that ≥75% adherence may be sufficient to achieve viral suppression.35,40 One of these studies included patients receiving NNRTI-based regimens,41 and the other included patients on NRTI-based regimens.46 The positive effects of lower (<80%) levels of adherence on viral suppression observed in these studies are consistent with findings from a small pilot study of a “five-on, two-off” treatment schedule demonstrating viral suppression with ~70% adherence.62 Additionally, a systematic review with meta-analysis attempted to disentangle this relationship further.22 In this review, adherence of 80%–90% appeared to be adequate for viral suppression, suggesting that some types of regimens may be more forgiving than others, owing to higher efficacy and lower toxicity (in newer regimens),63 and unique pharmacological properties such as longer half-life.64 Results of the meta-analysis by Bezabhe et al22 suggests that the level of adherence required for sustaining viral suppression may be lower than has previously been thought; however, data are inconclusive as to the minimum level necessary to ensure durable virologic suppression, and the relationship between adherence and virologic failure was impacted by study design and study region.
The majority of studies identified in our searches used higher adherence thresholds (≥95%) than those suggested to be necessary for sustaining viral suppression in the Bezabhe study.22 In that review, however, virologic failure was variably defined (<100 copies/mL vs 500 copies/mL and <1,000 copies/mL), and the likelihood of viral suppression differed for various adherence thresholds, with greater ORs for higher thresholds. Moreover, thresholds of adherence varied among their reported studies (from 100% to 80%),22 and the effect of adherence on viral suppression was significantly higher, with lower cut-offs for virologic failure (<100 copies/mL vs 500 copies/mL and 1,000 copies/mL).22 Thus, it is crucial for future research to use well-defined and clinically meaningful thresholds to better compare studies.
Only five studies comparing the effect of STR and MTR use on virologic outcomes were found (objective 3),3,5,32,58,59 three of which demonstrated no significant differences in viral suppression for patients using STRs compared with those using MTRs3,32,59 (the other two showed better viro-logic outcomes with STRs5,58). Thus, despite several studies showing that STRs are associated with greater adherence than MTRs, the impact of this improvement on virologic suppression has yet to be conclusively demonstrated. In the Clay et al21 meta-analysis that included only three studies, viral suppression at 48 weeks was significantly greater in patients using STRs than in those using MTRs (P=0.0003).
The low number of studies and the differing results suggest that further research is needed to determine whether adherence to STRs translates into improved virologic and clinical outcomes. Although a limited number of studies have demonstrated that STRs are associated with lower rates of disease progression and mortality compared with MTRs,9,65,66 it is crucial that future research addresses this gap in our understanding of how pill burden impacts viral suppression so that the needs of patients are better addressed.
As PLWH age and experience a higher burden of comorbidities in addition to HIV,25 it is crucial that they are helped to manage comorbidities and coordination of multiple therapies. Aside from pill burden, other potential barriers to adherence, such as inadequate housing, food insecurity, substance use, and psychiatric disorders including depression, should be addressed to better support PLWH.67 To improve treatment adherence in PLWH, it is critical to take multiple approaches, such as minimizing socio-structural barriers, improving management of health systems, strengthening patient–provider relationships, and working with patients to ensure that regimens are acceptable, feasible, and have minimal side-effects.
Despite the robust findings, our systematic literature review and meta-analyses are not without limitations. The studies reporting associations between STR vs MTR use and real-world adherence were non-randomized, and may be confounded by patient differences and medication characteristics between treatment groups. While the lack of ran-domization limits the internal validity, the approach taken in the current study is more applicable to real-world settings in which patients face barriers to treatment adherence and have less support and oversight than is present in a controlled setting. In most studies, adherence was calculated according to pharmacy records and reimbursement claims data, which may not reflect true adherence. The results of this meta-analysis, however, are generally consistent with those of sensitivity analyses involving identified studies using the more accurate PDC approach and those using MPR.
Although the results of a substantial number of studies suggest that STRs are associated with higher adherence to therapy than MTRs and show an association between adherence and viral suppression, only a small number of studies directly evaluated the association between STR use and clinical outcomes, and few data are available on INSTI regimens, which are now recommended.
Conclusion
This systematic review addresses an important gap in the HIV adherence literature, providing a quantitative analysis of the effect of STRs vs MTRs on the achievement of 90% and 95% adherence. The analysis re-affirms the significant association between the use of STRs and adherence, and the positive impact of adherence on virologic outcome. However, future research is still necessary to measure the association between specific adherence thresholds and specific levels of viral suppression. Although the existing literature shows similar treatment outcomes when adherence is defined using 95% and 90% thresholds, virologic outcomes have been mixed with thresholds below 90%. Overall, our systematic review and meta-analysis provides a quantitative estimate of the benefits of STRs over MTRs on adherence and virologic outcomes, and highlights the need for research assessing the impact of pill burden on viral suppression to meet patients’ needs more effectively.
Supplementary materials
Table S1.
MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946–present; search conducted on September 14, 2016a
| Search category | ID | Search terms |
|---|---|---|
| Disease | #1 | (“human immunodeficiency virus” OR “HIV”) |
| Intervention | #2 | (“once daily” OR “once a day” OR “QD” OR “single tablet” OR “single pill” OR “one pill” OR “fixed dose combination” OR “co-formulated”) |
| Adherence | #3 | (“adherence” OR “nonadherence” OR “non-adherence” OR “adherent” OR “nonadherent” OR “non-adherent” OR adhere* OR “compliance” OR “noncompliance” OR “non-compliance” OR “compliant” OR “noncompliant” OR “non-compliant”) |
| Viral suppression | #4 | (“viral load” OR “viral suppression” OR “virologic suppression” OR “virologic response” OR “virologic failure” OR “virologic success” OR “virological suppression” OR “virological response” OR “virological failure” OR “virological success” OR “RNA suppression” OR “RNA level” OR “RNA concentration” OR “undetectable” OR “undetectability”) |
| Association | #5 | (compare* OR “comparison” OR associate* OR relate* OR relation* OR correlate* OR “correlation” OR cause* OR “versus” OR “vs” OR “vs.” OR “lead to” OR “leads to” OR “led to”) |
| RCT | #6 | (“clinical trial” OR “clinical trials” OR “randomized controlled trial” OR “randomized controlled trials” OR “randomised controlled trial” OR “randomised controlled trials” OR random* OR enroll* OR “protocol” OR “open-label” OR “single blind” OR “double blind”) |
| Real world | #7 | (“observational” OR “longitudinal” OR “retrospective” OR “prospective” OR “follow up” OR “cohort” OR “insurance” OR electronic medic* OR “claims data” OR “naturalistic” OR “pragmatic” OR “medical records” OR “registry”) |
| Study objectives | ||
| 1. Real-world adherence with STR vs MTR | #8 | #1 AND #2 AND #3 AND #7 |
| 2. Association between adherence and viral outcomes | #9 | #1 AND #3 AND #4 AND #5 AND (#6 OR #7) |
| 3. Comparative effects of STR vs MTR on viral outcomes | #10 | #1 AND #2 AND #4 AND (#6 OR #7) |
| All | #11 | #8 OR #9 OR #10 |
| #12 | Limit 11 to yr=“2013-Current” | |
| #13 | Limit 12 to English language | |
Note:
An initial search of MEDLINE In-Process was completed on September 6, 2013 to identify any studies that were relevant to the three objectives that were published between 2006 and 2013.
Abbreviations: HIV, human immunodeficiency virus; MTR, multiple-tablet regimen; RCT, randomized controlled trial; STR, single-tablet regimen.
Table S2.
Eligibility criteria
| Criteria | Included | Excluded |
|---|---|---|
| Publication type | • Full-text original research published in English | • Non-English publications • Duplicate publication(s) of the same trials or studies • Case reports • Commentaries and letters • Recommendations/guidelines • Books/chapters/addresses/bibliographies/biographies/lectures • Non-systematic reviews • Systematic reviews and meta-analyses |
| Study design | • RCTs • Observational studies (retrospective studies, longitudinal studies, prospective studies, etc) |
• Non-human studies • Preclinical studies • Phase 1 studies • Short-term studies (study length #10 days) • Studies interrupted or prematurely terminated |
| Study location | • North America and the European Union | • Including any location not in North America or the European Union |
| Population | • Adults (aged $18 years) diagnosed with HIV and treated with ART | • Children and adolescents (aged <18 years) • Non-HIV-positive patients • Studies conducted only in HIV subpopulation, including: o patients infected with hepatitis B or C ○ patients with a history of drug abuse ○ patients with depression o pregnant women ○ homeless and marginally housed patients |
| Interventions | • Once-daily ART regimens | • Non-ART regimens • More-than-once-daily regimens |
| Outcomes | • Association between treatment regimen and adherence level, or • Association between adherence level and drug efficacy/effectiveness,a or • Association between treatment regimen and drug efficacy/effectivenessa |
• Studies that report neither treatment adherence nor drug efficacy/effectiveness • Studies that do not report the association or comparative results |
Note:
Drug effectiveness or efficacy was measured as viral load (RNA level), viral suppression rate, viral failure rate, and undetectable RNA level.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; RCT, randomized controlled trial.
Acknowledgments
The authors acknowledge the help of Reza Oskrochi who assisted with data analysis and interpretation. This study was funded by Gilead Sciences.
Abbreviations
- ABC-3TC
abacavir and lamivudine
- ACTG
AIDS Clinical Trials Group
- AIDS
acquired immune deficiency syndrome
- AMAF
antiviral medication adherence form
- ART
antiretroviral therapy
- EFV
efavirenz
- EU
European Union
- FTC
emtricitabine
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- HIV-1
type 1 HIV
- HRQoL
health-related quality of life
- INSTI
integrase strand transfer inhibitor
- M-MASRI
Modified Medication Adherence Self-Report Inventory
- MPR
medication possession ratio
- MTR
multiple-tablet regimen
- NA
not applicable
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- NVP
nevi-rapine
- OR
odds ratio
- PBMC
peripheral blood mononuclear cell
- PDC
proportion of days covered
- PI
protease inhibitor
- PLWH
people living with human immunodeficiency virus
- PPD
pills per day
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QD
once daily
- RCT
randomized controlled trial
- RPV
rilpivirine
- SMAQ
simplified medication adherence questionnaire
- SPNS
Special Project of National Significance
- STR
single-tablet regimen
- TDF
tenofovir disoproxil fumarate
- TDM
therapeutic drug monitoring
- VAS
visual analog scale
- WIHS
Women’s Interagency HIV Study
Footnotes
Author contributions
FA: manuscript review and final approval. OE: data inclusion assessment; data extraction; data analysis and interpretation; manuscript draft; manuscript review and final approval. SS: data inclusion assessment; data extraction; data analysis and interpretation; manuscript draft; manuscript review and final approval. GC: manuscript draft; manuscript review and final approval. ACB: concept and design; manuscript draft; manuscript review and final approval. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
Obaro Evuarherhe, Gemma Carter and Sophie Shina are employees of Oxford PharmaGenesis Ltd, Oxford UK, which was funded by Gilead Sciences. Anne Christine Beaubrun is an employee of Gilead Sciences, Foster City, CA, USA. The authors report no other conflicts of interest in this work.
References
- 1.Department of Health and Human Services; [Accessed March 23, 2017]. Panel on Antiretroviral Guidelines for Adults and Adolescents: guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [updated July 14, 2016]. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.Astuti N, Maggiolo F. Single-tablet regimens in HIV therapy. Infect Dis Ther. 2014;3:1–17. doi: 10.1007/s40121-014-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejesus E, Young B, Morales-Ramirez JO, et al. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified anti-retroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr. 2009;51:163–174. doi: 10.1097/QAI.0b013e3181a572cf. [DOI] [PubMed] [Google Scholar]
- 4.Glass TR, De Geest S, Hirschel B, et al. Self-reported non-adherence to antiretroviral therapy repeatedly assessed by two questions predicts treatment failure in virologically suppressed patients. Antivir Ther. 2008;13:77–85. [PubMed] [Google Scholar]
- 5.Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65:587–596. doi: 10.1097/QAI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48:484–488. doi: 10.1086/596482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) Adherence to long-term therapies. Evidence for action. Jan, 2003. [Accessed October 31, 2016]. Available from: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf.
- 8.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 9.Hogg RS, Yip B, Chan K, O’Shaughnessy M, Montaner J. Nonadherence to triple-combination ART is predictive of AIDS progression and death in HIV+ men and women. Presented at the XIII International AIDS Conference; July 9–14; 2000; Durban, South Africa. Abstract TuOrB419. [Google Scholar]
- 10.Kitahata MM, Reed SD, Dillingham PW, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS. 2004;15:803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 11.Yehia BR, Mehta JM, Ciuffetelli D, et al. Antiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adults. Clin Infect Dis. 2012;55:593–599. doi: 10.1093/cid/cis491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannheimer SB, Matts J, Telzak E, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- 13.Panel on treatment of HIV-infected pregnant women and prevention of perinatal transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States [updated October 26, 2016] [Accessed April 30, 2012]. Available from: http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf.
- 14.Gardner EM, Maravi ME, Rietmeijer C, Davidson AJ, Burman WJ. The association of adherence to antiretroviral therapy with healthcare utilization and costs for medical care. Appl Health Econ Health Policy. 2008;6:145–155. doi: 10.1007/bf03256129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leisegang R, Cleary S, Hislop M, et al. Early and late direct costs in a Southern African antiretroviral treatment programme: a retrospective cohort analysis. PLoS Med. 2009;6:e1000189. doi: 10.1371/journal.pmed.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor JL, Gardner EM, Mannheimer SB, et al. Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J Infect Dis. 2013;208:40–49. doi: 10.1093/infdis/jis731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweet DK, Zhong Y, Zhuo D, Signorovitch J. Real-world adherence among patients receiving single versus multiple tablet regimens for HIV-1 infection, and associations between adherence and viral suppression: a systematic literature review and meta-analysis. Presented at the 20th International AIDS Conference; July 20–25; 2014; Melbourne, Australia. Abstract MOPE054. [Google Scholar]
- 19.Uthman OA, Parienti DW, Dowdy DW, et al. Regimen simplification in HIV infection toward once-daily dosing and fixed-dose combinations: a meta-analysis and sequential analysis of randomized controlled trials. Poster presented at the XIX International AIDS Conference; July 22–27; 2012; Washington, DC, USA. Poster TUPE096. [Google Scholar]
- 20.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Clay PG, Nag S, Graham CM, Narayanan S. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore) 2015;94:e1677. doi: 10.1097/MD.0000000000001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016;95:e3361. doi: 10.1097/MD.0000000000003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Carmen T, Freeman R, Siegler EL, Singh H. A qualitative analysis of inpatient geriatric consults in the aging HIV population. Innovation in Aging. 2017;1(Suppl 1):835. [Google Scholar]
- 24.Martin-Iguacel R, Llibre JM, Friis-Moller N. Risk of cardiovascular disease in an aging HIV population: where are we now? Curr HIV/ AIDS Rep. 2015;12:375–387. doi: 10.1007/s11904-015-0284-6. [DOI] [PubMed] [Google Scholar]
- 25.Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. Antiret-roviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS. 2012;26(Suppl 1):S39–S53. doi: 10.1097/QAD.0b013e32835584ea. [DOI] [PubMed] [Google Scholar]
- 26.Cherry SB, Benner JS, Hussein MA, Tang SS, Nichol MB. The clinical and economic burden of nonadherence with antihypertensive and lipid-lowering therapy in hypertensive patients. Value Health. 2009;12:489–497. doi: 10.1111/j.1524-4733.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 27.van Onzenoort HA, Menger FE, Neef C, Verberk WJ, Kroon AA, de Leeuw PW, van der Kuy PH. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011;58:573–578. doi: 10.1161/HYPERTENSIONAHA.111.171074. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tennant SJ, Hester EK, Caulder CR, Lu ZK, Bookstaver PB. Adherence among rural HIV-infected patients in the deep south: a comparison between single-tablet and multi-tablet once-daily regimens. J Int Assoc Provid AIDS Care. 2015;14:64–71. doi: 10.1177/2325957414555228. [DOI] [PubMed] [Google Scholar]
- 33.Cohen CJ, Meyers JL, Davis KL. Association between daily antiret-roviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open. 2013;3:e003028. doi: 10.1136/bmjopen-2013-003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooke CE, Lee HY, Xing S. Adherence to antiretroviral therapy in managed care members in the United States: a retrospective claims analysis. J Manag Care Pharm. 2014;20:86–92. doi: 10.18553/jmcp.2014.20.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kauf TL, Davis KL, Earnshaw SR, Davis EA. Spillover adherence effects of fixed-dose combination HIV therapy. Patient Prefer Adherence. 2012;6:155–164. doi: 10.2147/PPA.S28482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langness J, Cook PF, Gill J, Boggs R, Netsanet N. Comparison of adherence rates for antiretroviral, blood pressure, or mental health medications for HIV-positive patients at an academic medical center outpatient pharmacy. J Manag Care Spec Pharm. 2014;20:809–814. doi: 10.18553/jmcp.2014.20.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogato F, Martinez C, Rodriguez Sagrado MA, et al. Adherence in HIV-positive patients treated with single tablet regimens versus recommended once daily multi-pill regimen in clinical practice: findings from the international iSTRAP study. Presented at the 21st International AIDS Conference; July 18–22; 2016; Durban, South Africa. Abstract THPEB072. [Google Scholar]
- 38.Raffi F, Yazdanpanah Y, Fagnani F, Laurendeau C, Lafuma A, Gourmelen J. Persistence and adherence to single-tablet regimens in HIV treatment: a cohort study from the French National Healthcare Insurance Database. J Antimicrob Chemother. 2015;70:2121–2128. doi: 10.1093/jac/dkv083. [DOI] [PubMed] [Google Scholar]
- 39.Sax PE, Meyers JL, Mugavero M, Davis KL. Adherence to antiret-roviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7:e31591. doi: 10.1371/journal.pone.0031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton SS, Hardin JW, Bramley TJ, D’Souza AO, Bennett CL. Single-versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care. 2016;22:242–248. [PubMed] [Google Scholar]
- 41.Taneja C, Juday T, Gertzog L, et al. Adherence and persistence with non-nucleoside reverse transcriptase inhibitor-based antiretroviral regimens. Expert Opin Pharmacother. 2012;13:2111–2118. doi: 10.1517/14656566.2012.719875. [DOI] [PubMed] [Google Scholar]
- 42.Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III random-ized trials. AIDS. 2013;27:939–950. doi: 10.1097/QAD.0b013e32835cee6e. [DOI] [PubMed] [Google Scholar]
- 43.Josephson F, Andersson MC, Flamholc L, et al. The relation between treatment outcome and efavirenz, atazanavir or lopinavir exposure in the NORTHIV trial of treatment-naive HIV-1 infected patients. Eur J Clin Pharmacol. 2010;66:349–357. doi: 10.1007/s00228-009-0763-z. [DOI] [PubMed] [Google Scholar]
- 44.Lathouwers E, De Meyer S, Dierynck I, et al. Virological characterization of patients failing darunavir/ritonavir or lopinavir/ritonavir treatment in the ARTEMIS study: 96-week analysis. Antivir Ther. 2011;16:99–108. doi: 10.3851/IMP1719. [DOI] [PubMed] [Google Scholar]
- 45.Nelson M, Girard PM, Demasi R, et al. Suboptimal adherence to daruna-vir/ritonavir has minimal effect on efficacy compared with lopinavir/ ritonavir in treatment-naive, HIV-infected patients: 96 week ARTEMIS data. J Antimicrob Chemother. 2010;65:1505–1509. doi: 10.1093/jac/dkq150. [DOI] [PubMed] [Google Scholar]
- 46.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobi-cistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 47.Baxi SM, Greenblatt RM, Bacchetti P, et al. Nevirapine concentration in hair samples is a strong predictor of virologic suppression in a prospective cohort of HIV-infected patients. PLoS One. 2015;10:e0129100. doi: 10.1371/journal.pone.0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viswanathan S, Justice AC, Alexander GC, et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;69:493–498. doi: 10.1097/QAI.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilkins EL, Cohen CJ, Trottier B, et al. Patient-reported outcomes in the single-tablet regimen (STaR) trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/teno-fovir disoproxil fumarate in antiretroviral treatment-naive adults infected with HIV-1 through 48 weeks of treatment. AIDS Care. 2016;28:401–408. doi: 10.1080/09540121.2015.1096890. [DOI] [PubMed] [Google Scholar]
- 50.Bonora S, Calcagno A, Vigano O, et al. Efficacy, tolerability and viro-logical consequences of long-term use of unboosted atazanavir plus 2 NRTIs in HIV-infected patients. Curr HIV Res. 2014;12:339–346. doi: 10.2174/1570162x12666140807151616. [DOI] [PubMed] [Google Scholar]
- 51.Dragovic G, Salemovic D, Ranin J, Nikolic J, Kusic J, Jevtovic D. Clinical and immunologic outcomes of HAART-treated HIV-infected women in resource constrain settings: the Belgrade study. Women Health. 2014;54:35–47. doi: 10.1080/03630242.2013.850465. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez Arroyo MJ, Cabrera Figueroa SE, Sepulveda Correa R, et al. Impact of a pharmaceutical care program on clinical evolution and antiretroviral treatment adherence: a 5-year study. Patient Prefer Adherence. 2013;7:729–739. doi: 10.2147/PPA.S47519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayaweera D, Dejesus E, Nguyen KL, Grimm K, Butcher D, Seekins DW. Virologic suppression, treatment adherence, and improved quality of life on a once-daily efavirenz-based regimen in treatment-Naive HIV-1-infected patients over 96 weeks. HIV Clin Trials. 2009;10:375–384. doi: 10.1310/hct1006-375. [DOI] [PubMed] [Google Scholar]
- 54.Julian FS, Martin P, Erickson SR. Validation of the special projects of national significance adherence tool in HIV/AIDS patients. Ann Pharmacother. 2010;44:1003–1009. doi: 10.1345/aph.1M690. [DOI] [PubMed] [Google Scholar]
- 55.Martin M, Del Cacho E, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008;24:1263–1268. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- 56.Podzamczer D, Rozas N, Domingo P, et al. ACTG-HIV symptoms changes in patients switched to RPV/FTC/TDF due to previous intolerance to CART. Interim analysis of the PRO-STR study. J Int AIDS Soc. 2014;17(4 Suppl 3):19814. doi: 10.7448/IAS.17.4.19814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres-Cornejo A, Benmarzouk-Hidalgo OJ, Gutierrez-Valencia A, et al. Cellular HIV reservoir replenishment is not affected by blip or intermittent viremia episodes during darunavir/ritonavir monotherapy. AIDS. 2014;28:201–208. doi: 10.1097/QAD.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 58.Chakraborty H, Weissman S, Duffus WA, et al. HIV community viral load trends in South Carolina. Int J STD AIDS. 2016;31:31. doi: 10.1177/0956462416642349. [DOI] [PubMed] [Google Scholar]
- 59.DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379:2429–2438. doi: 10.1016/S0140-6736(12)60918-0. [DOI] [PubMed] [Google Scholar]
- 60.Rice T, Rosenau P, Unruh LY, Barnes AJ, Saltman RB, van Ginneken E. United States of America: health system review. Health Syst Transit. 2013;15:1–431. [PubMed] [Google Scholar]
- 61.Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434. doi: 10.2147/PPA.S44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen CJ, Colson AE, Sheble-Hall AG, McLaughlin KA, Morse GD. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) study. HIV Clin Trials. 2007;8:19–23. doi: 10.1310/hct0801-19. [DOI] [PubMed] [Google Scholar]
- 63.Antiretroviral Therapy Cohort Collaboration Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trancart S, Charreau I, Marchou B, et al. Presence of lamivudine or emtricitabine is associated with reduced emergence of nonnucleoside reverse transcriptase inhibitor mutations in an efavirenz-based intermittent antiretroviral treatment regimen. Antimicrob Agents Chemother. 2012;56:1655–1657. doi: 10.1128/AAC.05452-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bangsberg DR, Ragland K, Monk A, Deeks SG. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS. 2010;24:2835–2840. doi: 10.1097/QAD.0b013e328340a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis AC, Prista Guerra M, Lencastre L. Treatment adherence, quality of life and clinical variables in HIV/AIDS infection. J Int AIDS Soc. 2000;13(Suppl 4):119. [Google Scholar]
- 67.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379–396. doi: 10.1001/jama.2018.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946–present; search conducted on September 14, 2016a
| Search category | ID | Search terms |
|---|---|---|
| Disease | #1 | (“human immunodeficiency virus” OR “HIV”) |
| Intervention | #2 | (“once daily” OR “once a day” OR “QD” OR “single tablet” OR “single pill” OR “one pill” OR “fixed dose combination” OR “co-formulated”) |
| Adherence | #3 | (“adherence” OR “nonadherence” OR “non-adherence” OR “adherent” OR “nonadherent” OR “non-adherent” OR adhere* OR “compliance” OR “noncompliance” OR “non-compliance” OR “compliant” OR “noncompliant” OR “non-compliant”) |
| Viral suppression | #4 | (“viral load” OR “viral suppression” OR “virologic suppression” OR “virologic response” OR “virologic failure” OR “virologic success” OR “virological suppression” OR “virological response” OR “virological failure” OR “virological success” OR “RNA suppression” OR “RNA level” OR “RNA concentration” OR “undetectable” OR “undetectability”) |
| Association | #5 | (compare* OR “comparison” OR associate* OR relate* OR relation* OR correlate* OR “correlation” OR cause* OR “versus” OR “vs” OR “vs.” OR “lead to” OR “leads to” OR “led to”) |
| RCT | #6 | (“clinical trial” OR “clinical trials” OR “randomized controlled trial” OR “randomized controlled trials” OR “randomised controlled trial” OR “randomised controlled trials” OR random* OR enroll* OR “protocol” OR “open-label” OR “single blind” OR “double blind”) |
| Real world | #7 | (“observational” OR “longitudinal” OR “retrospective” OR “prospective” OR “follow up” OR “cohort” OR “insurance” OR electronic medic* OR “claims data” OR “naturalistic” OR “pragmatic” OR “medical records” OR “registry”) |
| Study objectives | ||
| 1. Real-world adherence with STR vs MTR | #8 | #1 AND #2 AND #3 AND #7 |
| 2. Association between adherence and viral outcomes | #9 | #1 AND #3 AND #4 AND #5 AND (#6 OR #7) |
| 3. Comparative effects of STR vs MTR on viral outcomes | #10 | #1 AND #2 AND #4 AND (#6 OR #7) |
| All | #11 | #8 OR #9 OR #10 |
| #12 | Limit 11 to yr=“2013-Current” | |
| #13 | Limit 12 to English language | |
Note:
An initial search of MEDLINE In-Process was completed on September 6, 2013 to identify any studies that were relevant to the three objectives that were published between 2006 and 2013.
Abbreviations: HIV, human immunodeficiency virus; MTR, multiple-tablet regimen; RCT, randomized controlled trial; STR, single-tablet regimen.
Table S2.
Eligibility criteria
| Criteria | Included | Excluded |
|---|---|---|
| Publication type | • Full-text original research published in English | • Non-English publications • Duplicate publication(s) of the same trials or studies • Case reports • Commentaries and letters • Recommendations/guidelines • Books/chapters/addresses/bibliographies/biographies/lectures • Non-systematic reviews • Systematic reviews and meta-analyses |
| Study design | • RCTs • Observational studies (retrospective studies, longitudinal studies, prospective studies, etc) |
• Non-human studies • Preclinical studies • Phase 1 studies • Short-term studies (study length #10 days) • Studies interrupted or prematurely terminated |
| Study location | • North America and the European Union | • Including any location not in North America or the European Union |
| Population | • Adults (aged $18 years) diagnosed with HIV and treated with ART | • Children and adolescents (aged <18 years) • Non-HIV-positive patients • Studies conducted only in HIV subpopulation, including: o patients infected with hepatitis B or C ○ patients with a history of drug abuse ○ patients with depression o pregnant women ○ homeless and marginally housed patients |
| Interventions | • Once-daily ART regimens | • Non-ART regimens • More-than-once-daily regimens |
| Outcomes | • Association between treatment regimen and adherence level, or • Association between adherence level and drug efficacy/effectiveness,a or • Association between treatment regimen and drug efficacy/effectivenessa |
• Studies that report neither treatment adherence nor drug efficacy/effectiveness • Studies that do not report the association or comparative results |
Note:
Drug effectiveness or efficacy was measured as viral load (RNA level), viral suppression rate, viral failure rate, and undetectable RNA level.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; RCT, randomized controlled trial.