Abstract
Purpose
The aim of this study was to examine the effects of exposure to air pollution and cigarette smoke on respiratory function, respiratory symptoms, and the prevalence of COPD in individuals aged ≥50 years.
Patients and methods
We used spirometry and medical questionnaires to screen 433 individuals from Omuta City, Japan, an area with high levels of air pollution.
Results
Non smokers had a high estimated COPD prevalence rate of 16%. Among smokers, the estimated prevalence of COPD was 29% in seniors (50- to 74-years group) and 37% in the elderly (>75 years group). We also found a correlation between levels of suspended particulate matter and COPD.
Conclusion
Both smoking and chronic exposure to air pollution (>5 years) decreased respiratory function, exacerbated respiratory symptoms, and increased the prevalence of COPD. We strongly recommend periodic screening for the elderly patients to facilitate early detection of respiratory disease.
Keywords: diagnosis, primary care, screening
Introduction
COPD is a respiratory condition that develops from long-term exposure to air pollutants and through smoking.1 The worldwide prevalence of COPD is increasing, and the disease is expected to be the third leading cause of death by 2020.2 The Japanese Respiratory Society’s guidelines for the management of COPD list exogenous risk factors such as tobacco smoke, atmospheric pollution, passive smoking, exposure to chemical substances, and respiratory infection.3 Of these factors, fine particulate matter with an aerodynamic diameter of ≤2.5 µm (PM2.5) is of particular concern. PM2.5 can penetrate deep into the lung tissue and precipitate a number of respiratory diseases, although the research showing a clear causal relationship between particulate matter and COPD is scant.4 A few studies have reported a relationship between long-term exposure to suspended particulate matter and impairment of respiratory function, but no correlations were found between exposure to PM2.5 and changes in respiratory function.5 While there is no clear correlation between exposure to atmospheric pollution and the development of COPD, exposure has been reported to exacerbate respiratory symptoms such as cough; chronic respiratory symptoms and a history of asthma may increase the risk of developing COPD.6
Aging is a factor that increases the prevalence of COPD. With age, the respiratory system’s morphological and functional resistance weakens, leading to an increase in morbidity.7 According to the European Lung White Book, individuals aged 60–79 years display an increase in respiratory morbidity and a reduction in the ratio of FEV1/FVC.8
The current air pollution problem in Japan and its aging society are expected to result in increased COPD morbidity. In Omuta City (Fukuoka prefecture), the proportion of the population aged ≥75 years is 35%, representing a super-aging society.9 We constructed a system for coordinating local clinics, hospitals, and medical associations to raise awareness, improve opportunities for early diagnosis, and characterize the state of COPD in the city, which had been officially designated an air-polluted region because of coal-mining activities. Elderly residents are considered part of the coal-energy generation, affected by the burning of biomass fuel and other products of a high-growth period, such as dust and smoking. A high cumulative exposure rate leads to high rates of COPD morbidity. Although data on the prevalence of COPD in the elderly are available, there are few reports concerning respiratory symptoms, respiratory function, and morbidity among elderly individuals who have been chronically exposed to contaminants in air-polluted areas.
The purpose of this study was to examine respiratory function, respiratory symptoms, and the prevalence of COPD in elderly individuals exposed to air pollution, smoking, and related factors.
Patients and methods
Study design and setting
We carried out a total of 10 screenings for COPD in district 4 of Omuta City, among mass residential health screenings held between 2015 and 2018. Of the 55,316 residents who underwent a screening, 721 were aged >50 years, and 433 of those individuals consented to undergo an examination for COPD.
Clinical measurements
We collected information on age, sex, height, weight, and body mass index (BMI) and measured FVC, percentage vital capacity, FEV1, and the FEV1/FVC ratio. Diagnoses were classified as bronchial asthma, chronic bronchitis, both bronchial asthma and chronic bronchitis, and others. The categories used for establishing smoking status were current smoker, former smoker, passive smoker, and never-smoker.
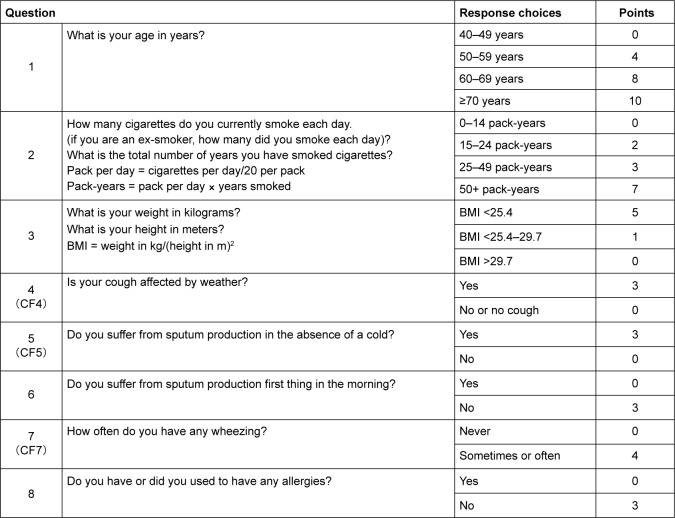
Subjects had to have resided or been employed in Omuta City for at least 5 years. We used a COPD questionnaire to classify the subjects suspected of having COPD (Figure 1) and performed a pulmonary function test, for which we used a spirometer (Autospiro AS-407; Minato Medical Science, Osaka, Japan), adhering to the guidelines of the Japanese Respiratory Society. Measurements were taken before providing a bronchodilator, and subjects with a FEV1/FVC ratio of ≤70% and a score of at least 17 points on the questionnaire were considered possible COPD patients.10 The estimated prevalence was calculated from the spirometry data. To assess the effect on the elderly, we classified the subjects further into two groups according to the Japanese health care system: the seniors (50- to 74-years group) and the elderly (>75 years group).
Figure 1.
IPAG Questionnaire.
Note: The figure was adapted from the study by Sichletidis L, Spyratos D, Papaioannou M, et al. A combination of the IPAG Questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim Care Respir J. 2011;20(2):184–189.10
Abbreviations: BMI, body mass index; IPAG, International Primary Care Airways Group.
Air pollution monitoring
We collected atmospheric data for concentrations of suspended particulate matter, PM2.5, nitrogen dioxide, and ozone for Omuta City for the period between December 2013 and March 2017. The data were obtained from Omuta City government.
Statistical analyses
The Shapiro–Wilk method was used to test for normality. The Mann–Whitney U test was used for comparisons between subjects with and without suspected COPD. For other comparisons between groups, we performed multiple-comparison tests using ANOVA and the Steel–Dwass test. The Cochran–Armitage test for trend was used for the categorical smoking data, and the chi-squared test was used for disease risk. The significance level was set at 5%, and PASW for Windows, version 18 (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent before data collection began. The study protocol was approved by the ethics committee of Teikyo University Faculty of Fukuoka Medical Technology (approval number: 08072424-2).
Results
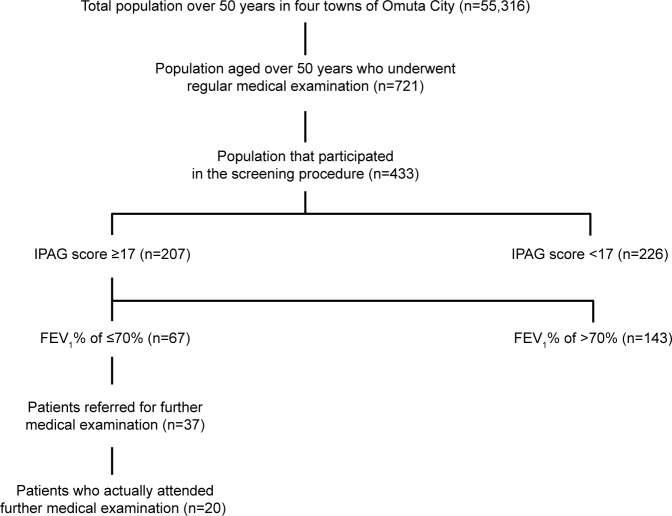
Four hundred and thirty-three individuals provided consent and participated in the COPD screening. Of these individuals, 67 (15%) subjects scored 17 or more points on the COPD questionnaire and had an FEV1 measurement of ≤70% (Figure 2). The estimated prevalence of COPD in smokers was 29% among seniors (50- to 74-years group) and 37% among the elderly (>75 years group). The estimated prevalence of COPD in the passive-smoking group was 8% among seniors and 17% among the >75 years group. In the non-smoking group, the estimated prevalence was 9% among the 50- to 74-years group and 16% among the >75 years group (Table 1). Overall, the prevalence of COPD differed significantly between 50- to 74-years group and >75 years group (OR: 2.4, 95% CI: 0.18–0.98; Table 2).
Figure 2.
Flowchart of the screening process.
Abbreviation: IPAG, International Primary Care Airways Group.
Table 1.
Prevalence of COPD among study participants
| Subjects | Total | 50- to 74-years group smokers and former smokers | ≥75 years group smokers and former smokers | 50- to 74-years group passive smokers | ≥75 years group passive smokers | 50- to 74-years group non smokers | ≥75 years group non smokers |
|---|---|---|---|---|---|---|---|
| Subjects with IPAG score indicating COPD | 207/433=0.48 | 47/75=0.63 | 13/26=0.53 | 21/61=0.34 | 15/25=0.64 | 67/180=0.37 | 44/66=0.66 |
| Ratio of those thought to have COPD after spirometry | 67/207=0.30 | 22/47=0.47 | 9/13=0.69 | 5/21=0.23 | 4/15=0.26 | 16/67=0.23 | 11/44=0.25 |
| Subjects with both IPAG score and spirometry indicating COPD | 55,316×0.48×0.30= | 55,316×0.63×0.47= | 55,316×0.53×0.69= | 55,316×0.34×0.23= | 55,316×0.64×0.26= | 55,316×0.37×0.23= | 55,316×0.66×0.25= |
| Prevalence of suspected COPD in subjects aged >50 years | 7,965/55,316 | 16,379/55,316 | 20,229/55,316 | 4,326/55,316 | 9,204/55,316 | 4,707/55,316 | 9,127/55,316 |
| ×100=14% | ×100=29% | ×100=37% | ×100=8% | ×100=17% | ×100=9% | ×100=16% |
Notes: Subjects with both IPAG score and spirometry indicating COPD. Total population over 50 years in four towns of Omuta City (n=55,316). Prevalence of suspected COPD in subjects aged >60 years.
Abbreviation: IPAG, International Primary Care Airways Group.
Table 2.
ORs of COPD by age group and smoking status
| Subjects | 50- to 74-years group | ≥75 years group | P-value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| (n=316) | (n=117) | ||||||
| Smokers and former smokers | 22/53 | 41% | 9/17 | 52% | 0.56 | 1.07 | 0.35–2.46 |
| Passive smokers | 5/56 | 9% | 4/21 | 19% | 0.1 | 0.3 | 0.09–1.36 |
| Non smokers | 16/164 | 10% | 11/55 | 20% | <0.04 | 2.4 | 0.18–0.98 |
Note: The chi-squared test was used for calculating disease risk.
Respiratory function commonly deteriorates with age, and the FEV1/FVC ratio was lower in the elderly and lowest among >75 years group smokers (72.7%±11.2%, P<0.05; Table 3). As for respiratory symptoms (Table 4), it shows the ratio of the number of people who answered Yes. There was a significant difference between 50- to 74-years group and >75 years group smokers in wheezing, but not in coughing or sputum (Table 4).
Table 3.
Characteristics of study participants and respiratory measurements
| Characteristics or measurements | Group A | Group B | Group C | Group D | Group E | Group F | P-value |
|---|---|---|---|---|---|---|---|
| 50- to 74-years group smokers and former smokers | ≥75 years group smokers and former smokers | 50- to 74-years group passive smokers | ≥75 years group passive smokers | 50- to 74-years group never-smokers | ≥75 years group never-smokers | ||
| Sex (male/female) | 56/19 | 9/17 | 6/55 | 3/22 | 48/132 | 11/55 | 0.75 |
| Age (years) | 69.2±2.6 | 77.8±2.5 | 69.5±19.0 | 78.6±4.1 | 69.5±2.8 | 78.9±3.1 | <0.001 a, c, e, f, h, i, n, <0.001 |
| Height (cm) | 163.6±7.5 | 161.0±10.2 | 154.5±15.4 | 153.1±7.6 | 155.3±7.6 | 152.5±7.5 | <0.001 b, c, e |
| Weight (kg) | 59.3±8.6 | 60.9±10.2 | 53.4±14.7 | 52.4±7.0 | 55.3±10.2 | 53.4±8.9 | 0.39 |
| Body mass index | 22.2±7.9 | 23.5±9.8 | 22.3±14.2 | 22.38±7.3 | 23.2±8.6 | 23.11±8.9 | 0.12 |
| FVC (L) | 3.4±0.7 | 3.1±0.9 | 3.4±0.6 | 2.4±0.7 | 2.7±0.7 | 2.4±0.7 | 0.31 |
| Percentage vital capacity | 102.9±27.6 | 101.3±14.8 | 102.9±24.4 | 104.0±22.5 | 99.8±16.7 | 98.5±18.0 | 0.12 |
| FEV1 (L) | 2.4±0.6 | 2.1±0.6 | 2.0±0.5 | 1.8±0.6 | 2.1±0.5 | 1.7±0.5 | <0.001 b, c, d, e, i, l, n, o |
| FEV1/FVC (%) | 75.3±13.1 | 72.7±11.2 | 86.4±20.5 | 77.8±15.3 | 78.7±11.3 | 77.7±11.9 | <0.05 f, l |
| Suspected COPD (male/female) | 22/53 | 9/17 | 5/56 | 4/21 | 16/164 | 11/55 | <0.05 |
Notes: IPAG score, the COPD questionnaire score mean ± SD of the group. CF4, IPAG fourth question about cough in the IPAG questionnaire. CF5, IPAG fourth question about sputum production in the IPAG questionnaire. CF7, IPAG fourth question about wheezing in the IPAG questionnaire. These show the ratio of the people who answered “Yes.” A significant difference was seen only in wheezing (P<0.05, Group A vs Group E). a, Group A vs. Group B; b, Group A vs. Group C; c, Group A vs. Group D; d, Group A vs. Group E; e, Group A vs. Group F; f, Group B vs. Group C; h, Group B vs. Group E; i, Group B vs. Group F; j, Group C vs. Group D; k, Group C vs. Group E; l, Group C vs. Group F; n, Group D vs. Group E; o, Group E vs. Group F. Values are presented as mean ± SD. Differences in sample characteristics were analyzed for significance using ANOVA and a post-hoc test, the Mann-Whitney U test, the Cochran-Armitage test, and the χ2 test.
Abbreviation: IPAG, International Primary Care Airways Group.
Table 4.
Respiratory symptoms of study subjects and scores on the chronic obstructive pulmonary disease questionnaire
| Finding | Group A | Group B | Group C | Group D | Group E | Group F | P-value |
|---|---|---|---|---|---|---|---|
| 50- to 74-years group | ≥75 years group | 50- to 74-years group | ≥75 years group | 50- to 74-years group | ≥75 years group | ||
| Smokers and former smokers | Smokers and former smokers | Passive smoker | Passive smoker | Non-smoker | Non-smoker | ||
| n=75 | n=26 | n=61 | n=25 | n=180 | n=66 | ||
| IPAG score | 19.8±4.7 | 19.4±4.8 | 17.5±4.4 | 18.7±2.7 | 17.2±4.1 | 18.5±3.4 |
P<0.00 b, d, h |
| Cough | 5/70 | 3/23 | 3/58 | 1/24 | 19/161 | 4/62 | 0.18 |
| CF4 | 7% | 13% | 5% | 4% | 11% | 6% | |
| Sputum | 22/53 | 9/17 | 19/42 | 6/19 | 68/112 | 23/43 | 0.29 |
| CF5 | 29% | 35% | 31% | 24% | 38% | 35% | |
| Wheezing | 7/68 | 2/24 | 4/57 | 2/23 | 10/170 | 6/60 |
P<0.05 d |
| CF7 | 10% | 8% | 7% | 8% | 6% | 9% |
Notes: b, Group A vs Group C; d, Group A vs Group E; h, Group B vs Group E. IPAG score, the chronic obstructive pulmonary disease questionnaire score mean ± SD of the group. CF4, IPAG 4th question about cough in the IPAG questionnaire. CF5, IPAG 4th question about sputum production in the IPAG questionnaire. CF7, IPAG 4th question about wheezing in the IPAG questionnaire. These show the ratio of the people who answered ‘Yes.’ A significant difference was seen only in wheezing (p<0.05, Group A vs Group E)
Of the 37 patients with suspected COPD who were referred to local hospitals, only 20 actually underwent a formal diagnosis process. Follow-up showed that seven patients were diagnosed with COPD (35%), six with bronchial asthma (30%), two with lung cancer (10%), and one with restrictive ventilatory impairment (5%). The remaining four subjects were diagnosed with other conditions or with no abnormalities (20%) (Table 5). Of the seven patients diagnosed with COPD, four were from Group A, two were from Group B, and one was from Group C. Of those diagnosed with bronchial asthma, one was from Group A, three were from Group B, one was from Group C, and one was from Group E.
Table 5.
Medical diagnoses of 20 study participants
| Diagnoses | Number of patients, n (%) |
|---|---|
| Bronchial asthma | 6 (30) |
| Lung cancer | 2 (10) |
| Pulmonary emphysema | 6 (30) |
| COPD | 1 (5) |
| Restrictive ventilatory impairment | 1 (5) |
| No abnormality | 2 (10) |
| Others | 2 (10) |
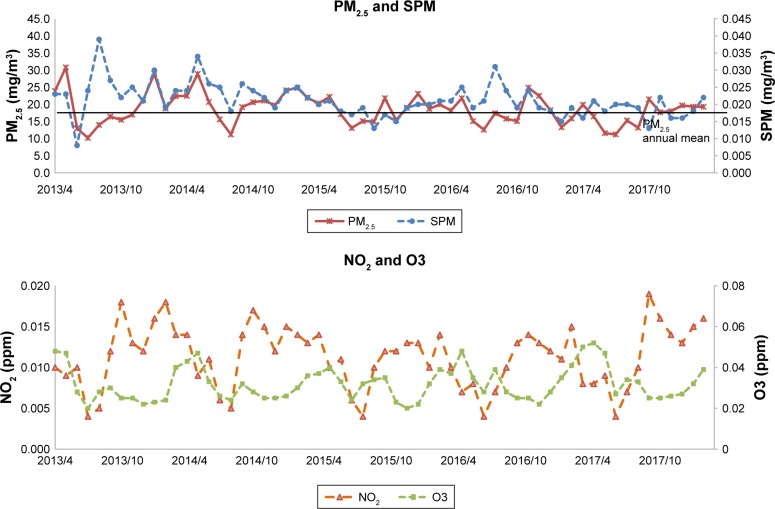
The average PM2.5 values in Omuta City were 23.3, 19.4, and 20.9 µg/m3 in 2012, 2013, and 2014, respectively. The mean values of suspended particulate matter in those years were 24.4, 23.8, and 24.1 µg/m3. The Japanese environmental standards specify an average PM2.5 daily value below 35 µg/m3 and an annual average value below 15 µg/m3. The annual average value for Omuta City was therefore higher in 2012–2014 than the environmental standard (Figure 3). The WHO standard for PM2.5 is 25 µg/m3/day, and the standard for the annual average is 10 µg/m3, lower than the Japanese standard. Japanese standards for daily average concentrations of nitrogen dioxide are within the range of 0.04–0.006 ppm or less, and the workplace health and safety standard for ozone is 0.1 ppm or less; the concentrations measured in Omuta City were well within the limits.
Figure 3.
Air pollution monitoring.
Notes: The annual daily mean levels of PM2.5, SPM, NO2, and O3 were recorded from 2013 to 2017. Information dissemination: Omuta City environment safeguard section.
Abbreviations: NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter with an aerodynamic diameter of ≤2.5 µm; SPM, suspended PM2.5.
Discussion
We examined respiratory function, respiratory symptoms, and the prevalence of COPD in 50- to 74-years group and >75 years group individuals with chronic exposure to air pollution. An epidemiological survey of COPD prevalence conducted in Japan in 2001 reported an estimated prevalence of 8.5% overall, 12.4% for smokers, and 5% for non smokers. COPD prevalence was reported to be 5.1% for individuals in their 50s, 12.2% for those in their 60s, and 17.4% for those aged >70 years.11 The FEV1/FVC ratio of non smokers was 79.9% and 77.5% for smokers.
In the present study, elderly non-smoking individuals had a high estimated prevalence rate of 16%, and rates in elderly individuals increased significantly from rates in seniors. We also found a correlation between levels of suspended particulate matter and COPD. Smokers and former smokers had an estimated prevalence rate of >25% regardless of age, clearly showing the negative impact of smoking. The magnitude of the association we found between pollutant exposure and lung function is comparable to associations reported by studies that included pollution levels above the standards of the US Environmental Protection Agency.12 A study of 4,757 women in Germany found that a 7 µg/m3 increase in PM10 was associated with an increased prevalence of COPD (OR: 1.33, 95% CI: 1.03–1.72).13
Morbidity, respiratory function, and respiratory symptoms are exacerbated with age.14 The prevalence of COPD among men aged >40 years is considered high and tends to increase rapidly aged >60 years.15 We found that the prevalence of COPD was high even among non smokers. With age, respiratory muscle strength is reduced, chest walls stiffen, leading to a reduction in thoracic compliance, and a decrease in lung capacity, and FEV1 is observed. In the elderly, pulmonary aging causes a decrease in FEV1 and enlargement of alveolar airspaces even in healthy individuals.16 Smokers, who are exposed to both air pollution and cigarette smoke, had an estimated prevalence of >25% in our study.
We did not measure previous-day air pollution exposure or whether FEV1 varied by smoking status. We had sufficient power to evaluate effect modification by former smoking and found no evidence of heightened susceptibility to air pollution among former smokers.
The average annual values of PM2.5 concentrations in Omuta City are higher than international standards, and the proportion of the elderly in the population is >35.7%; the city is expected to see an increase in COPD patients. More than half of the subjects examined exceeded the cut off value in the COPD questionnaire, which was developed in Europe and the US, and so its usefulness in Japan has not been validated.17 It has been noted that using BMI as a variable is inappropriate in Japanese individuals and tends to yield higher results. In the subjects not suspected of having COPD, however, the proportion of complaints of cough, sputum, and wheezing exceeded 50%, and it has been reported that these symptoms increase when PM2.5 concentrations exceed 20 µg/m3.18 In the US, COPD accounts for higher medical expenditures than other pulmonary diseases,19 and it is expected that it will also become a substantial economic burden in Omuta City. Early intervention and early diagnosis at the local-government level may reduce medical expenses.20
In this study, only 20 of the patients with suspected COPD who were referred to the central hospital for further diagnosis actually underwent a further medical examination. The number of COPD cases was relatively low (35%). However, chronic bronchitis, bronchial asthma, emphysema, and other respiratory conditions were included in the COPD classification. Making a definitive diagnosis may be difficult, and an overlap of symptoms may be observed because of the complex presentation of these diseases.
There is evidence that the rate of awareness of COPD is lower among older individuals, who typically do not seek medical help until their symptoms are severe.21 Improving awareness of the causes and symptoms of COPD is essential for early detection of the disease.22 Furthermore, difficulty in accessing health care may have been a factor for older patients. It is likely that many COPD patients go undiagnosed; therefore, the elderly should undergo regular COPD screenings.
Limitations
As a limitation of this study, differences in air pollution exposure may have influenced the prevalence, and we did not have a control group. In addition, the exposure conditions, the levels of exposure in residential district environments, and the influence of the exposure period have not yet been investigated. The specific smoke-exposure history of current smokers, former smokers, and passive smokers was not taken into account, and neither had a family history of endogenous COPD factors. Nonetheless, the conclusions of the study are supported by appropriate evidence; the claims are not exaggerated.
Conclusion
We examined the effects of air pollution and smoking on respiratory function, respiratory symptoms, and trends of COPD prevalence in the 50- to 74-years group and the >75 years group individuals. In addition to the impact of smoking, we verified that the elderly who were chronically exposed to air pollution had exacerbated respiratory symptoms and impaired respiratory function. Japan, with its aging population and air pollution issues, is expected to see an increase in the number of elderly individuals diagnosed with respiratory diseases. We strongly recommend that the elderly undergo periodic health screenings to facilitate early detection and that local governments enhance the provision of social security services such as patient education and smoking cessation programs.
Acknowledgments
The authors thank the study participants, technical staff, administrative support team, and our co-workers for their assistance. We thank the Omuta Public Health Center and Sugi Hospital for their support. We thank Dean Meyer, PhD, ELS from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported by the Omuta Public Health Center and the National Hospital Organization Omuta Hospital.
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Price DB, Tinkelman DG, Halbert RJ, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73(3):285–295. doi: 10.1159/000090142. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Respiratory Society The Japanese Respiratory Society guidelines for management of respiratory infections. 2018. [Accessed February 12, 2018]. Available from: http://www.jrs.or.jp/modules/guidelines/index.php?content_id=20.
- 4.Yang JY, Xin JY, Ji DS, Zhu B, Ds J. Variation analysis of background atmospheric pollutants in North China during the summer of 2008 to 2011. Huan Jing Ke Xue. 2012;33(11):3693–3704. Chinese. [PubMed] [Google Scholar]
- 5.Götschi T, Sunyer J, Chinn S, et al. Air pollution and lung function in the European community respiratory health survey. Int J Epidemiol. 2008;37(6):1349–1358. doi: 10.1093/ije/dyn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu G, Zhong N, Ran P. Air pollution and COPD in China. J Thorac Dis. 2015;7(1):59–66. doi: 10.3978/j.issn.2072-1439.2014.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The European Respiratory Society’s White Book [homepage on the Internet] [Accessed February 12, 2018]. Available from: https://www.erswhitebook.org/
- 9.Omuta-shi synthesis page the number of the households. [Accessed February 12, 2018];Population. 2018 Available from: https://www.city.omuta.lg.jp. [Google Scholar]
- 10.Sichletidis L, Spyratos D, Papaioannou M, et al. A combination of the IPAG Questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim Care Respir J. 2011;20(2):184–189. doi: 10.4104/pcrj.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the nippon COPD epidemiology study. Respirology. 2004;9(4):458–465. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide – global update. 2005. [Accessed August 21, 2018]. Available from: https://apps.who.int/iris/bitstream/handle/10665/69477/WHO_SDE_PHE_OEH_06.02_eng.pdf?sequence=1.
- 13.Schikowski T, Sugiri D, Ranft U, et al. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6:152. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Sun S, Tang R, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079–3091. doi: 10.2147/COPD.S122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbeken EK, Cauberghs M, Mertens I, et al. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest. 1992;101(3):800–809. doi: 10.1378/chest.101.3.800. [DOI] [PubMed] [Google Scholar]
- 17.Kawayama T, Minakata Y, Matsunaga K, et al. Validation of symptom-based COPD questionnaires in Japanese subjects. Respirology. 2008;13(3):420–426. doi: 10.1111/j.1440-1843.2008.01241.x. [DOI] [PubMed] [Google Scholar]
- 18.Zemp E, Elsasser S, Schindler C, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA team. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1257–1266. doi: 10.1164/ajrccm.159.4.9807052. [DOI] [PubMed] [Google Scholar]
- 19.Pope CA, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119(11):1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawara Y, Senjyu H, Tanaka K, et al. Value of systematic intervention for chronic obstructive pulmonary disease in a regional Japanese City based on case detection rate and medical cost. Int J Chron Obstruct Pulmon Dis. 2015;10:1531–1542. doi: 10.2147/COPD.S82872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornmann O, Beeh KM, Beier J, et al. Newly diagnosed chronic obstructive pulmonary disease. Clinical features and distribution of the novel stages of the global initiative for obstructive lung disease. Respiration. 2003;70(1):67–75. doi: 10.1159/000068417. [DOI] [PubMed] [Google Scholar]
- 22.Asai M, Tanaka T, Kozu R, et al. Effect of a chronic obstructive pulmonary disease (COPD) intervention on COPD awareness in a regional city in Japan. Intern Med. 2015;54(2):163–169. doi: 10.2169/internalmedicine.54.2916. [DOI] [PubMed] [Google Scholar]