Abstract
Objective: We explored the effectiveness of preventive home visits on the health-related quality-of-life (HRQoL) and mortality among independently community-dwelling older adults.
Design: A randomised controlled trial.
Subjects: Independently home-dwelling older adults 75 years and older, consisting of 211 in the intervention and 211 in the control group.
Setting: Hyvinkää town municipality, Finland.
Main outcome measures: We used the change in HRQoL measured by the 15D scale as our primary outcome. Mortality at two years was retrieved from central registers.
Results: At the one-year time point, the HRQoL according to the 15D scores deteriorated in the control group, whereas we found no change in the intervention group. The difference between the 15D score changes between the groups was −0.015 (95% CI −0.029 to −0.0016; p = 0.028, adjusted for age, sex, and baseline value). At the two-year time point as the visits ended, that difference diminished. There was no difference in mortality between the groups during the 24-month follow-up.
Conclusion: Preventive home visits implemented by a multidisciplinary team with CGA appear to help slow down the decline in HRQoL among older adults, although the effect diminishes when the visits end.
Key points
We are exploring preventive home visits as means to support the health-related quality-of-life (HRQoL) of home-dwelling older adults
Multiprofessional preventive home visits in this intervention study helped to maintain the HRQoL when measured using 15D
The effects on HRQoL diminished when the intervention ended, so could further benefits be attained with a longer intervention?The clinical trial registration number: ACTRN12616001411437
Keywords: Prevention, preventive home visit, older adults, health-related quality-of-life, comprehensive geriatric assessment
1. Introduction
As the population ages, societies face challenges regarding how to best offer social and health care services for older adults. Effective prevention is needed to support older adults' quality-of-life and to prevent their disability. However, older people have often multiple and complex health care needs. Thus, assessing and caring older people is challenging [1]. Therefore, comprehensive assessments, multiprofessional approaches, and individualised interventions are crucial when planning health care services for older adults.
Preventive home visits for older people have already been studied for decades [2]. Earlier studies indicated that preventive home visits might positively affect old people’s functioning, institutionalisations, and mortality, but the heterogeneity of interventions, participants, and outcome measures in previous studies renders comparisons challenging [3,4]. Studies targeted to unselected older populations are needed, since earlier studies failed to show clear effects when preventive home visits were targeted solely to older adults at risk [3,4]. Only a few studies have investigated multiprofessional preventive home visit programs [5–9], although multidimensional interventions with comprehensive assessment and cooperation between several professionals may be more effective than one nurse performing preventive home visits [4]. There are a few preventive programmes which have been performed in primary care [10–12]. However, in all of them the home visits were based on one professional performing the visits.
The aim of our randomised controlled trial was to investigate the effects of a multiprofessional preventive home visit intervention on independently home-dwelling older adults’ (75+) health-related quality-of-life (HRQoL) by 15D scale, and their mortality.
2. Methods
2.1. Design
Briefly, our study was a two-year randomised, controlled, single-center trial investigating the effectiveness of multidisciplinary preventive home visits on independently community-dwelling older people. Our primary outcome measure is HRQOL according to 15D. Secondary outcome measures are use of health and social services, and mortality. The intervention was delivered during a six- to nine-month period by a nurse, a physiotherapist, and a social worker. We described the methods and baseline findings in detail in an earlier paper [13]. In this paper we described the feasibility of our intervention, whilst the feedback from participants was mostly favorable [13]. The study protocol was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR). Ethics approval was provided by the Ethics Committee of the Helsinki University Central Hospital. Written and oral information was provided to all participants, and participants provided their written informed consent.
2.2. Participants
A total of 422 participants were recruited to the study in 2013. A study letter was sent to all residents of the Hyvinkää area aged 75 years or older. The inclusion criteria for this study consisted of being 75 years old or older, home dwelling, not receiving home help or nursing services, Finnish speaking, and living permanently in the Hyvinkää area. Hyvinkää is a mid-size Finnish town with 46,600 inhabitants. Among those who returned a letter showing an interest in participating and fulfilling the inclusion criteria (n = 968), we recruited to the study the first consecutive 422 individuals who met the inclusion criteria and provided their informed consent (Figure 1). Among those older people not recruited of respective age in Hyvinkää, the mean age was slightly higher than those recruited (81.6 years), the proportion of females was similar (65%), whereas the proportion of married older people was lower (41% vs. 51%).
Figure 1.
Flowchart of study participant selection and randomisation.
2.3. Measures and study procedures
Data were collected through the same postal survey at baseline and at one- and two-year time points. The survey included items about demography (gender, age, marital status, and education), diagnoses, current weight and height, current medications, use of assistive devices, health habits, and risk factors (smoking, use of alcohol, exercise habits, and falls during the past six months). Assessments and interventions were performed in 2013 and 2014. The intervention and control groups were compared with respect to changes in their HRQoL using a 15-dimensional assessment scale (15D) [14] from baseline at the one- and two-year time points.
We used 15D as the primary outcome measure. The 15D can be measured by independently filling a questionnaire or by interview. We used a postal questionnaire. As a generic assessment measurement, 15D can be used as a profile measurement on its 15 dimensions as well as a single index. The index varies between 0 (poorest HRQoL) and 1 (excellent HRQoL). The domains of the 15D scale consist of mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. 15D has a very good discriminant validity and prognostic validity in different-aged populations [15]. In addition, it is sensitive to changes following a health care intervention [16].
Furthermore, mortality dates were retrieved from central registers at the end of the study. The follow-up on mortality in the Finnish register system is 100% complete.
2.4. Randomisation
We randomised participants into the intervention and control groups using computer-generated random numbers after the baseline assessment. To avoid dilution of the intervention effect, spouses (n = 128) were randomised together. The intervention group received the preventive home visits in addition to their typical care, while the control group received their typical care including the normal health and social care offered by the municipality.
2.5. Intervention
The intervention was based on the comprehensive geriatric assessment (CGA) and consisted of three multiprofessional preventive home visits performed by a nurse, a physiotherapist, and a social worker during a six- to nine-month time period. The nurse visit was first, followed by the visit from a physiotherapist, and the social worker visit was last. The professionals performing the intervention visits were trained and given oral and written instructions on how to perform the home visits. They had the possibility of consulting with a physician from a geriatric ward if needed (Table 1).
Table 1.
Components of the home visit intervention, specific instructions given, and the number of participants who received specific instructions in addition to general information.
| Individual delivering the home visit | Intervention | Duration of visit | Tailored guidance | Participants receiving tailored guidance (n) |
|---|---|---|---|---|
| Nurse |
Structured assessment: RAI-HCa, MMSEb, MNAc, Barthel scale [38], GDS-15d, and IADLe Measurements: Blood pressure and blood glucose levels Information: health and social services offered by the municipality, and local volunteer and third-party organizations |
1.0–1.5 h | Contact family doctor, health nurse, service needs assessment, physiotherapy, memory clinic, exercise group, or third-party service provider | n = 104 |
| Physiotherapist |
Structured assessment: barriers of mobility, FROP-Comf screen, and home safety assessment Measurements: a hand grip strength test (Jamar) and CS-5g Information: physical activity recommendations, physiotherapy services of the municipality for individuals and groups, and services offered by local volunteer and third-party organizations |
1.0–1.5 h | Personalized guidance for physical training, instructions to register for group training, and instructions to book a personal physiotherapist appointment | personalized training instructions, n = 211 physiotherapy group, n = 3 physiotherapist appointment, n = 2 |
| Social worker |
Structured assessment: social functioning, ADLh, IADLe, and service needs Information: financial and other benefits for older people, social services provided by the municipality, services and benefits from SIIi, and local volunteer and third-party organizations |
0.5–1.5 h | Instructions/help to contact case management, day activities for older adults, home delivered services, and applying for financial benefits | n = 76 |
The nurse home visit comprised a structured assessment relying on validated measures (Table 1). The nurse measured the participants’ blood glucose and blood pressure levels and distributed information on the social and health services offered by the municipality, as well as local third-party organizations and voluntary groups. If any concerns regarding participant's health or well-being arose during the assessment, the nurse directed participants to contact their family doctor or other suitable health or social service.
The physiotherapist’s home visit comprised a structured assessment focusing on the barriers to mobility, fall risk, and home safety. It relied on validated tests and measurements (Table 1). S/he assessed the need for aids and compiled individual exercise instructions based on the test results as well as the participant’s motivation and wishes. Participants were given information on the physiotherapy and individual and group exercise services offered by the municipality and local voluntary and third-party organizations.
The social worker home visit comprised a structured assessment on social functioning, activities of daily living (ADL), instrumental activities of daily living (IADL), and service needs (Table 1). The social worker also distributed information on social services, and financial and other benefits provided to older adults, and left contact information when needed. The social worker helped the participant to contact a service provider if other services or a financial need arose during a visit.
2.6. Statistical analyses
We calculated the needed sample size based on 15D, our primary outcome measure. The calculation was based on a change of 0.03 points in the 15D score [14]. In prior studies the standard deviation of 15D was 0.15 [27]. We calculated that we would need a minimum sample size of 196 study participants per study arm, for a type 1 error of 5% and a power of 80%.
We analysed our results according to the intention-to-treat principle, including all participants who completed the baseline and at least one follow-up assessment. We described the group characteristics at baseline as proportions for categorical variables and means with standard deviations for continuous variables. Statistical comparison between groups at baseline relied on the t-test, the Mann-Whitney U-test, the permutation test, or the chi-square test when appropriate. Multiple imputations were performed for some missing 15D items using the chained equations method. Mean changes in the 15D score were assessed using the mixed-model repeated measure methods with treatment, visit, and treatment–visit interaction as fixed effects; the model included age, sex, and the baseline score as covariates. We used the Cox proportional hazards regression for the estimation of age, gender, and Carlson adjusted hazard ratios. All statistical analyses were performed using Stata statistical software version 15.0 (StataCorp, College Station, Texas, USA).
3. Results
Of the 422 participants, we randomised 211 into the intervention group and 211 into the control group. The dropout rate was moderate at 11.8% of the intervention and 14.7% of the control group lost to follow-up at the one-year time point and 23.2% of the intervention and 30.3% of the control group lost to follow-up at the two-year time point. At the baseline, the intervention and control groups were similar (Table 2) [13]. The mean age of participants was 81 years and 35% were male (Table 2). The groups were similar with respect to their comorbidities, prescription medications taken, and years of education. They had no significant difference in their HRQoL according to the 15D score at the baseline.
Table 2.
Characteristics of participants at baseline.
| Intervention (n = 211) |
Control (n = 211) |
p valuef | |
|---|---|---|---|
| Age, mean (SDa) | 80.8 (4.3) | 81.3 (4.3) | 0.20 |
| Male, n (%) | 73 (35) | 75 (35) | 0.84 |
| Less than 9 years of education completed | 96 (47) | 99 (50) | 0.49 |
| Cohabiting | 110 (52) | 105 (51) | 0.70 |
| Satisfaction with life, n (%): | 0.55 | ||
| Satisfied | 187 (90) | 185 (89) | |
| Unsatisfied | 11 (6) | 12 (5) | |
| Cannot say | 9 (4) | 12 (6) | |
| HRQoLb: 15Dc score (SD) | 0.82 (0.11) | 0.82 (0.11) | 0.87 |
| BMId (SD) | 26.5 (5.0) | 26.5 (4.9) | 0.62 |
| Current or ex-smoker, n (%) | 45 (22) | 42 (20) | 0.72 |
| Fallen during last 6 months, n (%) | 68 (32) | 54 (26) | 0.14 |
| Exercise ≥30 min at least once per week, n (%) | 149 (71) | 145 (71) | 0.88 |
| Uses a walker, n (%) | 21 (10) | 35 (17) | 0.05 |
| Subjective well-being good to moderate | 176 (83) | 170 (81) | 0.45 |
| 15D usual activities 1–2 | 173 (82) | 172 (82) | 0.90 |
| Charlson comorbidity indexe (SD) | 1.29 (1.3) | 1.44 (1.5) | 0.61 |
| Prescription medications regularly taken | 4.4 (2.6) | 4.5 (2.9) | 0.77 |
SD: standard deviation.
HRQoL: health-related quality-of-life.
Sintonen 2001 [14].
BMI: body mass index.
Charlson et al. 1987 [28].
Differences between groups tested using X2 or Fisher’s exact test for categorical variables and the Mann-Whitney test or permutation test for continuous variables.
Over time, the HRQoL using the 15D score declined significantly slower in the intervention group compared to the control group (p for group 0.18, for time <0.001 and for group#time 0.043 adjusted for age and sex). At the one-year time point, the difference between changes in the 15D score between groups was −0.015 (95% CI −0.029 to −0.0016; P = 0.028 adjusted for age, sex, and baseline value). However, the favorable effect was lost once the visits ended, and at the two-year time point the difference between groups (−0.0093) was no longer significant (95% CI −0.031 to 0.013; p = 0.41 adjusted for age, sex, and baseline value; Figure 2).
Figure 2.
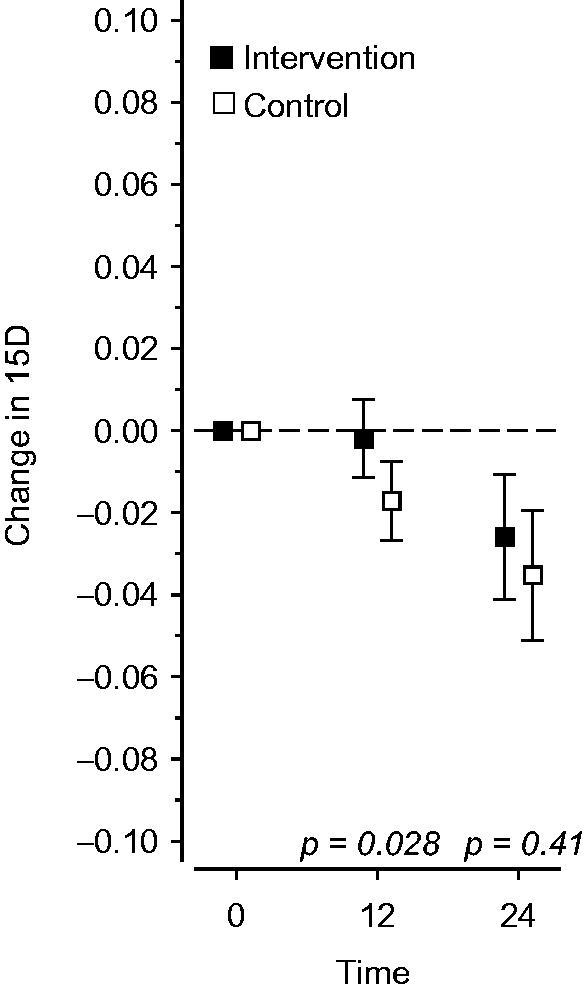
Effects of the intervention on the change in health-related quality-of-life on a 15D scale during a two-year follow-up period.
At the one-year time point, the dimensions in the 15D score showed that the favorable differences stemmed from sleeping, mental functioning, discomfort/symptoms, and vitality. However, when adjusting for age, sex, and baseline values, the differences for the individual areas of 15D did not reach significance (Figure 3).
Figure 3.

Effects of the intervention on the dimensions of a 15D scale at the one-year time point.
During the 24-month follow-up period, 13 participants died: five in the intervention group and eight in the control group. In the Cox regression analysis, the hazard ratio in the control group was 2.4 (95% CI 0.7 to 9.1, p = 0.19 adjusted for age, sex, and the Charlson comorbidity index).
4. Discussion
In this study examining the effects of three home visits delivered by a nurse, a physiotherapist, and a social worker, at the one-year time point the HRQoL had deteriorated faster in the control group than in the intervention group measured by 15D. The effect diminished at the two-year time point, however, as the visits ended. We found no significant difference in mortality between groups.
Our study consisted of a randomised controlled trial with a large sample of participants, representing a strength of this study. Our pragmatic trial applied few exclusion criteria and the willingness to participate was high among older people. Thus, our participants probably fairly well represent their background population – that is, independently home-dwelling older people. In earlier trials the independently living older people have benefitted most from preventive home visits [2]. Thus, we chose this population as a target of our intervention. This also explains the difference between our sample and the background Hyvinkää population. In addition, we had a small number of dropouts. Our primary outcome measure, 15D, is well-validated and has worked well in several earlier intervention studies [16,27,29]. HRQoL is a patient-relevant outcome measure. In addition, we used an intervention which is simple and based on CGA [30], thus designing an intervention that is feasible, affordable, and transferable to other contexts in primary care.
One limitation is that we were not able to blind the participants of the study or the deliverers of the intervention due to the nature of this study. Furthermore, the data were not blinded at the time of analysis, representing a limitation of our study. However, we performed our analysis strictly following the intention-to-treat principle. Although we had a large number of participants, the power of the study was not sufficient to detect differences in mortality. We do not have data on how the participants' elaborated their needs and wishes or how they adhered to the intervention. A qualitative study may have been able to answer these questions.
Some earlier studies showed that preventive home visits may favorably affect the quality-of-life of older adults, a finding our study confirms [6,31–34]. Although previous findings with positive effects typically had a relatively high number of home visits, some trials applying a light intervention have also shown favorable effects [31]. In older people HRQoL tends to deteriorate over time [7,9,34]. Thus, our hypothesis was that we could slow down this deterioration. Although the difference between changes in the 15D score between groups in our study was small (−0.015), it is considered to be clinically significant. Earlier research states that generic minimally important change in 15D is −0.015 for deterioration in HRQoL [35].
Most likely several reasons explain why the preventive home visit intervention was successful in our study. Those performing the home visits were uniformly trained professionals. Therefore, the intervention visits were as standardised as possible. We used validated tests during the home visits for measurement, upon which the professionals based their instructions to participants. Home visits delivered by a multiprofessional team compared to home visits delivered only by a nurse appear more effective [36]. Yet, CGA performed by a multiprofessional team reinforcing one anothers’ messages to participants might be the key to effectiveness. In addition, we involved the participants to tell about their wishes and aims and relied on their ability to follow instructions and take further steps to support their own health and well-being, which might have empowered these older adults [37]. Furthermore, all professionals delivering the home visits gave information on local social and group activities, potentially helping to support social interactions and alleviating loneliness.
However, the effects on HRQoL diminished when the intervention ended. Thus, we wonder if a further postponement to declines in HRQOL could be attained with a longer intervention. Would it be beneficial to continue these home visits? What kinds of elements should these additional visits include? Furthermore, the power of our study was too small to detect differences in mortality between the trial arms. These dimensions should be studied further.
4.1. Conclusions
To conclude, preventive home visits implemented through a multidisciplinary approach yielded favorable results in maintaining HRQoL among older adults when measured using 15D, but the effect diminished when home visits were discontinued. The intervention was simple and economical, and it may be transferred to other settings of primary care. The power of our trial was insufficient to detect differences in mortality between groups.
Funding Statement
This study was funded by a university-level health research grant from HYYKS's area of responsibility (ERVA) (HYKS:n erityisvastuualue) [four grants for the research group between 2013 and 2016] and supported by the Medical Officer Uulo Arhio Foundation [grant for corresponding author Heini Liimatta in 2016], the Finnish Medical Foundation (Suomen Lääketieteen Säätiö) [grant for corresponding author Heini Liimatta in 2016 and 2018], the Paulo Foundation [grant for corresponding author Heini Liimatta in 2018], and the Päivikki and Sakari Sohlberg Foundation [grant for corresponding author Heini Liimatta in 2018].
Acknowledgements
The authors wish to thank Janne Kasurinen, Marja-Liisa Mustonen, Kaarina Moisio, and Tarja Laitila-Mphande for their work with the study subjects and data collection, and Hannu Kautiainen for his help in the analysis of the data.
Ethics approval
Ref number 15/13/03/00/12, provided 15.8.2012 by the Ethics Committee of the Helsinki University Central Hospital.
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- 1.Metzelthin SF, van Rossum E, Hendriks MR, et al. . Reducing disability in community-dwelling frail older people: cost-effectiveness study alongside a cluster randomised controlled trial. Age Ageing. 2015;44:390–396. [DOI] [PubMed] [Google Scholar]
- 2.Stuck AE, Egger M, Hammer A, et al. . Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022–1028. [DOI] [PubMed] [Google Scholar]
- 3.Mayo-Wilson E, Grant S, Burton J, et al. . Preventive home visits for mortality, morbidity, and institutionalization in older adults: a systematic review and meta-analysis. PLoS One. 2014;9:e89257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liimatta H, Lampela P, Laitinen-Parkkonen P, et al. . Effects of preventive home visits on older people's use and costs of health care services: a systematic review. Eur Geriatri Med. 2016;7:571–580. [Google Scholar]
- 5.Sommers LS, Marton KI, Barbaccia JC, et al. . Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med. 2000;160:1825–1833. [DOI] [PubMed] [Google Scholar]
- 6.Counsell SR, Callahan CM, Clark DO, et al. . Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298:2623–2633. [DOI] [PubMed] [Google Scholar]
- 7.Kono A, Kanaya Y, Fujita T, et al. . Effects of a preventive home visit program in ambulatory frail older people: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2012;67:302–309. [DOI] [PubMed] [Google Scholar]
- 8.Brettschneider C, Luck T, Fleischer S, et al. . Cost-utility analysis of a preventive home visit program for older adults in Germany. BMC Health Serv Res. 2015;15:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairhall N, Sherrington C, Kurrle SE, et al. . Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc. 2015;16:41–48. [DOI] [PubMed] [Google Scholar]
- 10.Vass M, Avlund K, Kvist K, et al. . Structured home visits to older people. Are they only of benefit for women? A randomised controlled trial. Scand J Prim Health Care. 2004;22:106–111. [DOI] [PubMed] [Google Scholar]
- 11.Luukinen H, Lehtola S, Jokelainen J, et al. . Prevention of disability by exercise among the elderly: A population-based, randomized, controlled trial. Scand J Prim Health Care. 2006;24:199–205. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen L, Fokdal S, Gjørup T, et al. . Can municipality-based post-discharge follow-up visits including a general practitioner reduce early readmission among the fragile elderly (65+ years old)? A randomized controlled trial. Scand J Prim Health Care. 2015;33:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liimatta H, Lampela P, Laitinen-Parkkonen P, et al. . Preventive home visits to promote the health-related quality of life of home-dwelling older people: Baseline findings and feasibility of a randomized, controlled trial. Eur Geriatr Med. 2017;8:440–445. [Google Scholar]
- 14.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33:328–336. [DOI] [PubMed] [Google Scholar]
- 15.Strandberg T, Pitkälä K, Sintonen H, et al. . Usability, discriminant and prognostic validity of 15D instrument for health-related quality-of-life in older population samples In: T Huusko, T Strandberg, K Pitkala, editors. Can older people’s quality of life be measured? Helsinki: The Central Union for the Welfare of the Aged, 2006, pp 42-42-61. [Google Scholar]
- 16.Pitkala KH, Juola AL, Kautiainen H, et al. . Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc. 2014;15:892–898. [DOI] [PubMed] [Google Scholar]
- 17.Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med. 2002;18:737–757. [DOI] [PubMed] [Google Scholar]
- 18.Version information on validated Finnish translations of the instruments used [Internet]. TOIMIA database, Finland; [cited 2018. March 6]. Available at: http://www.thl.fi/toimia/tietokanta/mittari/tulokset/
- 19.Hirdes JP, Ljunggren G, Morris JN, et al. . Reliability of the interRAI suite of assessment instruments: a 12-country study of an integrated health information system. BMC Health Serv Res. 2008;8:277-6963–278-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 21.de Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry. 2003;18:63–66. [DOI] [PubMed] [Google Scholar]
- 22.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 23.FROP-Com Screen, validated Finnish version [Internet]. Finnish national institute for health and welfare; [cited 2018. March 6]. Available at: https://thl.fi/documents/567861/598779/Lyhyt_kaatumisvaaran_arviointi_FROP-Com.pdf.
- 24.Russell MA, Hill KD, Day LM, et al. . Development of the Falls Risk for Older People in the Community (FROP-Com) screening tool. Age Ageing. 2008;38:40–46. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills. 2006;103:215–222. [DOI] [PubMed] [Google Scholar]
- 26.Community Health Assessment [Internet]. InterRAI; [cited 2018. March 6]. Available at: http://www.interrai.org/community-health.html.
- 27.Pitkala KH, Laurila JV, Strandberg TE, et al. . Multicomponent geriatric intervention for elderly inpatients with delirium: effects on costs and health-related quality of life. J Gerontol A Biol Sci Med Sci. 2008;63:56–61. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 29.Pitkala KH, Routasalo P, Kautiainen H, et al. . Effects of psychosocial group rehabilitation on health, use of health care services, and mortality of older persons suffering from loneliness: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:792–800. [DOI] [PubMed] [Google Scholar]
- 30.Bernabei R, Landi F, Onder G, et al. . the birth of standardization in geriatric care. J Gerontol A Biol Sci Med Sci. 2008;63:308–313. Second and third generation assessment instruments [DOI] [PubMed] [Google Scholar]
- 31.Byles JE, Tavener M, O'Connell RL, et al. . Randomised controlled trial of health assessments for older Australian veterans and war widows. Med J Aust. 2004;181:186–190. [DOI] [PubMed] [Google Scholar]
- 32.Markle-Reid M, Weir R, Browne G, et al. . Health promotion for frail older home care clients. J Adv Nurs. 2006;54:381–395. [DOI] [PubMed] [Google Scholar]
- 33.Pathy MS, Bayer A, Harding K, et al. . Randomised trial of case finding and surveillance of elderly people at home. Lancet. 1992;340:890–893. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro A, Taylor M. Effects of a community-based early intervention program on the subjective well-being, institutionalization, and mortality of low-income elders. Gerontologist. 2002;42:334–341. [DOI] [PubMed] [Google Scholar]
- 35.Alanne S, Roine RP, Räsänen P, et al. . Estimating the minimum important change in the 15D scores. Qual Life Res. 2015;24:599–606. [DOI] [PubMed] [Google Scholar]
- 36.Ellis G, Whitehead MA, Robinson D, et al. . Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laakkonen ML, Kautiainen H, Holtta E, et al. . Effects of self-management groups for people with dementia and their spouses–randomized controlled trial. J Am Geriatr Soc. 2016;64:752–760. [DOI] [PubMed] [Google Scholar]
- 38.Collin C, Wade DT, Davies S, et al. . The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10:61–63. [DOI] [PubMed] [Google Scholar]



