Abstract
Background
Helminth parasites cause morbidity and mortality in both humans and animals. Most anthelmintic drugs used in the treatment of parasitic nematode infections act on target proteins or regulate the electrical activity of neurons and muscles. In this way, it can lead to paralysis, starvation, immune attack, and expulsion of the worm. However, current anthelmintics have some limitations that include a limited spectrum of activity across species and the threat of drug resistance, which highlights the need for new drugs for human and veterinary medicine.
Purpose
Present study has been conducted to determine the anthelmintic activity of silver nanoparticles (AgNPs) synthesized from the extract of nematophagous fungus, Duddingtonia flagrans, on the infecting larvae of Ancylostoma caninum (L3).
Methods
The nanoparticles were characterized by visual, ultraviolet, Fourier-transform infrared spectroscopy, transmission electron microscopy (TEM) analysis, and X-ray diffraction. The in vitro study was based on experiments to inhibit the motility of infective larvae (L3), and the ultrastructural analysis of the nematode was performed by images obtained by TEM.
Results
The XRD studies revealed the crystalline nature of the nanoparticles, and FTIR results implied that AgNPs were successfully synthesized and capped with compounds present in the extract. The results showed that the green synthesis of AgNPs exhibited nematicidal activity, being the only ones capable of penetrating the cuticle of the larvae, causing changes in the tegmentum, and consequently, the death of the nematode.
Conclusion
The extract of the fungus D. flagrans is able to synthesize AgNP and these have a nematicidal action.
Keywords: nanotechnology, biomedicine, nanoparticles, helminths, biological control
Introduction
More than a quarter of the world’s population is susceptible to infection caused by geohelminths through contact with contaminated soil.1 Geohelmintosis is caused by the agents like Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis, and ancylostomides. Ancylostomiasis is a much neglected tropical disease that is caused by nematodes of the genus Ancylostoma, which are pathogenic to dogs and generate a burden of four million US dollars in treatment costs. In humans, Ancylostoma spp. is responsible for skin inflammation or Larva Migrans Cutaneous (LMC), an infection that is seen mainly in “travelers” who return to beaches in tropical areas and who are contaminated by the larvae of this nematode.2
While it is observed that helminth parasites cause morbidity and mortality in both humans and animals, we are also seeing advances in technologies for the treatment and prevention of these agents, particularly nanotechnology. Nanotechnology is a field of science that is constantly developing, as its applications both in medical sciences and biological sciences are broad: medical diagnosis, biosensors, health care, drug delivery, and water purification.3 Within the field of nanotechnology, silver nanoparticles (AgNPs) have been widely studied due to their exclusive physical, chemical, and optical properties.4
Several studies have investigated a wide variety of chemical, physical, and biological synthesis methods with the objective of establishing a technique that makes morphological regulation of the AgNPs possible. Physical and chemical methods are the most used for the synthesis of the NPs; however, these methods require the use of toxic compounds, minimizing their applications.5 In contrast, the biological method for the synthesis of AgNPs, called “green synthesis”, is the most advantageous given that it is cost effective, ecologically correct, reproducible, and energy efficient.6
In this context, the use of fungi in the green synthesis of NPs has been highlighted, as the process to synthesize and culture them is easier, and large scale synthesis is possible using a small quantity of biomass, making it economically feasible.7,8 Additionally, filamentous fungi produce a large quantity of extracellular enzymes and organic compounds needed for NP green synthesis.9,10
Recently, Costa Silva et al11 published a new approach to the biological synthesis of AgNPs in which the NP in question was synthesized from the fungus Duddingtonia flagrans (AC001). D. flagrans is a filamentous nematophagous fungus that has excellent predatory capacity, and is considered the most promising species for the biological control of gastrointestinal parasites.12–14 It produces conidia and chlamydospores that ensure the propagation and survival of the organism under environmental and laboratory conditions.14 In addition, it is possible to observe the production of extracellular enzymes, such as proteases and chitinases, in the crude extract of D. flagrans, which besides being necessary for NP production, as mentioned earlier, are able to hydrolyze the cuticle of the nematode, resulting in the death of the parasite.12,15 Also, these authors suggest that in the future this fungal NP could be used in the control of infective helminth larvae.
Thus, in this work, we investigated the nematicidal action of silver particles synthesized from the fungus D. flagrans on the L3 stage of Ancylostoma spp. It is also worth mentioning that this study will open a new door based on pharmacology for the treatment of geohelmintosis.
It should also be emphasized that this is the first report that proves the antihelmintic action of AgNPs synthesized from the extract of the nematophagous fungus D. flagrans.
Methods
The green synthesis of AgNPs
Obtaining conidia and chlamydospores
An isolate of the nematophagous fungus D. flagrans (AC001) was used to produce a solution containing the fungal structures. This isolate comes from Brazilian soil in the locality of Viçosa, Minas Gerais. The fungus was maintained in 9 cm Petri dishes containing culture medium based on 2% water agar (2% WA) at 4°C and in the dark.
Obtaining the fungal filtrate
To prepare the biomass for the biosynthesis studies, fungal mycelia were obtained by transferring disks (~5 mm in diameter) of the fungal isolate cultures maintained in 2% WA to Erlenmeyer flasks (250 mL) containing 200 mL of nutrient-poor liquid medium (low nutrient medium), composed of bacteriological peptone 2 g/L, yeast extract 3 g/L, potassium phosphate dibasic 0.1 g/L, magnesium sulfate hexahydrate 0.05 g/L, and lactic acid 100 µL, pH=9. The flasks were then incubated in an orbital agitator (120 rpm) at 25°C for 10 days.
The biomass formed was removed after 10 days of growth using a previously autoclaved stainless steel strainer and then rinsed extensively with ultrapure water to remove any component of the medium from the biomass. Then 100 mL of ultrapure water, 10 g (wet weight) of the biomass, and 0.1 g of tick shells, a natural source of chitin, were added to each flask.
The flasks were incubated again in an orbital agitator under the same previously mentioned conditions for 15 days. After incubation, the cellular filtrate was obtained using 0.22 µm membrane filters.
Analysis of the fungal filtrate for protein content
The fungal filtrate was analyzed for total protein content using the Bradford method. Bovine serum albumin (BSA [98%]; Sigma-Aldrich Co.) was used for the standard curve and the readings were taken in an ultraviolet-visual (UV-vis) absorption spectrophotometer (Spectramax 190 Spectrophotometer) at 595 nm.
The green synthesis of the AgNPs from the fungal filtrate
One hundred milliliters of 1 mM AgNO3 solution was added to the fungal filtrate in a concentration of 1:50, and the new solution was kept in an agitator at 120 rpm, 60°C, in the dark. The formation of AgNPs was first seen primarily by the change in the color of the solution and was confirmed by optical absorption (UV-vis).
Characterization of the AgNPs
Ultraviolet-visual absorption spectography
Confirmation of the formation of the AgNPs after the change in the color of the solution and determination of the localized surface plasmon resonance (LSPR) of the AgNPs were accomplished using a UV-vis spectrophotometer (Spectramax 190 Spectrophotometer) at a resolution of 1 nm and sweep of 200–600 nm.
Transmission electron microscopy (TEM)
The morphology of the NPs was confirmed by TEM. The AgNPs were placed on a copper grid coated with Formvar (Ted Pella Inc., Tustin, CA, USA) and examined by TEM using a JOEL microscope (JEOL, Inc., Peabody, MA, USA), model JEM1400 operating at 120 kV with a lanthanum hexaboride (LAB6) filament.
Fourier-transform infrared (FTIR) spectroscopy
After the green synthesis of the AgNPs, the samples were centrifuged at 15,000 rpm for 30 minutes and the pellets formed were used for FTIR analysis. The FTIR was conducted in the ATR mode (FT-MIR FTLA 2000 Bomem) to investigate which organic components could be associated with the AgNPs.
X-ray diffraction (XRD)
The crystalline structures of the synthesized AgNPs were determined by XRD studies. The AgNP samples were prepared by reverse coating the pelletized AgNPs on a glass slide and sweeping in region 2θ of 30°–80° at 0.01° per minute with a time constant of 2 seconds, using a DB Advance (CuKα) (Bruker-AXS, Billerica, MA, USA) X-ray diffractometer.
Experimental assay
Cytotoxicity assay
An analysis was performed of the cytotoxic action of the AgNPs synthesized from the fungus D. flagrans, of the AgNPs chemically synthesized from reduction by sodium borohydride, of the fungal filtrate, and of the chemotherapeutic agent doxorubicin, and the chemotherapeutic agent doxorubicin on a line of non-cancerous L929 fibroblast cells (L929 cell line, ATCC®-CCL1™; Cell Line Service, Rio de Janeiro, Brazil) was analyzed by the MTT colorimetric assay according to Mosmann.16
A suspension of 7 × 104 cells/mL was used for the MTT experiments. The cells were cultured in 96-well plates, and after 24 hours were exposed to different concentrations of the samples diluted in the culture medium itself. Then the plates were incubated for exactly 24 hours at 37°C in an atmosphere of 5% CO2. Doxorubicin was used as positive control. After incubation, the content of each well was removed and 100 µL of MTT (5 mg/mL) reagent was added, followed by incubation for 2 hours for mitochondrial metabolism of the MTT by the live cells. Subsequently, the excess MTT was removed and the formazan crystals were dissolved with 100 µL of dimethyl sulfoxide. The absorbance (595 nm) of the plates was read by a microplate reader (SpectraMax 190 Microplate Reader; Molecular Devices, Palo Alto, CA, USA).
Cellular viability was determined by comparing the absorbance values obtained for the treated cells with those obtained for the non-treated cells, and the result was expressed as IC50 in µg/mL (concentration necessary to cause the death of 50% of the cells) with the aid of linear regression.
Obtaining the L3 of Ancylostoma caninum
Infective larvae (L3) of A. caninum were obtained from the fresh feces of naturally infected dogs. For the processing of the feces, the Willis test was performed to find positive samples. Subsequently, coprocultures were prepared with ~20 g of feces and kept in an incubation chamber for 10 days at 26°C. After this period, the larvae were extracted using the Baermann technique17 and identified as A. caninum, according to the criteria described by Bevilaqua et al.18
Preparation of the solution of D. flagrans conidia and chlamydospores
The 9 cm Petri dishes containing 2% WA medium in which the fungus was grown for over 10 days were bathed with 10 mL of distilled water. Using a glass slide, the surface of the agar was scraped to remove the fungal structures (conidia and chlamydospores) without removing the culture medium from the dish. Then, the solutions were poured into a sterile beaker and homogenized. Finally, aliquots of 10 µL of the solutions were made for the counting of conidia and chlamydospores, in a Neubauer chamber.
Analysis of the nematicidal action of the biosynthesized AgNPs on L3 of A. caninum
Initially, 50 µL of the solution of L3s of A. caninum (13 L3/µL) was added to each of the 27 microtubes. Then these microtubes were divided into nine groups:
Group I (negative control): solution of L3 larvae +1 mL of ultrapure water;
Group II (positive control): solution of L3 larvae +1 mL of levamisole 22.3%;
Group III (positive control): solution of L3 larvae +1 mL of ivermectin 1%;
Group IV: solution of L3 larvae +1 mL of AgNO3 solution (1 mM);
Group V: solution of L3 larvae +1 mL of D. flagrans conidia and chlamydospores solution at a concentration of 126 conidia/10 µL;
Group VI: solution of L3 larvae +1 mL of AgNPs chemically synthesized from reduction by sodium borohydride;
Group VII: solution of L3 larvae +1 mL of biosynthesized AgNP solution at a concentration of 43.40 µg/mL;
Group VIII: solution of L3 larvae +1 mL of biosynthesized AgNP solution at a concentration of 21.70 µg/mL;
Group IX: solution of L3 larvae +1 mL of biosynthesized AgNP solution at a concentration of 10.85 µg/mL.
The microtubes were placed in a benchtop agitator, where they remained in the dark for 24 hours at 28+C. After this period, the samples were placed in 12-well microplates, under a heating plate at 37+C for 2 minutes. One aliquot of 280 µL was removed from each group for counting of the live larvae using an optical microscope (10× objective). The tests were performed in triplicate.
Ultrastructural analysis of the A. caninum larvae
The ultrastructural analysis of the L3s of A. caninum was performed by TEM. The treated larvae and controls were washed in PBS and fixed in 0.1 M of cacodylate buffer for 24 hours. Then, they were washed again in PBS and post-fixed with a solution of 1% osmium tetroxide, OsO4, in 0.1 M cacodylate buffer with the addition of potassium ferrocyanide 1.25%. The specimens were then washed with 0.1 mol/L of cacodylate buffer and ultrapure water. The samples were then dehydrated with increasing degrees of ethanol concentration (30%, 50%, 70%, 90%, and 100%) and dried by critical point method using liquid CO2 as the transitional fluid and air dried at 25°C for 15 minutes. The dry material was placed on a metal point according to the required orientation, and sprayed with gold in a fine coat ion sputter (JFC-1100; JEOL). The gold-coated specimens were observed using a Philips SEM (model no LEO 435 VP 501B) with electron acceleration varying between 10 and 20 kV.
Results and discussion
Based on the results obtained using the Bradford method, the concentration of total proteins present in the fungal filtrate was 0.80 mg/mL. In order to increase the protein concentration of the filtrate, the medium was enriched with tick shells, a natural source of chitin, which stimulates the production of chitinase by the fungus, following the method used by Costa Silva et al.11
The formation of NPs could initially be observed by the change in color of the fungal filtrate solution, from yellow to yellowish brown 24 hours after the addition of the AgNO3 solution. Basavaraja et al10 stated that AgNPs have strong absorption of visible electromagnetic waves due to LSPR. This phenomenon is due to the collective oscillations of the NP conductor electrons on irradiation by visible light. Therefore, the formation of NPs was established by the UV-vis spectroscope. The AgNPs have a characteristic peak between 380 and 450 nm. In Figure 1, the peak of the sample can be observed at 408 nm.
Figure 1.
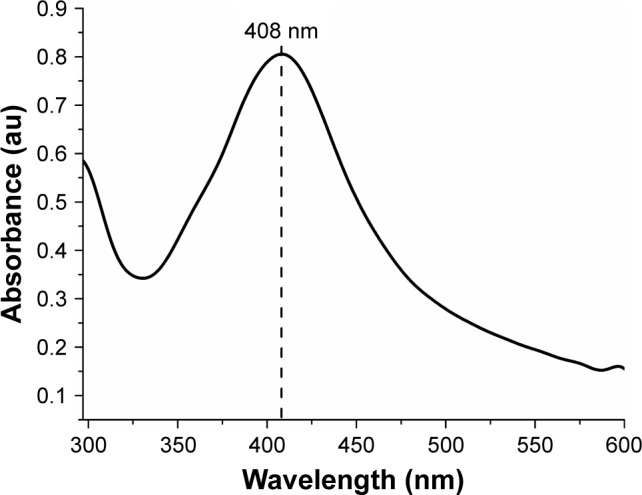
UV-vis spectrum of the biosynthesized AgNPs using the free extract of cells of the fungus Duddingtonia flagrans.
Abbreviations: AgNPs, silver nanoparticles; UV-vis, ultraviolet-visual.
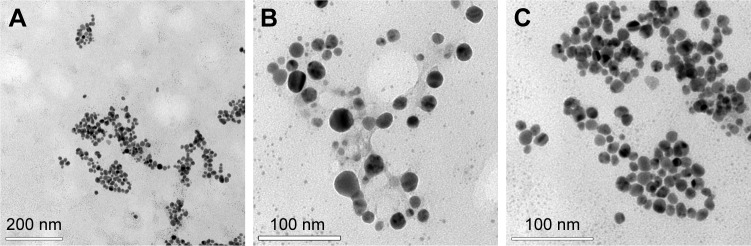
It was possible to observe the monodispersion of the AgNPs in the images obtained by the TEM (Figure 2A–C). With the assistance of the ImageJ 1.51i program, these images were also analyzed to determine the size and spherical form of the AgNPs by calculating the average diameter and the aspect ratio of the AgNPs (n=500), which were 14.51±3.25 nm and 1.18±0.20, respectively.
Figure 2.
Images obtained by TEM showing the shape and size of the AgNPs biosynthesized from the free extract of cells of the fungus Duddingtonia flagrans. Amplification: (A) 1,00,000×, (B) 2,00,000×, and (C) 3,00,000×.
Abbreviations: AgNPs, silver nanoparticles; TEM, transmission electron microscopy.
To characterize the molecules that can be absorbed on the surface of the biosynthesized NPs, the FTIR spectroscopy was used.19 When Costa Silva et al11 analyzed the samples of the synthesized AgNPs from the fungus D. flagrans in the FTIR, they reported two specific bands of proteins at 1,640 and 1,540 cm−1 identified as amide I and amide II, respectively, in addition to the band at 2,665 cm−1, which is characteristic of the axial O−H deformation of carboxylic acid, and stretch bands at 730 cm−1 (CH2−S−S, possibly related to the cross-linking of disulfide between the amino acid cysteine), 1,000 cm−1 (C−O), 1,811 cm−1 (anhydrous C=O), 2,100 cm−1 (C≡O), 2,326 cm−1, and at 2,665, 3,600, and 3,751 cm−1 (O−H).
The bands identified as amide I and amide II occur because of the vibrations of axial C=O deformation and angular N−H deformation in the amide links of the proteins.20,21
The FTIR spectroscopy of the AgNPs synthesized by green synthesis in this study (Figure 3) and the filtrate used for the synthesis, similarly to the study by Costa Silva et al,11 showed bands at 1,640 and 1,540 cm−1 related to amide I and amide II groups, respectively; band at 996 cm−1 for the stretching of typical C−O−C bonds of acyclic saturated aliphatic anhydrides;22 band at 1,344 cm−1 related to asymmetric axial deformations of the group SO2;23 and weak band at 1,998 cm−1 associated to the group.22
Figure 3.
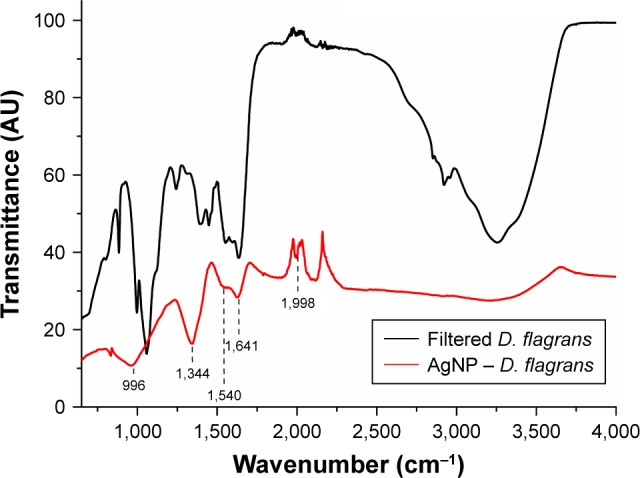
FTIR spectrum of the free extract of cells of the fungus Duddingtonia flagrans (black) and of the AgNP (D. flagrans) (red).
Abbreviations: AgNPs, silver nanoparticles; FTIR, Fourier-transform infrared.
The pattern obtained from the XRD of the synthesized AgNPs by green synthesis showed four intense peaks in spectrum of 2θ from 30° to 80°. The XRD ratified the reduction of Ag+ (protonated silver) to Ag+ (reduced silver) carried out by the fungal filtrate of D. flagrans.
The data obtained were then combined with the database of the Joint Committee on Powder Diffraction Standards (No 04-0783), and it was observed that the biosynthesized AgNPs exhibited peaks of silver at 2θ=39°, 43.45°, 64.5°, and 77.9°, relating to the facets (111), (200), (220), and (311) of the silver, respectively (Figure 4). According to Rathod et al,24 these values agree with the values reported for face-center-cubic silver nanocrystals.
Figure 4.
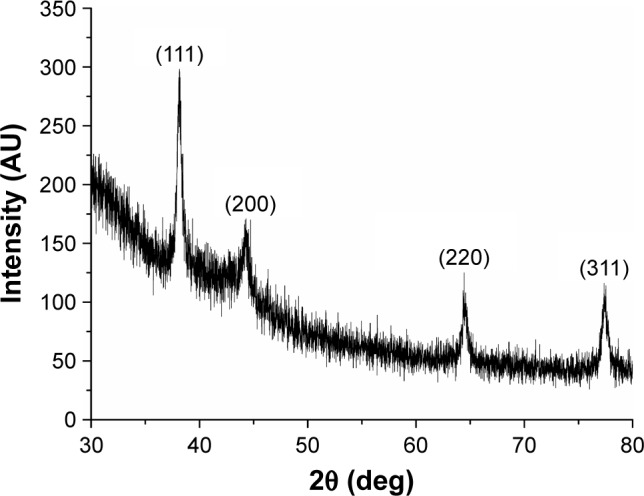
Crystalline structure analysis by XRD of the AgNPs (Duddingtonia flagrans).
Abbreviations: AgNPs, silver nanoparticles; XRD, X-ray diffraction.
From the patterns of diffraction obtained by the XRD, it was also possible to confirm the size of the crystallites using the Scherrer equation,26
where DNP is the average particle size (nm), k is the shape factor (0.9), λ is the wavelength of the X-ray (λ=0.15418 nm for CuKα radiation), θ is the Bragg angle of diffraction (°), and γ is the widening of the peak of diffraction measured at its maximum intensity (in radians). Thus, the average size of the AgNPs calculated from the XRD results was 17.33 nm. It should be emphasized that this value is close to that found in the analyses of the images obtained by the TEM (Figure 2A–C).
In the cytotoxicity assay of the AgNPs in noncancerous cells (L929 fibroblasts), the MTT revealed the following IC50 values (µg/mL): doxorubicin 64.2±5.2, fungal filtrate 444.1±12.3, AgNPs (chemical synthesis) 228.2±6.7, and AgNPs (D. flagrans) 43.4±6.8 (Figure 5). This result indicated the toxic dose for L929 fibroblasts.25 From the MTT results, we determine the maximum concentration of AgNPs (D. flagrans) to be used in the tests.
Figure 5.
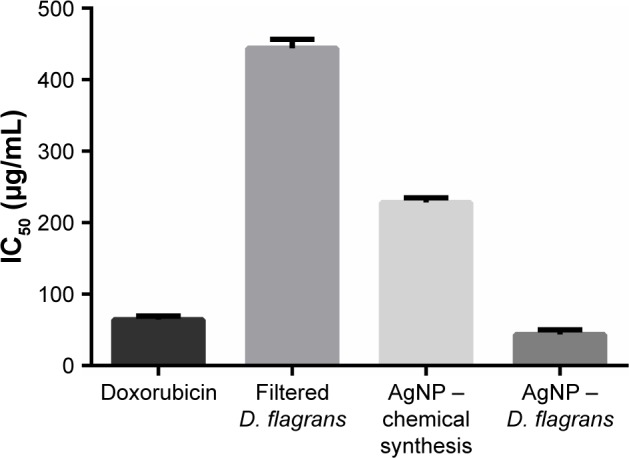
Cell survival was evaluated by the MTT assay. The results represent the averages of at least three independent experiments. Cellular viability was observed after treatment with MTT in the L929 fibroblast cell line.
Abbreviations: AgNPs, silver nanoparticles; D. Flagrans, Duddingtonia flagrans.
The tests to determine the nematicidal action of the biosynthesized AgNPs in A. caninum larvae revealed that the AgNPs, at all concentrations tested (10.85 µg/mL, 21.70 µg/mL, and 43.4 µg/mL), demonstrated nematicidal effect (P<0.01) compared to levamisole and the conidia and chlamydospore solution, but with no difference (P>0.01) in relation to ivermectin.
Based on the data obtained, we observed that the AgNPs (chemical synthesis) did not cause the death of 100% of the larvae as observed for the AgNP (D. flagrans). Therefore, the cause of larval death was not due to the reduced silver alone, since both NP solutions contained silver. The efficacy of the AgNP (D. flagrans) was probably due to the absorption of D. flagrans proteins on the surface of the AgNPs, facilitating the penetration of the NP into the nematode.11 Costa Silva et al11 suggest that the protein present on the surface of the AgNP synthesized from the fungal filtrate of D. flagrans is chitinase.
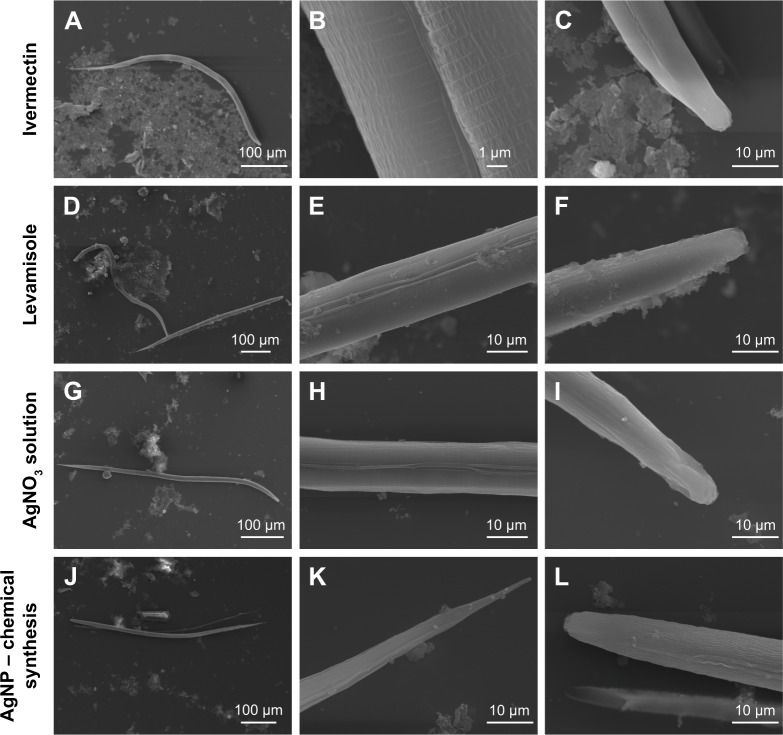
Analyses of the effects of AgNPs on the nematode cuticle were conducted using the images obtained by the TEM. In Figure 6A–F, we can observe that when the larvae are exposed to the antihelmintics ivermectin and levamisole, there are no visual changes in the cuticle, since these medications act on the GABAergic receptors and cause spastic paralysis of the nematodes. Therefore, neither of the drugs acts on the cuticle of the parasite.27,28
Figure 6.
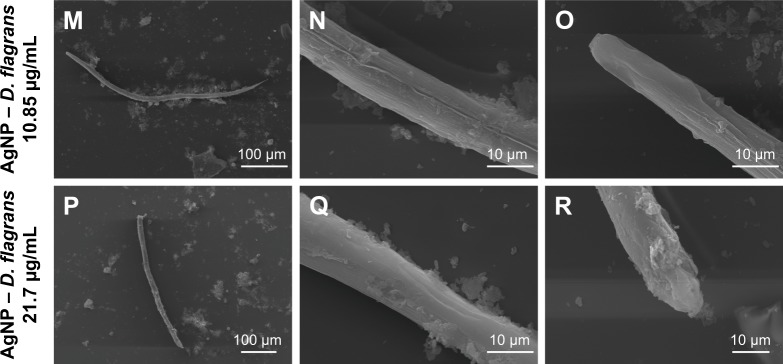
Images obtained by TEM of the larvae of Ancylostoma spp., treated with ivermectin (A–C), levamisole (D–F), AgNO3 solution (G–I), AgNP (chemical synthesis) (J–L), AgNP (Duddingtonia flagrans) 10.85 µg/mL (M–O), and AgNP (D. flagrans) 21.7 µg/mL (P–R). Amplification: 160× for D; 190× for J, P; 230× for G; 250× for A, M; 2,000× for R; 2,200× for E, H; 2,500× for C, F, I, K, L, N, O, Q; and 11,000× for B.
Abbreviations: AgNPs, silver nanoparticles; TEM, transmission electronic microscopy.
Analyzing the larvae treated with the AgNO3 solution and AgNP (chemical synthesis) (Figure 6G–L), no morphological changes were observed in the cuticle of the nematodes. However, the larvae treated with AgNP (D. flagrans) presented extensive morphological changes in the cuticle (Figure 6M–R). These results corroborate the hypothesis of the efficiency of the AgNP (D. flagrans) solution due to the presence of chitinases produced by D. flagrans absorbed on their surface, which facilitates the penetration of the NP into the nematode, leading to the death of the parasite. Surface differences of the larvae in response to the different treatments tested can be seen in Figure 6.
The AgNPs (D. flagrans) were highly effective in their nematicidal action, producing more satisfactory results than the chemical synthesis, the D. flagrans solution, and levamisole, besides being the only substances to trigger integumentary changes in the A. caninum larvae. Because of their effectiveness, and because they are new substances, we can say that they have a promising future in the antihelmintic treatment of animals and humans.
Conclusion
To the best of our knowledge, this is the first report of myconanotechnology originating from the fungus D. flagrans (AC001) as a nematicidal “tool”. It should be emphasized that the resistance of A. caninum to conventional drugs is already well known,29 and results like those presented in this study may help make control more effective.
Finally, the authors of this paper report that AgNP (D. flagrans) showed nematicidal effectiveness, being the only treatment capable of penetrating the cuticles of the larvae and causing their subsequent death. New experimental designs are already being constructed that will enable a better understanding of the nematicidal myconanoactivity of the fungus D. flagrans and a better focus on control of infective larvae of the potentially zoonotic nematodes that are ravaging the poorest parts of the world.
Acknowledgments
The authors extend their thanks to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the Fabio Ribeiro Braga’s Productivity Grant, the FAPES (Fundação de Amparo à Pesquisa e Inovação do Espírito Santo) for the scholarship grant, LabPetro and Luccar Ufes (Laboratório de Ultraestrutura Celular Carlos Alberto Redins), Fapemig (Fundação de Amparo à Pesquisa de Minas Gerais), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (Finance Code 001). Anna Claudia Mombrini Silva Barbosa and Laryssa Pinheiro Costa Silva are equal first authors.
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391(10117):252–265. doi: 10.1016/S0140-6736(18)30302-7. [DOI] [PubMed] [Google Scholar]
- 2.Figueiredo SDP, Taddei JAAC, Menezes JJC, et al. Clinical-epidemiological study of toxocariasis in a pediatric population. J Pediatr (Rio J) 2005;81(2):126–132. doi: 10.2223/JPED.1414. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Peng X, Daley J, et al. Inhibition of DNA2 nuclease as a therapeutic strategy targeting replication stress in cancer cells. Oncogenesis. 2017;6(4):e319. doi: 10.1038/oncsis.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Shen W, Xue J, et al. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res Lett. 2018;13(1):54. doi: 10.1186/s11671-018-2450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashid MMO, Ferdous J, Banik S, Islam MR, Uddin AHMM, Robel FN. Anthelmintic activity of silver-extract nanoparticles synthesized from the combination of silver nanoparticles and M. charantia fruit extract. BMC Complement Altern Med. 2016;16(1):242. doi: 10.1186/s12906-016-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ovais M, Khalil AT, Islam NU, et al. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechnol. 2018;102(16):6799–6814. doi: 10.1007/s00253-018-9146-7. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury S, Basu A, Kundu S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multi-drug-resistant bacteria. Nanoscale Res Lett. 2014;9(1):365. doi: 10.1186/1556-276X-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghareib M, Tahon MA, Saif MM, El-Sayed Abdallah W. Rapid extracellular biosynthesis of silver nanoparticles by Cunninghamella phaeospora culture supernatant. Iran J Pharm Res. 2016;15(4):915–924. [PMC free article] [PubMed] [Google Scholar]
- 9.Moazeni M, Rashidi N, Shahverdi AR, Noorbakhsh F, Rezaie S. Extracellular production of silver nanoparticles by using three common species of dermatophytes: Trichophyton rubrum, Trichophyton mentagrophytes and Microsporum canis. Iran Biomed J. 2012;16(1):52–58. doi: 10.6091/IBJ.1001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basavaraja S, Balaji SD, Lagashetty A, Rajasab AH, Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull. 2008;43(5):1164–1170. doi: 10.1016/j.materresbull.2007.06.020. [DOI] [Google Scholar]
- 11.Costa Silva LP, Pinto Oliveira J, Keijok WJ, et al. Extracellular bio-synthesis of silver nanoparticles using the cell-free filtrate of nematophagous fungus Duddingtonia flagrans. Int J Nanomedicine. 2017;12:6373–6381. doi: 10.2147/IJN.S137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braga FR, Araujo JM, Araújo JV, et al. In vitro predatory activity of conidia of fungal isolates of the Duddingtonia flagrans on Angiostrongylus vasorum first-stage larvae. Rev Soc Bras Med Trop. 2013;46(1):108–110. doi: 10.1590/0037-86829612013. [DOI] [PubMed] [Google Scholar]
- 13.Buzatti A, Santos CP, Fernandes MAM, et al. Duddingtonia flagrans in the control of gastrointestinal nematodes of horses. Exp Parasitol. 2015;159:1–4. doi: 10.1016/j.exppara.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Hiura E, Lopes ACG, Da Paz JS, et al. Fungi predatory activity on embryonated Toxocara canis eggs inoculated in domestic chickens (Gallus gallus domesticus) and destruction of second stage larvae. Parasitol Res. 2015;114(9):3301–3308. doi: 10.1007/s00436-015-4459-2. [DOI] [PubMed] [Google Scholar]
- 15.Braga FR, Soares FEF, Giuberti TZ, et al. Nematocidal activity of extracellular enzymes produced by the nematophagous fungus Duddingtonia flagrans on cyathostomin infective larvae. Vet Parasitol. 2015;212(3–4):214–218. doi: 10.1016/j.vetpar.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Staniland LN. A modification of the Baermann funnel technique for the collection of nematodes from plant material. J Helminthol. 1954;28(1–2):115. doi: 10.1017/S0022149X00032739. [DOI] [PubMed] [Google Scholar]
- 18.Bevilaqua C, Rodrigues M, Concordet D. Identification of infective larvae of some common nematode strongylids of horses [Strongylus vulgaris, S. equinus, S. edentatus, Triodontophorus spp., Poteriostomum spp., Gyalocephalus capitatus, Cylicocyclus radiatus, C. nassatus, C. minutus, C. poculatu] Rev Med Vet. 1993;144(12):985–989. [Google Scholar]
- 19.Ţucureanu V, Matei A, Avram AM. FTIR spectroscopy for carbon family study. Crit Rev Anal Chem. 2016;46(6):502–520. doi: 10.1080/10408347.2016.1157013. [DOI] [PubMed] [Google Scholar]
- 20.Stuart B. Infrared Spectroscopy: Fundamentals and Applications. Austrália: John Wiley & Sons, Ltd; 2004. [Google Scholar]
- 21.Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne JA. Comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol. 2007;98(16):3000–3011. doi: 10.1016/j.biortech.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa LCA. Espectroscopia no infravermelho: na caracterização de compostos orgânicos. Viçosa, MG: Ed. UFV; 2007. [Google Scholar]
- 23.Silverstein RM, Webster FX, Kiemle DJ. Identificação espectrométrica de compostos orgânicos. Vol. 7. Rio de Janeiro: LTC; 2006. [Google Scholar]
- 24.Rathod V, Banu A, Ranganath E. Biosynthesis of highly stabilized silver nanoparticles by Rhizopus stolonifer and their anti-fungal efficacy. Int J Curr Biomed Pharm Res. 2012;2(1):241–245. [Google Scholar]
- 25.Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari MR. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomedicine. 2018;13:8013–8024. doi: 10.2147/IJN.S189295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojnarowicz J, Opalinska A, Chudoba T, et al. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. J Nanomater. 2016;2016:1–15. doi: 10.1155/2016/2789871. [DOI] [Google Scholar]
- 27.Walker RJ, Colquhoun L, Holden-Dye L. Pharmacological profiles of the GABA and acetylcholine receptors from the nematode, Ascaris suum. Acta Biol Hung. 1992;43(1–4):59–68. [PubMed] [Google Scholar]
- 28.Köhler P. The biochemical basis of anthelmintic action and resistance. Int J Parasitol. 2001;31(4):336–345. doi: 10.1016/s0020-7519(01)00131-x. [DOI] [PubMed] [Google Scholar]
- 29.Kopp SR, Coleman GT, McCarthy JS, Kotze AC. Application of in vitro anthelmintic sensitivity assays to canine parasitology: detecting resistance to pyrantel in Ancylostoma caninum. Vet Parasitol. 2008;152(3–4):284–293. doi: 10.1016/j.vetpar.2007.12.014. [DOI] [PubMed] [Google Scholar]