Abstract
Clinical and consumer health informatics interventions promise to transform health care, yielding higher quality, more accessible care at a lower cost. However, the potential of these interventions cannot be achieved if they are developed and rolled out in a disconnected way: clinic-based systems typically do not interface with home-based systems that capture patient-generated health-related data. The fragmentation between these interventions severely limits the benefits of all interventions; given that health care is a continuum between clinical and daily-living settings. We introduce the Infinicare framework, which posits that clinical health-related activities “shape” daily-living-based health-related activities and, conversely, that daily-living-based health-related activities “inform” activities in clinics. Non-alignment of activities across these diverse contexts yields systemic gaps. Workflow studies that capture health-related activities and characterise gaps between clinical and daily-living contexts can inform the design and implementation of gap-filling, collaborative health information technologies. To inform these technologies, workflow studies should be patient-oriented, include both clinical and daily-living settings and subsume both process and structure variables. Novel methodologies are needed to effectively and efficiently capture health-related activities across both clinical and daily-living settings and their contexts. Guidelines for applying these recommendations in developing collaborative health information technologies are provided.
Keywords: Workflow, daily-living, patient-oriented, context, health-related activities
1. The current state of health care and health information technologies
Health care delivery practices have changed in response to fragmented care delivery services (Chassin & Galvin, 1998; Medicine IOMIo, 2001), increased pressure for earlier discharge, transformation of daily-living contexts (e.g., home, community) into primary settings for health (Aliotta & Andre, 1997; Wagner et al., 2001), increased clinical and home use of health information technologies (HIT) (Brennan, Downs, & Casper, 2010; National Center for Health Statistics, 2009; Koch, 2006), and increased patient-engagement expectations (Carman et al., 2013; Gruman et al., 2010). Consequently, health-related activities are not bounded within hospitals or clinics. The fragmented nature of HIT and non-integration of clinical and consumer systems cannot adequately support care delivery in light of these changes. Clinic-oriented systems are typically closed to patient-generated data. Patient access to health information (and patient ability to get information captured using home technologies into the hands of clinicians) is limited. Tethered personal health records (PHRs), which seek to bridge clinical and daily-living settings, show patients only subsets of their health information, rarely allow patients to input data (Cahill, Gilbert, & Armstrong, 2014; Marquard et al., 2013), and limit access to patients and one other designee (Sarkar & Bates, 2014). But even beyond all of these technological issues is a human-use issue: HIT has not been designed in a way that maximises either use or potential benefit (Ancker et al., 2014; Kaziunas & Ackerman, 2015; Simon et al., 2009).
From a patient perspective – especially a patient with a chronic condition – health management is a continuous effort across diverse clinical and “real world” loci (Miller et al., 2009; Naithani, Gulliford, & Morgan, 2006). Any inconsistency or disconnect between these contexts can generate suboptimal patient outcomes (Rogers, Kennedy, Nelson, & Robinson, 2005). For example, if therapy plans developed in clinical settings do not accommodate unique daily-living situations in which health-related activities (e.g., acquiring and taking medication) occur, the probability of non-adherence increases (Beach, Keruly, & Moore, 2006). Therefore, HIT designers and researchers need frameworks, theories, methods, and guidelines that enable holistic, cross-setting understanding of patient care to inform collaborative HIT solutions (Valdez, Holden, Novak, & Veinot, 2015).
Development of HIT has traditionally focused either solely on clinical settings (e.g., electronic health records [EHRs], computerised provider order entry systems, scheduling systems) or solely on consumer use (e.g., home glucose devices, patient portals). On the one hand, the design of clinical information systems aims to effectively use clinical information such as lab results, radiological/other tests, and previous diagnoses by health care professionals. On the other hand, consumer health information systems are designed to provide information to patients for self-management at home. Therefore, existing HIT generally fits exclusively into a clinical-solution bucket or a consumer-solution bucket. The disconnected development of clinical and consumer HIT, or lack of platforms that facilitate data exchange across clinical and consumer HIT, prevents the unleashing of full HIT potential. Collaborative HIT solutions (Valdez et al., 2015) that integrate clinical and consumer informatics are needed to bridge clinical and daily-living settings and ensure access to needed data by both clinicians and patients, regardless of where the data are generated.
In this article, we propose a design-oriented framework called Infinicare to guide the broad design goals of collaborative HIT solutions. The Infinicare framework promotes the integration of health-related activities across care delivery settings (i.e., locations). Infinicare can be operationalised using the patient-oriented workflow approach through the incorporation of contexts (i.e., physical, social, organisational, and cultural dimensions of locations). In so doing, Infinicare both uniquely recognises the interrelationships in health care provision and uniquely isolates health care provision gaps; therefore, requirements of HIT can be elicited, analysed, specified, validated, and managed in a complete, consistent, and relevant manner (Sommerville & Sawyer, 1997). These requirements can then be translated into design specifications.
2. Interrelationships and gaps in the current health care delivery paradigm
2.1. Interrelationships among health-related activities across diverse settings
Clinic-based diagnostic and treatment activities (e.g., consultations, laboratory orders, emergency visits, or providers’ clinical decisions) are expected to shape patients’ health-related activities in environments very different from the clinic (i.e., homes and communities). Consequently, providers are increasingly expected to create therapy plans and make clinical decisions jointly with patients (Barry & Edgman-Levitan, 2012; Légaré et al., 2011), commonly known as “shared decision-making”. These shared therapy plans should reflect the patient’s prior clinical history, capabilities, and daily-living-based situations.
Health-related activities in clinical settings shape those in daily-living settings in two ways: clinical setting-based activities determine (a) what health-related activities patients perform in daily-living settings (e.g., what medications are prescribed) and (b) how patients perform health-related activities in daily life (e.g., patient adherence to prescriptions). For example, while a patient from a remote rural setting may be prescribed a clinically appropriate drug on hospital discharge, if the patient’s local pharmacy does not carry the drug, treatment may be delayed or not adopted.
Health-related activities in daily-living settings also inform clinic-based activities. This dynamic manifests in two ways: (a) consideration of home/community-based facilitators and barriers allows clinicians to tailor feasible, effective treatment plans and (b) daily-living-based health outcomes provide feedback that providers can incorporate into subsequent treatment plans. For example, dietary plans for patients should factor in cultural and social proclivities (Batalden et al., 2015; Denford, Frost, Dieppe, Cooper, & Britten, 2014; Evert et al., 2013). Moreover, understanding of other contextual factors (e.g., supportive or negative influences) or attitudes towards technology can help craft appropriate treatment regimens (Flynn et al., 2013; O’Leary, Vizer, Eschler, Ralston, & Pratt, 2015). Because successful chronic disease self-management and other self-care tasks depend on contexts in which the individual is embedded (Sallis, Owen, & Fisher, 2008), treatment plans should be tailored accordingly; failing to provide tailoring may be considered a “contextual error” (Weiner et al., 2010).
Lack of understanding of these symbiotic mechanisms (i.e., clinical shaping non-clinical and non-clinical informing clinical) may lead to poor patient outcomes such as medication non-adherence and appointment no-shows. Previous studies show that, among patients with HIV, levels of family and social support were related to levels of medication adherence and appointment attendance (Godin, Côté, Naccache, Lambert, & Trottier, 2005; van Servellen, Chang, Garcia, & Lombardi, 2002). Furthermore, patients’ work commitments, long-distance travel, and unavailability of transportation can be other important reasons for no-shows (Spikmans et al., 2003). Therefore, one strategy providers can use to maximise patient adherence to protocols is responsiveness to patients’ unique cultural and socio-economic circumstances. The hope is that by better understanding these daily-living circumstances, providers can better connect with, and more effectively administer to, their patients (Stein, 2009). Collaborative health technologies can potentially support this understanding by collecting and analysing data and presenting it across diverse contexts (i.e., relevant clinical data are presented in daily-living environments, and relevant data related to daily living are captured and presented in clinical settings). Therefore, these technologies should be able to handle various types of data (e.g., sound, smell, text, picture, number, and sequence) and analyse these various types to yield meaningful results. Moreover, these technologies should also have the ability to present data (raw and processed) and result through various media when needed.
2.2. Gaps among health-related activities across diverse settings
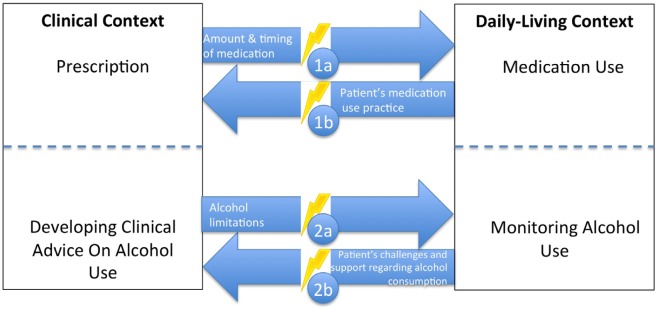
The term “gap” refers to a “break in continuity” between health-related activities across diverse settings. Gaps can disturb care delivery and lead to poor patient outcomes (Booth, Lowis, Dean, Hunter, & McKinley, 2013; Nagelkerk, Reick, & Meengs, 2006). Figure 1 provides examples of gaps, depicting two health-related activities in a clinical setting (i.e., medication prescription and alcohol consumption protocol) and two health-related activities in a daily-living setting (i.e., actual medication use and actual alcohol use). These examples were chosen because of their relevance to a wide variety of treatments. Ideally, medication prescription should shape actual medication use, and actual medication use should inform medication prescription. A similar reciprocal relationship should exist with respect to alcohol consumption. However, if information from one setting is not conveyed or enacted as intended in the other, gaps occur.
Figure 1.
Examples of gaps between clinical and daily-living settings.
Gap Cycle, Example No. 1: Medication Administration
Gap No. 1a (Clinic-to-daily-living Flow): medication prescription is a part of the clinical workflow, but not incorporating daily-living information could lead to non-adherence.
Gap No. 1b (Daily-living-to-clinic Flow): clinician unfamiliarity with patient daily-living factors could lead to poorly tailored therapy (Does the patient require assistance? Are there scheduling conflicts? Is the medication affordable?).
Gap Cycle, Example No. 2: Alcohol Intake
Gap No. 2a (Clinic-to-daily-living Flow): treatments such as anticoagulation therapy require alcohol consumption protocols. If protocols provided in clinical settings are not implemented by patients in daily-living settings, patient safety can be compromised.
Gap No. 2b (Daily-living-to-clinic Flow): patient social environments and routines (e.g., going to bars to socialise, camping) may lead to non-adherence. If these patient-related factors are unknown to the clinician, the protocols may be ineffective.
These gaps should inform collaborative HIT design and implementation to connect cross-setting health-related activities. Specifically, HIT should be developed to narrow or minimise gaps. Well-designed and well-implemented collaborative HIT (e.g., patient portals yielding patient-generated data that is integrated into clinical EHR decision-support features) can be key in bridging the gaps. Design and implementation of these technologies require an understanding of both health-related activities in diverse settings and the relationships among these activities (Moen & Brennan, 2005). However, there is a paucity of research about cross-setting health-related activities, cross-setting relationships among health-related activities, and ways that connections/disconnections among these activities can inform the design and implementation of a HIT. Moreover, the extant literature reveals an absence of a framework that can (a) capture the inherently all-of-a-piece nature of every cross-setting health-related activity and (b) guide design/implementation of a HIT.
3. Infinicare principles and theoretical foundation
The Infinicare framework links clinical and daily-living contexts of health management. Previously developed conceptual frameworks (e.g., the Transitional Care Model [TCM]) aim to ensure post-hospitalisation continuity through interventions such as home visits and follow-up calls by nurses (Naylor et al., 2004). Infinicare augments TCM’s transition-period focus, encompassing all patient health management activities and including more daily-living settings and setting-to-setting links. Moreover, by considering the constant interaction between clinical and daily-living settings, Infinicare aims to pre-empt hospitalisation.
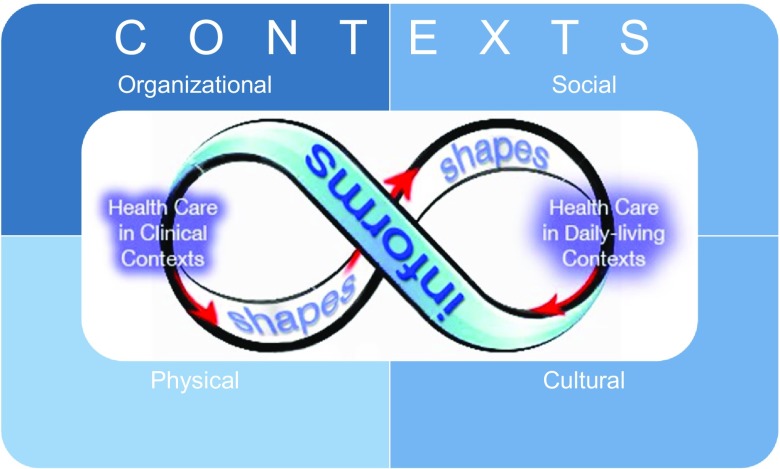
The Infinicare framework is built on a foundational premise that clinic-based health-related activities shape (e.g., influence, necessitate) activities in daily-living contexts and that health-related activities within daily life inform activities in clinical contexts (Figure 2). The Infinicare framework posits that health and health care have no strict temporal or physical boundaries. Although we focus on two mechanisms (i.e., shaping and informing), others (e.g., facilitating, constraining) are not precluded. The Infinicare framework highlights the importance of context and operationalises it by incorporating four different contextual dimensions: physical, organisational, social, and cultural. These four dimensions of context interdependently affect health-related activities across diverse settings (Bate, 2014).
Figure 2.
Visual depiction of Infinicare.
Table 1 provides examples of the Infinicare dimensions of context.
Table 1. Dimensions of context.
| Dimensions of context | Examples |
|---|---|
| Physical |
|
| Organisational |
|
| Social |
|
| Cultural |
|
A key element of Infinicare is the contextual elements in which work process is embedded (Unertl, Novak, Johnson, & Lorenzi, 2010). Sociotechnical systems are hierarchically arranged structures that function both as inputs into workflow and as the entities within which the workflow occurs (Karsh, Holden, Alper, & Or, 2006), and the four contextual dimensions represented in Figure 2 emerge from the interaction of sociotechnical system components (Bate, 2014; Carayon, 2006) – people, technology, tasks, organisation, internal environment, and external environment (Carayon et al., 2006; Holden et al., 2013). Sociotechnical systems theory also highlights the importance of precisely defined boundaries. Infinicare is informed by sociotechnical systems theory in asserting that the boundaries of health-related activities encompass both clinical and daily-living settings. Over time, structural interactions recur across settings in a way that gives the impression of routine or repeated patterns of work. Variability in health-related activities (Carayon et al., 2007; Grigg, Garrett, & Craig, 2011) is influenced by people (e.g., physician vs. nurse vs. patient), task conditions (routine vs. emergent), technologies (paper vs. electronic), organisational factors (workload, incentives), physical factors (pod vs. hallway layout), social factors (alone vs. with others), and temporal factors (time of day vs. season). Overall, evidence that “context matters” are seen across nearly every health-related domain including health behaviour (“ecology matters”), health geography (“place matters”), health systems engineering (“organisation matters”), and medical genetics (“environment matters”).
In sum, Infinicare is a design-oriented framework. In other words, it is prescriptive in nature and provokes the development of new strategies and procedures for interaction and development of collaborative HIT. Infinicare formalises a theoretical perspective that (a) highlights the integration of health-related activities across care delivery settings and (b) accounts for patient-specific contextual features.
4. Empirical testing of Infinicare
We tested Infinicare through a qualitative field study involving 39 patients from a hospital-based outpatient anticoagulation clinic. This field study was a part of a broader study, which aims to examine the possible disconnect between activities in clinical and daily-living settings and the impact of context. Anticoagulation treatment is an appropriate test case for demonstrating the value of Infinicare in that the therapy typically is long-term and complex. Further, although the therapy plan originates and is adjusted by professionals in formal healthcare settings, patients are responsible for therapy-plan adherence in informal, daily-living settings. Anticoagulation treatment therapy-plan activities include daily vitamin K intake, daily monitoring of alcohol consumption, and a regimen of multiple antibiotics. Maintenance of all aspects of these intricate, customised therapy plans must be coordinated regularly with an anticoagulation provider.
The age of the participants in our field study ranged from 26 to 83. They had been undergoing anticoagulation therapy for between 2 weeks and 26 years. Data were collected through one-hour semi-structured interviews. Interview data included background information about participants and their treatment plans, participants’ health-related activities, challenges faced by participants, and facilitators to their self-management practices. Qualitative analysis was accomplished using Dedoose® to support applied qualitative (Gale, Heath, Cameron, Rashid, & Redwood, 2013) content analytic techniques. Interviews were coded, main themes determined, and relationships between main themes examined. Data analysis revealed the applicability of Infinicare in framing and capturing study participants’ cross-setting treatment experiences. This is demonstrated in the next three subsections by reporting study findings according to the three principles of the Infinicare framework. Section 6 also presents a case study from these findings to highlight these three principles from the perspective of a single patient and provide a guideline how to apply the Infinicare framework in three steps.
4.1. Principle 1: clinic-based health-related activities shape activities in daily-living contexts
Study participants reported that clinicians taking time to frequently talk to them were essential to treatment success. When prescribed dosages were tailored to their food consumption habits, participants reported greater adherence and time in therapeutic range, an important clinical outcome. Participants also reported that customised therapy calendars provided by clinic staff were helpful with medication adherence. In fact, some participants who had changed clinics reported having done so to receive customised therapy. There were also undesirable shaping effects of clinic-based activities. Younger participants who recently started therapy reported the impact of clinic appointments on their work schedules, e.g., “How am I going to come in every week? They are not going to let me off every single week. The clinic is not open before and after work”.
4.2. Principle 2: daily-living-based health-related activities inform activities in clinical contexts
Study participants who enjoyed gardening reported a proactive change of dosage before harvest season. Patients who had had a recent unusual or one-off episode that took them out of therapeutic range in their daily life setting required a subsequent period of more vigilant clinical monitoring. As reported by participants, patients’ daily routines are important in clinical decision-making and developing therapy plans.
the first year that I went in, they said “there are other drugs you can use, If you don’t want to come in every 6 weeks”. I’d remind them that I have animals and that I have horse wrecks and then they [said] “you need to stay on something that is potentially reversible.”
The patient is on warfarin because it is the only reversible option although it requires regular office visits.
4.3. Principle 3: context is critical
All four contextual dimensions listed in Table 1 were represented in the accounts of study participants: physical context (e.g., availability of private space, line of sight); organisational context (e.g., transportation to clinic, coordinating busy work schedules, and appointments); social context (e.g., dietary expectations associated with the Christmas season, accommodating other household members); and cultural context (e.g., incorporating religious perspectives into the therapy process).
To conclude, our analysis of interviews with 39 anticoagulation patients underscored the applicability and relevance of the Infinicare framework. The framework provided a holistic data analysis perspective for examining the interaction of activities across diverse settings.
5. Operationalising infinicare via patient-oriented workflow
The Infinicare framework can be operationalised by incorporating contextual elements into patient-oriented workflow. In general, workflow can be defined as “the flow of work through space and time” (Karsh, 2009) – i.e., a cross-setting, temporally organised activity sequence. Studying workflow enables understanding of how work elements (including information, resources, and influence) are organised. Workflow models can help explain patient interactions (Unertl, Weinger, Johnson, & Lorenzi, 2009) and reveal design directions for technology supporting user performance (Yen & Bakken, 2012). Operationalisation of the Infinicare framework through patient-oriented workflow facilitates understanding of how work elements (hereafter referred to collectively as “activities”) traverse clinical and daily-living settings and by explicitly incorporating contextual elements. As a result, care delivery gaps across settings can be understood, guiding gap-bridging HIT solutions.
HIT literature indicates that explicating workflow across settings is essential to obtaining desired results (Brennan & Casper, 2015; Kaufman et al., 2009; Moen & Brennan, 2005; Ozkaynak & Brennan, 2013; Valdez, Holden, Novak, & Veinot, 2014). Existing workflow studies have a limited scope, typically (a) single settings (e.g., emergency departments and operating rooms) or (b) isolated processes (e.g., barcode medication administration). However, health-related activities occur beyond a single setting and include multiple interacting processes. Unnuanced workflow models may cause non-adoption of new technology (Tang, Ash, Bates, Overhage, & Sands, 2006), lack of contextual awareness (Unertl, Johnson, Gadd, & Lorenzi, 2013), unintended consequences (Koppel et al., 2005), and operational ineffectiveness (Abraham & Reddy, 2010).
A workflow model that tracks patient trajectory across settings and incorporates patient-specific contexts can be called “patient-oriented”. Patient-oriented workflow differs from traditional clinician-oriented approaches, where workflow is defined as a collection of activities by a single type of clinician (e.g., physician workflow). Patient-oriented workflow makes the patient, rather than the clinician, the protagonist of the care episode story arc (Ozkaynak et al., 2013) and captures health-related cooperation related to the care of a single patient. Encompassing health-related activities in daily-living contexts necessitates capturing the work by all key players – patient, informal caregivers, “care partners” (Sarkar & Bates, 2014), and clinicians – in the “coproduction of healthcare delivery” (Batalden et al., 2015). In short, Infinicare can be operationalised a patient-oriented workflow approach, which follows the patient “out the door” of the formal healthcare setting rather than stopping at the door. Patient-oriented workflow guided by Infinicare allows researchers and designers to depict the flow across all health-related activities, regardless of setting. In doing so, it reveals the presence of gaps.
Workflow is also organised differently in various clinical and daily-living settings, based on the personnel available, cultural norms, performance criteria (e.g., goals), or physical conditions. In some clinical settings, many short cycles of activity occur during the day in a restricted space (e.g., orthopaedic surgical clinic) whereas in others, the workflow is distributed over time and space (e.g., paediatric oncology clinic) (Stange & Glasgow, 2013). In some daily-living settings, people visit the doctor or dentist when they are acutely ill or pregnant, but not for preventive care. In addition to research on the contextual or structural factors shaping healthcare workflow, several recent investigations have begun to examine the structural factors constraining or enabling patients’ and families’ workflows. These studies have examined the factors that affect self-care of patients with heart failure (Holden, Schubert, & Mickelson, 2015), or the conditions of the home environment and community that affect health behaviour (Zayas-Cabán & Valdez, 2012) and self-care performance (Holden, Valdez, Schubert, Thompson, & Hundt, in press). The representation of workflow in daily-living settings should particularly be responsive to physical, organisational, social, and cultural contexts because patients’ and clinicians’ behaviours depend heavily on them (Ozkaynak, Jones, Weiss, Klem, & Reeder, 2016).
Understanding context yields understanding of (a) why workflow unfolds as it does and (b) how workflow might change when context changes. Because of the former, an understanding of health-related process is incomplete without accounting for contextual factors (Siemieniuch & Sinclair, 2005). Because of the latter, contextual data are integral to evaluating and planning workflow interventions (Carayon et al., 2010) and must be captured simultaneously with activity-related data.
6. Guidelines for applying the Infinicare framework
We developed a three-step set of guidelines to help researchers and designers develop HIT and make organisational changes informed by the Infinicare framework. The following case study, a composite of field study interviews (see the methodological details in Section 4), provides a context for applying these guidelines:
Jane is 28 years old. During an emergency room visit about 3 months ago, a clot was identified in her left leg. Since then, she has been taking warfarin (the generic name for Coumadin) daily at 10:00 p.m. and visiting an anticoagulation clinic 2-4 times per month, depending on her INR value (a measure of blood clotting).
Jane lives with her older sister in a large, 2-bedroom apartment and works full-time as an executive assistant. She and her sister have a close, supportive relationship. Jane will be getting married in 10 days, so much of her non-work time has been focused on wedding preparations and socializing/celebrating with her friends.
Jane likes to cook at home, but because of her busy work schedule, she has been dining out often. Her vegetarian diet is heavy in green, leafy vegetables and broccoli. However, her therapy regimen requires a consistent diet, particularly in terms of Vitamin K, which counters the effect of warfarin. Jane usually monitors her green, leafy vegetable consumption but as a result consumes more carbohydrates. Thus, she has been gaining weight. She is upset about it, particularly with the wedding approaching. As a result, Jane eats primarily spinach salads and broccoli for 2 days before her dress fitting.
Jane enjoys going out and socializing with friends on occasion, and with her wedding approaching, she has been getting more invitations than usual. However, recently, she has been put in a slightly awkward position because warfarin use limits her alcohol consumption. Her friends keep asking her why she is reluctant to drink alcohol and celebrate freely with them – last week, she gave in and drank excessively (it was her best friend’s birthday) 2 days before her anticoagulation clinic appointment. Moreover, she forgot her to take her medication that night. This is not the first time she forgot her medication after coming home late.
She is also becoming concerned about arranging an INR test during her upcoming honeymoon in Cancun.
Step 1: Identify patient-oriented workflow and contextual elements
Step 1a: Define boundaries. Patient-oriented workflow highlights the organisation of all activities related to the patient’s treatment. All essential settings where these activities take place should be taken into consideration. The boundaries are defined for a specific temporal period, and new boundaries should be defined as living condition changes. For example, the boundaries of a high school student with type 1 diabetes will change when he graduates and goes to college in another city. In our case study, the boundaries of care delivery not only include the clinic but also Jane’s home, her workplace, the restaurants where she dines, and the clubs she occasionally visits with friends. Defining boundaries can start by capturing and listing relevant clinical and daily-living settings through interviewing patients, their relatives, and clinicians. Additional inquiry can be performed by using time-stamped GPS devices.
Step 1b: Define activities, the temporal relationship of activities, and other building blocks of the patient-oriented workflow. What the patient does, what is done for the patient, and how these activities are ordered temporally should now be examined. “Activities” here connote the physical aspects (behaviours) associated with managing a chronic health condition. Depending on the research question and type of disease, some other work elements such as information flow or mood state of the patient can be important when studying patient-oriented workflow. Jane performs various anticoagulation therapy-related activities as stated in the case. Some of these activities include:
-
•
Using medication (warfarin) daily
-
•
Visiting the anticoagulation clinic
-
•
Monitoring and managing diet
-
•
Monitoring alcohol consumption (when going out with friends)
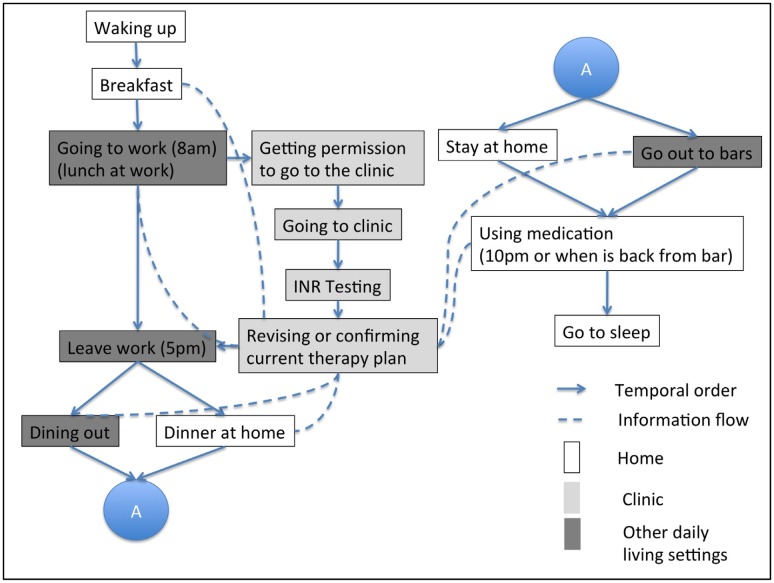
These activities can be arranged temporally within Jane’s daily routine (Figure 3). The figure also shows the information flow (in dashes) between clinical and daily-living settings.
Figure 3.
Workflow representation of Jane’s daily routine.
Capturing workflow-related data and overcoming methodological challenges in diverse settings require multiple data collection methods. Industrial and systems engineering methods such as process mapping, work sampling, task analysis, and social network analysis can provide rich information about activities and their temporal organisation (Carayon et al., 2012; Ozkaynak, Unertl, Johnson, Brixey, & Haque, 2015). Traditional methods from industrial engineering and qualitative research can be enriched by novel methodologies such as observation of patient daily-living practices through the distribution of mobile devices (Valdez & Brennan, 2013).
Step 1c: Identify important context-related factors. Context-related factors are categorised as physical, organisational, social, and cultural. In Jane’s case, some important context variables are as follows:
-
•
Physical context: her own bedroom in a home she shares
-
•
Organisational context: hectic work schedule, no time to cook
-
•
Social context: close sister, active friends, fiancée
-
•
Cultural context: standards of beauty, behavioural expectations at ritual celebrations (e.g., alcohol consumption), and beliefs about what is healthy (e.g., green leafy vegetables)
An essential feature of the Infinicare framework is its inclusion of daily-living settings, clinical settings, and the cross-setting interaction (informing and shaping) of health-related activities using patient-oriented workflow. Relevance of settings and context variables is study-specific, research question-specific, and/or target population-specific. Once the relevant settings are specified, health-related activities in these settings can be captured and patient-oriented workflow defined. The main challenge here is a level of granularity: too much granularity in abstracting health-related activities leads to too much variability and obviation of meaningful analysis; too little granularity omits potentially important details. Context-related factors may be subjective or objective. The selection of context variables can be accomplished by examining what context variables best inform the patient-oriented workflow blocked out in Step 1b. Qualitative research techniques such as interviews and focus group studies with patients and analysing their social media activities can be fruitful concerning the four dimensions of context variables. Satisfactory completion of the three components of Step 1 is essential to satisfactory completion of Step 2.
Step 2: Identify gaps. This step refers to the gaps between therapy plans formulated in the clinic and activities in daily-living settings. Gaps occur when health-related activities across settings do not interact, and shaping and informing mechanisms do not work optimally. Gaps typically show where informatics and other interventions are needed. This case includes gaps related to food consumption, alcohol consumption, and medication adherence.
During appointments in clinical settings, patients undergoing anticoagulation treatment are advised both to eat regularly and not to eat excessive amounts of high-vitamin K foods such as spinach and broccoli. However, these elements of the therapy plan can meet resistance when confronted with the exigencies of daily life: in our case study, Jane’s hectic work schedule does not allow for routine meals, and Jane is a vegetarian. Furthermore, Jane’s salad-intensive diet prior to her dress fitting will lessen the impact of the medication she is taking.
Excessive alcohol use also affects the therapeutic range in warfarin administration. In the case study, Jane’s social schedule and cultural beliefs make alcohol monitoring and restriction difficult.
Lastly, she sometimes forgets to take her medication when she stays out late. Identification of gaps can start by examining adherence difficulties, unmet information needs and perceived challenges by patients and their clinicians.
Step 3: Translate gaps into design specifications. The three gaps identified in our case can be addressed by a number of designs, including a collaborative HIT intervention that has an integrated decision-support system and convenient data-entry components for patients. The data-entry component allows patients to enter and record alcohol and food consumption in near-real time. Although self-reported variables are subject to limitations such as memory lapses, the accuracy of data entry can be improved by Chassin and Galvin (1998) better technology design that also collects objective data (e.g., GPS, to capture whether the patient goes to a bar or liquor store) and/or (Medicine IOMIo, 2001) technology support via motivational techniques such as Screening, Brief Intervention, and Referral to Treatment (SBIRT) (SAMHSA-HRSA Center for Integrated Health Solutions, 2016). The decision-support system makes clinicians aware – again, in near-real time – of the patient’s recent food and alcohol consumption. Therefore, the clinician is able to generate or modify therapy plans based on robust data. In short, the user-friendly design and near-real-time implementation of the decision-support system maximise the likelihood that clinicians and patients will act on data.
The first design research cycle (as described in Hevner, 2007), the relevance cycle, involves capturing requirements in all relevant contexts. The Infinicare framework allows for better utilisation of requirements engineering in the design of collaborative health information technologies by suggesting more comprehensive (and realistic) system boundaries. It further supports examination of interconnections between health-related activities and the four dimensions of context. Therefore, elicitation, analysis, specification, validation, and management of requirements will be complete, consistent, and relevant (Sommerville & Sawyer, 1997).
In the case study, patient input/clinician receipt of documentation of high-vitamin K food consumption, excessive alcohol use, or skipping a scheduled instance of medication administration may change INR temporarily. Timely, accurate communication of daily-living instances that conflict with therapy plan guidelines can yield a better understanding of variant INR values and inform more tailored plans going forward. Moreover, HIT could play an important role in helping patients and clinicians come up with daily-living alternatives. For example, if it is known that an individual eats green salads to lose weight, the decision-support system could offer other low-calorie meal suggestions that would have a smaller negative impact. Translating gaps into design specification can be accomplished by employing user-centered design approaches such as design thinking (Brown & Wyatt, 2010), participatory design (Sjoberg & Timpka, 1998), personas (Pruitt, 2006), or engineering techniques such as quality function deployment (Chan & Wu, 2002).
7. HIT implications
The Infinicare framework is intended to accelerate the development and use of disruptive approaches holding promise for reducing gaps through collaborative HIT. One such approach is linkAges™, which aims to address social determinants of health (e.g., loneliness) that are outside traditional medical delivery by creating a broad, community-based model to support successful in-community ageing (Sutter Health Palo Alto Medical Foundation, 2013). The linkAges intervention aims to narrow the gap between settings by adding an EHR order option, enabling a physician to prescribe linkAges. Adding patient context/social history (e.g., physical, organisation, cultural, and social) to EHR systems (which are typically limited to clinical data) will similarly allow clinicians to provide better, more patient-centered care (Institute of Medicine [IOM], 2014).
Informatics innovations should not merely provide more information – they should facilitate action (e.g., making a decision, initiating a service, or a coordination). For example, technology interfaces could show care providers a prescription to disability-related transportation, English language classes, or information about pharmacies that have interpreter services. Patient-generated data (e.g., food logs, sleep/exercise patterns, and mood) could be integrated into clinical workflow appropriately, to algorithmically recommend primary prevention activities. These integrations may require new interoperability and representation standards to aggregate data from different sources, and innovative visualisation techniques to present data at bedside. All these interventions require integrated, cross-setting understanding of health management. Technology design guided by the Infinicare perspective ensures continuity of activities across diverse settings.
Gregory (2006) proposed a taxonomy that classifies information systems theories with respect to the manner in which four central goals are addressed: analysis, explanation, prediction, and prescription. Five interrelated types of theory are distinguished: (1) theory for analysing, (2) theory for explaining, (3) theory for predicting, (4) theory for explaining and predicting, and (5) theory for design and action. Design and action theories address how to do something. The theory gives explicit prescriptions (e.g., methods, techniques, principles of form, and function for constructing an artefact). Infinicare framework is an example of the fifth category of theory. It redefines boundaries in chronic disease management and highlights the relationships of activities across diverse settings.
Chatterjee and Price (2009) identified three main domains of persuasive technologies: technology, persuasion, and health care. The effectiveness of these technologies increases as they are more integrated to clinical information technologies. Frameworks such as Infinicare and methods such as patient-oriented workflow can support this integration by emphasising the relationships of activities across diverse settings.
8. Discussion
We have proposed a framework, Infinicare, which promotes systematic health-related activity evaluation by incorporating clinic-based activities, daily-living-based activities, the interrelationship of both sets of activities, and the various contexts in which all activities and interactions are embedded. The theoretical aspect of Infinicare highlights the need for integration of health-related activities across care delivery settings. The operationalisation of Infinicare is accomplished by using patient-oriented workflow with the incorporation of contextual elements.
Infinicare can be used to identify the cross-setting links and gaps between health-related activities. In turn, characterising the gaps can reveal important design guidelines for collaborative informatics interventions that (a) bridge consumer and clinical domains and (b) leverage both provider- and patient-generated data to narrow these gaps (Valdez et al., 2014). Reducing gaps across settings is particularly important for chronic disease management, in which communication, continuity, and vigilance are essential.
Infinicare is the first conceptual framework that explicitly includes both (a) home and clinical settings as important locations for health and (b) continuity between settings from a patient perspective. These two important considerations have been implicit rather than explicit in previous models (Holden et al., 2015; Valdez et al., 2015). March and Smith (1995) and Hevner, March, Park, and Ram (2004) identify criteria for framework evaluation, such as a utility to a community of users and the persuasiveness of claims that it is effective. Models and methods can be evaluated for completeness, simplicity, consistency, ease of use, and quality of results obtained. The Infinicare framework must be subjected to scrutiny and evaluation of its efficacy, and this manuscript represents an important step in so doing.
Traditional methodologies focus on observable activities in community (i.e., less private) settings. However, health-related activities are mostly cognitive, not directly observable, and conducted by patients mostly in private settings. Innovative methodologies are needed to overcome these challenges and fully utilise the Infinicare framework.
Overall, the Infinicare framework contributes to health informatics and care delivery systems by guiding the design of collaborative health IT systems. Infinicare does this by highlighting continuous, cross-setting interaction between health-related activities. This design-oriented framework closes a gap in the literature by operationalising the broad design goals of collaborative HIT solutions. This framework can benefit, in particular, individuals with chronic conditions, as described in previous sections. Moreover, this framework can guide interventions for individuals with acute conditions (by ensuring discharge plans are realised at home) and promote wellness for all individuals (by codifying the exercise and diet regimens critical to a healthy lifestyle). This framework can be useful to academicians in designing field studies that address and respond to significant questions (e.g., “Under what circumstances would patients be willing to enter information that can be shared with physicians?” and “What kinds of information would patients be willing to share?” and “How can such information be integrated with other information on clinical/daily activities to provide more patient-centred care?”). This framework can be useful to designers by redefining the boundaries of health-related activities and helping them consider workflow and context in an integrated manner (Dourish, 2004).
Future studies should address the challenges related to (a) aggregation of disparate data streams and (b) presentation of clinical and patient-generated data to all relevant audiences.
9. Conclusion
We have proposed the Infinicare framework, which supports examining activities that go beyond only clinical or only daily-living settings, encouraging the conceptualisation of health-related activities across diverse settings and in context. The framework informs the design, implementation, and evaluation of collaborative health information technologies.
Funding
Development of the Infinicare framework was supported by AHRQ under award number R03HS024092 (PI: Ozkaynak).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abraham J., & Reddy M. C. (2010). Challenges to inter-departmental coordination of patient transfers: A workflow perspective. International Journal of Medical Informatics , 79(2), 112–122. PubMed PMID: 20005771. doi: 10.1016/j.ijmedinf.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Aliotta S., & Andre J. A. (1997). Case management and home health care: An integrated model. Home Health Care Management & Practice , 9(2), 1–12. doi: 10.1177/108482239700900204 [DOI] [Google Scholar]
- Ancker J. S., Kern L. M., Edwards A., Nosal S., Stein D. M., Hauser D., … Investigators H. (2014). How is the electronic health record being used? Use of EHR data to assess physician-level variability in technology use. Journal of the American Medical Informatics Association , 21(6), 1001–1008. . PubMed PMID: 24914013; PMCID: PMC4215048. doi: 10.1136/amiajnl-2013-002627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M. J., & Edgman-Levitan S. (2012). Shared decision making–pinnacle of patient-centered care. The New England Journal of Medicine , 366(9), 780–781. doi: 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- Batalden M., Batalden P., Margolis P., Seid M., Armstrong G., Opipari-Arrigan L., & Hartung H. (2015). Coproduction of Healthcare Service. BMJ Quality & Safety. doi: 10.1136/bmjqs-2015-004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate P. (2014). Context is everything In Bate P., Robert G., Fulop N., Ovretveit J., & Dixon-Woods M. (Eds.), Perspectives on context (1–30). London: Health Foundation. [Google Scholar]
- Beach M. C., Keruly J., & Moore R. D. (2006). Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? Journal of General Internal Medicine. , 21, 661–665. doi: 10.1111/j.1525-1497.2006.00399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. O., Lowis C., Dean M., Hunter S. J., & McKinley M. C. (2013). Diet and physical activity in the self-management of type 2 diabetes: Barriers and facilitators identified by patients and health professionals. Primary Health Care Research & Development , 14(03), 293–306 . PubMed PMID: 23739524. doi: 10.1017/S1463423612000412 [DOI] [PubMed] [Google Scholar]
- Brennan P., & Casper G. (2015). Observing health in everyday living: ODLs and the care-between-the-care. Personal and Ubiquitous Computing , 19(1), 3–8. doi: 10.1007/s00779-014-0805-0 [DOI] [Google Scholar]
- Brennan P. F., Downs S., & Casper G. (2010). Project health design: Rethinking the power and potential of personal health records. Journal of Biomedical Informatics , 43(5 Suppl), S3–S5. doi: 10.1016/j.jbi.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Brown T., & Wyatt J. (2010). Design thinking and social innovation. Stanford Social Innovation Review , 8(1), 30–35. [Google Scholar]
- Cahill J. E., Gilbert M. R., & Armstrong T. S. (2014). Personal health records as portal to the electronic medical record. Journal of Neuro-Oncology , 117(1), 1–6 . PubMed PMID: 24477621. doi: 10.1007/s11060-013-1333-x. [DOI] [PubMed] [Google Scholar]
- Carayon P. (2006). Human factors of complex sociotechnical systems. Applied Ergonomics , 37(4), 525–535. doi: 10.1016/j.apergo.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Carayon P., Schoofs Hundt A., Karsh B. T., Gurses A. P., Alvarado C. J., Smith M., & Flatley Brennan P. (2006). Work system design for patient safety: The SEIPS model. Quality & Safety in Health Care , 15(Suppl 1), i50–i58. doi: 10.1136/qshc.2005.015842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayon P., Wetterneck T. B., Hundt A. S., Ozkaynak M., DeSilvey J., Ludwig B., … Rough S. S. (2007). Evaluation of nurse interaction with bar code medication administration technology in the work environment. Journal of Patient Safety , 3(1), 34–42. doi: 10.1097/PTS.0b013e3180319de7 [DOI] [Google Scholar]
- Carayon P., Karsh B.-T., Cartmill R., Hoonakker P., Hundt A. S., Krueger D., Wetterneck T. B. (2010). Incorporating health it into workflow redesign. Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Carayon P., Cartmill R., Hoonakker P., Hundt AS., Karsh BT., Krueger D., … Wetterneck T. B. (2012). Human factors analysis of workflow in health information technology implementation In Carayon P. (Ed.), Handbook of Human Factors and Ergonomics in Health Care and Patient Safety (2nd ed., pp. 507–522). Boca Raton: CRC Press. [Google Scholar]
- Carman K. L., Dardess P., Maurer M., Sofaer S., Adams K., Bechtel C., & Sweeney J. (2013). Patient and family engagement: A framework for understanding the elements and developing interventions and policies. Health Affairs , 32, 223–231. doi: 10.1377/hlthaff.2012.1133 [DOI] [PubMed] [Google Scholar]
- Chan L.-K., & Wu M.-L. (2002). Quality function deployment: A literature review. European Journal of Operational Research , 143(3), 463–497. doi: 10.1016/S0377-2217(02)00178-9 [DOI] [Google Scholar]
- Chassin M. R., & Galvin R. W. (1998). The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality, 280(11), 1000–1005. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., & Price A. (2009). Healthy living with persuasive technologies: Framework, issues, and challenges. Journal of the American Medical Informatics Association , 16(2), 171–178. PubMed PMID: 19074300; PMCID: PMC2649327. doi: 10.1197/jamia.M2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denford S., Frost J., Dieppe P., Cooper C., & Britten N. (2014). Individualisation of drug treatments for patients with long-term conditions: A review of concepts. BMJ Open , 4(3), e004172. PubMed PMID: 24670429; PMCID: PMC3975745. doi: 10.1136/bmjopen-2013-004172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourish P. (2004). What we talk about when we talk about context. Personal and Ubiquitous Computing , 8(1), 19–30. PubMed PMID: WOS:000207560700002. doi: 10.1007/s00779-003-0253-8 [DOI] [Google Scholar]
- Evert A. B., Boucher J. L., Cypress M., Dunbar S. A., Franz M. J., Mayer-Davis E. J., … Yancy W. S. Jr (2013). American Diabetes A. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care , 36(11), 3821–3842. PubMed PMID: 24107659; PMCID: PMC3816916. doi: 10.2337/dc13-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn S. J., Ameling J. M., Hill-Briggs F., Wolff J. L., Bone L. R., Levine D. M., … Boulware L. E. (2013). Facilitators and barriers to hypertension self-management in urban African Americans: Perspectives of patients and family members. Patient Prefer Adherence , 7, 741–749. PubMed PMID: 23966772; PMCID: PMC3743518. doi: 10.2147/PPA.S46517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. K., Heath G., Cameron E., Rashid S., & Redwood S. (2013). Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology , 13, 117. PubMed PMID: 24047204; PMCID: PMC3848812. doi: 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G., Côté J., Naccache H., Lambert L. D., & Trottier S. (2005). Prediction of adherence to antiretroviral therapy: A one-year longitudinal study. AIDS Care , 17(4), 493–504 . PubMed PMID: 16036235. doi: 10.1080/09540120412331291715 [DOI] [PubMed] [Google Scholar]
- Gregor S. (2006). The nature of theory in information systems. MIS Quarterly , 30(3), 611–642. [Google Scholar]
- Grigg S. J., Garrett S. K., & Craig J. B. (2011). A process centered analysis of medication administration: Identifying current methods and potential for improvement. International Journal of Industrial Ergonomics , 41, 380–388. doi: 10.1016/j.ergon.2011.01.014 [DOI] [Google Scholar]
- Gruman J., Rovner M. H., French M. E., Jeffress D., Sofaer S., Shaller D., & Prager D. J. (2010). From patient education to patient engagement: Implications for the field of patient education. Patient Education and Counseling , 78, 350–356. doi: 10.1016/j.pec.2010.02.002 [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (2009). Health, United States, 2009: With special feature on medical technology. Hyattsville, MD. [PubMed] [Google Scholar]
- Hevner A. (2007). A three cycle view of design science research. Scandinavian Journal of Information Systems , 19(2), 87–92. [Google Scholar]
- Hevner A. R., March S. T., Park J., & Ram S. (2004). Design science in information systems research. MIS Quarterly , 28(1), 75–105. [Google Scholar]
- Holden R. J., Carayon P., Gurses A. P., Hoonakker P., Hundt A. S., Ozok A. A., & Rivera-Rodriguez A. J (2013). SEIPS 2.0: A human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics, 56(11), 1669–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden R. J., Schubert C. C., & Mickelson R. S. (2015). The patient work system: An analysis of self-care performance barriers among elderly heart failure patients and their informal caregivers. Applied Ergonomics , 47, 133–150. doi: 10.1016/j.apergo.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden R. J., Valdez R. S., Schubert C. C., Thompson M. J., & Hundt A. S. (in press). Macroergonomic factors in the patient work system: Examining the context of patients with chronic illness. Ergonomics. doi: 10.1080/00140139.2016.1168529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) (2014). Capturing social and behavioral domains and measures in electronic health records: Phase 2. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Karsh B.-T. (2009, June). Clinical practice improvement and redesign: How change in workflow can be supported by clinical decision support. AHRQ Publication No. 09-0054-EF. Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Karsh B. T., Holden R. J., Alper S. J., & Or C. K. L. (2006). A human factors engineering paradigm for patient safety: Designing to support the performance of the healthcare professional. Quality & Safety in Health Care , 15(Suppl 1), i59–i65. doi: 10.1136/qshc.2005.015974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. R., Pevzner J., Rodriguez M., Cimino J. J., Ebner S., Fields L., … Starren J. (2009). Understanding workflow in telehealth video visits: Observations from the IDEATel project. Journal of Biomedical Informatics , 42(4), 581–592. PubMed PMID: 19358897. doi: 10.1016/j.jbi.2009.03.012 [DOI] [PubMed] [Google Scholar]
- Kaziunas E., & Ackerman M. S. (2015). Designing for lived health: A practice-based approach for person-centered health information technologies In Wulf V., Schmidt K., & Randall D. (Eds.), Designing socially embedded technologies in the real-world (pp. 357–381). London: Springer; 10.1007/978-1-4471-6720-4 [DOI] [Google Scholar]
- Koch S. (2006). Home telehealth – Current state and future trends. International Journal of Medical Informatics , 75, 565–576. doi: 10.1016/j.ijmedinf.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Koppel R., Metlay J. P., Cohen A., Abaluck B., Localio A. R., Kimmel S. E., & Strom B. L. (2005). Role of computerized physician order entry systems in facilitating medication errors. JAMA , 293(10), 1197–1203. PubMed PMID: 15755942. doi: 10.1001/jama.293.10.1197 [DOI] [PubMed] [Google Scholar]
- Légaré F., Stacey D., Pouliot S., Gauvin F.-P., Desroches S., Kryworuchko J., … Graham I. D. (2011). Interprofessionalism and shared decision-making in primary care: A stepwise approach towards a new model. Journal of Interprofessional Care , 25, 18–25. doi: 10.3109/13561820.2010.490502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. T., & Smith G. F. (1995). Design and natural science research on information technology. Decision Support Systems , 15(4), 251–266. doi: 10.1016/0167-9236(94)00041-2 [DOI] [Google Scholar]
- Marquard J. L., Garber L., Saver B., Amster B., Kelleher M., & Preusse P. (2013). Overcoming challenges integrating patient-generated data into the clinical EHR: Lessons from the CONtrolling Disease Using Inexpensive IT – Hypertension in Diabetes (CONDUIT-HID) Project. International Journal of Medical Informatics , 82(10), 903–910 . PubMed PMID: 23800678. doi: 10.1016/j.ijmedinf.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Medicine IOMIo (2001). Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Miller A. R., Condin C. J., McKellin W. H., Shaw N., Klassen A. F., & Sheps S. (2009). Continuity of care for children with complex chronic health conditions: Parents’ perspectives. BMC Health Services Research , 9, 248. doi: 10.1186/1472-6963-9-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen A., & Brennan P. F. (2005). Health@Home: The work of health information management in the household (HIMH): Implications for consumer health informatics (CHI) innovations. Journal of the American Medical Informatics Association , 12(6), 648–656. PubMed PMID: 16049230; PMCID: PMC1294036. doi: 10.1197/jamia.M1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerk J., Reick K., & Meengs L. (2006). Perceived barriers and effective strategies to diabetes self-management. Journal of Advanced Nursing , 54(2), 151–158. doi: 10.1111/j.1365-2648.2006.03799.x . PubMed PMID: 16553701. [DOI] [PubMed] [Google Scholar]
- Naithani S., Gulliford M., & Morgan M. (2006). Patients’ perceptions and experiences of ‘continuity of care’ in diabetes. Health Expectations: An International Journal of Public Participation in Health Care and Health Policy. , 9(2), 118–129. doi: 10.1111/j.1369-7625.2006.00379.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M. D., Brooten D. A., Campbell R. L., Maislin G., McCauley K. M., & Schwartz J. S. (2004). Transitional care of older adults hospitalized with heart failure: A randomized, controlled trial. Journal of the American Geriatrics Society , 52(5), 675–684 . PubMed PMID: 15086645. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- O’Leary K., Vizer L., Eschler J., Ralston J., Pratt W.. Understanding patients’ health and technology attitudes for tailoring self-management interventions. AMIA Annual Symposium Proceedings. 2015;2015, 991–1000. PubMed PMID: PMC4765611. [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak M., & Brennan P. F. (2013). Revisiting sociotechnical systems in a case of unreported use of health information exchange system in three hospital emergency departments. Journal of Evaluation in Clinical Practice , 19(2), 370–373. PubMed PMID: 22420774. doi: 10.1111/j.1365-2753.2012.01837.x [DOI] [PubMed] [Google Scholar]
- Ozkaynak M., Flatley Brennan P. F., Hanauer D. A., Johnson S., Aarts J., Zheng K., & Haque S. N. (2013). Patient-centered care requires a patient-oriented workflow model. Journal of the American Medical Informatics Association , 20(e1), e14–e16. doi: 10.1136/amiajnl-2013-001633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak M., Unertl K. M., Johnson S. A., Brixey J. J., & Haque S. N. (2015). Clinical workflow analysis, process redesign, and quality improvement In Finnell J. T. & Dixon B. E. (Eds.), Clinical Informatics Study Guide (pp. 135–161). New York, NY: Springer. [Google Scholar]
- Ozkaynak M., Jones J., Weiss J., Klem P., & Reeder B. (2016). A workflow framework for health management in daily living settings. Studies in Health Technology and Informatics , 225, 392–396 [PubMed] [Google Scholar]
- Pruitt J. T. A. (2006). The persona lifecycle: Keeping people in mind throughout product design. San Francisco, CA: Morgan Kauffmann Publishers. [Google Scholar]
- Rogers A., Kennedy A., Nelson E., & Robinson A. (2005). Uncovering the limits of patient-centeredness: Implementing a self-management trial for chronic illness. Qualitative Health Research , 15, 224–239. [DOI] [PubMed] [Google Scholar]
- Sallis J. F., Owen N., & Fisher E. B. (2008). Ecological models of health behavior In Glanz K., Rimer B. K., & Viswanath K. (Eds.), Health Behavior and Health Education Theory, Research, and Practice (4th ed., pp. 465–485). San Francisco, CA: John Wiley & Sons. [Google Scholar]
- SAMHSA-HRSA Center for Integrated Health Solutions (2016, November 28). SBIRT: Screening, brief intervention, and referral to treatment. Retrieved 28 November, 2016, from https://www.integration.samhsa.gov/clinical-practice/sbirt [Google Scholar]
- Sarkar U., & Bates D. W. (2014). Care partners and online patient portals. JAMA , 311(4), 357–358. doi: 10.1001/jama.2013.285825 [DOI] [PubMed] [Google Scholar]
- van Servellen G., Chang B., Garcia L., & Lombardi E. (2002). Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus-infected men and women. AIDS Patient Care and STDs , 16(6), 269–281 . PubMed PMID: 12133262. doi: 10.1089/10872910260066705. [DOI] [PubMed] [Google Scholar]
- Siemieniuch C. E., & Sinclair M. A. (2005). The analysis of organisational processes In: Wilson J. R. & Corlett N. (Eds.), Evaluation of human work (3rd ed., pp. 977–1008). Boca Raton, FL: CRC Press. [Google Scholar]
- Simon S. R., Soran C. S., Kaushal R., Jenter C. A., Volk L. A., Burdick E., … Bates D. W. (2009). Physicians’ Use of key functions in electronic health records from 2005 to 2007: A statewide survey. Journal of the American Medical Informatics Association , 16(4), 465–470. PubMed PMID: 19390104; PMCID: PMC2705248. doi: 10.1197/jamia.M3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg C., & Timpka T.. Participatory design of information systems in health care. Journal of the American Medical Informatics Association. 1998. 5(2), 177–183. PubMed PMID: 9524350; PMCID: PMC61288 10.1136/jamia.1998.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville I., & Sawyer P. (1997). Requirements engineering: A good practice guide. Chichester: John Wiley & Sons. [Google Scholar]
- Spikmans F. J., Brug J., Doven M. M., Kruizenga H. M., Hofsteenge G. H., & van Bokhorst-van der Schueren M. A. (2003). Why do diabetic patients not attend appointments with their dietitian? Journal of Human Nutrition and Dietetics , 16(3), 151–158 . PubMed PMID: 12753108 10.1046/j.1365-277X.2003.00435.x [DOI] [PubMed] [Google Scholar]
- Stange K. C., & Glasgow R. E (2013). Considering and reporting important contextual factors in research on the patient-centered medical home. Rockville, MD: Agency for Healthcare Research and Quality; Contract No.: 13-0045-EF. [Google Scholar]
- Stein K. (2009). Cultural competency: Where it is and where it’s headed. Journal of the American Dietetic Association , 109(3), 388–394 . PubMed PMID: 19248847. doi: 10.1016/j.jada.2009.01.018 [DOI] [PubMed] [Google Scholar]
- Sutter Health Palo Alto Medical Foundation (2013). linkAges. Retrieved October 10, 2015 from, https://newsroom.pamf.org/2014/01/pamf-robert-wood-johnson-foundation-grant/ [Google Scholar]
- Tang P. C., Ash J. S., Bates D. W., Overhage J. M., & Sands D. Z. (2006). Personal health records: Definitions, benefits, and strategies for overcoming barriers to adoption. Journal of the American Medical Informatics Association , 13(2), 121–126. PubMed PMID: 16357345; PMCID: PMC1447551. doi: 10.1197/jamia.M2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unertl K. M., Weinger M. B., Johnson K. B., & Lorenzi N. M. (2009). Describing and modeling workflow and information flow in chronic disease care. Journal of the American Medical Informatics Association , 16(6), 826–836. PubMed PMID: 19717802; PMCID: PMC3002133. doi: 10.1197/jamia.M3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unertl K. M., Novak L. L., Johnson K. B., & Lorenzi N. M. (2010). Traversing the many paths of workflow research: Developing a conceptual framework of workflow terminology through a systematic literature review. Journal of the American Medical Informatics Association , 17(3), 265–273. doi: 10.1136/jamia.2010.004333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unertl K. M., Johnson K. B., Gadd C. S., & Lorenzi N. M. (2013). Bridging organizational divides in health care: An ecological view of health information exchange. JMIR Medical Informatics , 1(1), e3. PubMed PMID: 25600166; PMCID: PMC4288076. doi: 10.2196/medinform.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R. S., & Brennan P. F. (2013). Using iPod touch journals to capture patients’ health information communication practices. Washington, DC. [Google Scholar]
- Valdez R. S., Holden R. J., Novak L. L., & Veinot T. C. (2014). Transforming consumer health informatics through a patient work framework: Connecting patients to context. Journal of the American Medical Informatics Association. doi: 10.1136/amiajnl-2014-002826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R. S., Holden R. J., Novak L. L., & Veinot T. C. (2015). Technical infrastructure implications of the patient work framework. Journal of the American Medical Informatics Association , 22(e1), e213–e215. doi: 10.1093/jamia/ocu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. H., Glasgow R. E., Davis C., Bonomi A. E., Provost L., McCulloch D., … Sixta C. (2001). Quality improvement in chronic illness care: A collaborative approach. The Joint Commission Journal on Quality Improvement , 27, 63–80. 10.1016/S1070-3241(01)27007-2 [DOI] [PubMed] [Google Scholar]
- Weiner S. J., Schwartz A., Weaver F., Goldberg J., Yudkowsky R., Sharma G., … Abrams R. I. (2010). Contextual errors and failures in individualizing patient care: A multicenter study. Annals of Internal Medicine , 153(2), 69–75. doi: 10.7326/0003-4819-153-2-201007200-00002 . PubMed PMID: 20643988. [DOI] [PubMed] [Google Scholar]
- Yen P. Y., & Bakken S. (2012). Review of health information technology usability study methodologies. Journal of the American Medical Informatics Association , 19(3), 413–422. PubMed PMID: 21828224; PMCID: PMC3341772. doi: 10.1136/amiajnl-2010-000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas-Cabán T., & Valdez R. S. (2012). Human factors and ergonomics in home care In Carayon P. (Ed.), Handbook of human factors and ergonomics in health care and patient safety (2nd ed., pp. 743–762) Boca Raton, FL: CRC Press. [Google Scholar]