Abstract
The discovery of genetic forms of Parkinson’s disease (PD) has highlighted the importance of the autophagy/lysosomal and mitochondrial/oxidative stress pathways in disease pathogenesis. However, recently identified PD-linked genes, including DNAJC6 (auxilin), SYNJ1 (synaptojanin 1), and the PD risk gene SH3GL2 (endophilin A1), have also highlighted disruptions in synaptic vesicle endocytosis (SVE) as a significant contributor to disease pathogenesis. Additionally, the roles of other PD genes such as LRRK2, PRKN, and VPS35 in the regulation of SVE are beginning to emerge. Here we discuss the recent work on the contribution of dysfunctional SVE to midbrain dopaminergic neurons’ selective vulnerability and highlight pathways that demonstrate the interplay of synaptic, mitochondrial, and lysosomal dysfunction in the pathogenesis of PD.
Dopaminergic Neurodegeneration in PD
Dopaminergic neurons of the ventral midbrain substantia nigra pars compacta (SNc) play an important role in the regulation of voluntary movements. Degeneration of these neurons leads to the development of the cardinal motor symptoms of PD, such as tremor, rigidity, and slowed movements [1]. The identification of several genetic forms of PD has strongly implicated mitochondrial and lysosomal dysfunction as key cellular processes that contribute to PD pathogenesis [1]. However, the recent discovery of several synaptic genes linked to PD pathogenesis or predicted to alter it has highlighted the need to further investigate the contribution of synaptic dysfunction in disease pathogenesis. This review aims to summarize the compelling experimental and genetic evidence implicating deficits in SVE in PD. We also briefly discuss the relationship between synaptic, mitochondrial, and lysosomal dysfunction in dopaminergic neurodegeneration and the compounding effects of their interactions.
Modes of Synaptic Vesicle Retrieval
SVE is the regeneration of synaptic vesicles from the plasma membrane following neurotransmission [2]. One of the common modes of SVE is clathrin-mediated endocytosis, and given its relevance to the discussions in the following sections, we outline first the steps involved in this process. SVE begins with the recruitment of clathrin by adaptor proteins such as adaptor protein 2 (AP-2), AP180, and epsin to the cytoplasmic surface of the plasma membrane to areas where PtdsIns(4,5)P2 lipids are concentrated [3]. These adaptor proteins regulate cargo sorting to ensure that the proper proteins are internalized along with the vesicle. Epsin contains ubiquitin-interaction motifs and is responsible for binding ubiquitinated cargo during endocytosis [2]. Next, membrane benders such as FCHo and endophilin A1 are recruited to the plasma membrane where they begin to mold and direct the invagination of the nascently forming vesicle by inserting into the lipid membrane via its BAR domain [4] (Figure 1). FCHo also contains an additional cargo-binding subunit similar to AP-2 for an unknown cargo, implicating its role in the early stages of SVE [2]. As the PtdsIns(4,5)P2 lipid-enriched vesicle takes on a round and uniform shape, endophilin A1 subsequently interacts with dynamin and recruits synaptojanin 1 to the membrane interface [5–9]. Through its GTPase activity, dynamin stimulates the fission of the clathrin-coated vesicle (CCV) from the plasma membrane [7]. Once the CCV is free, synaptojanin 1 uses its phosphatase function to dephosphorylate PtdsIns(4,5)P2 to PtdsIns4P and subsequently PtdsIns [10,11]. These dephosphorylation events release AP-2, which relies on PtdsIns(4,5)P2 for its vesicle binding, and allow auxilin to bind the CCV through its PTEN-like and clathrin-binding domains [2,12]. Auxilin is a cofactor for hsc70 and its J domain is responsible for recruitment of the ATPase to stimulate clathrin-coat removal [2,12]. Once the CCV is uncoated, the nascent vesicle is then packaged with neurotransmitters and quickly transported to various synaptic vesicle pools in anticipation of the next neuronal stimulation.
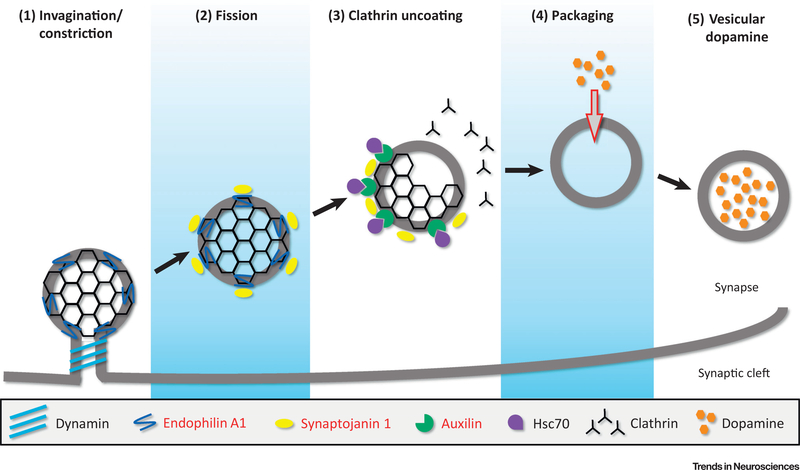
Figure 1. Recently Identified Parkinson’s Disease Genes Play a Role in Synaptic Vesicle Endocytosis (SVE).
The regeneration of synaptic vesicles following neurotransmission involves the concerted effort of various synaptic proteins, some of which have been recently linked to Parkinson’s disease or identified as risk factors for it (indicated in red in the legend, bottom). Clathrin-mediated endocytosis, a common mode of SVE, involves five key steps, illustrated here in the context of dopamine-loaded vesicles. (1) Invagination/constriction: Following the recruitment of adaptor and clathrin-coat proteins to the plasma membrane, endophilin A1 regulates the curvature of the emerging vesicle. Endophilin A1 is also responsible for the recruitment of dynamin to the neck of the clathrin-coated vesicle (CCV). (2) Fission: Dynamin then constricts the neck and mediates CCV fission from the plasma membrane. Endophilin A1 also recruits synaptojanin 1, whose phosphatase activity dephosphorylates synaptic vesicle membrane lipids to release adaptor proteins, allowing auxilin to bind to the CCV. (3) Clathrin uncoating: Auxilin is a cofactor for hsc70, which simulates the removal of the clathrin coat through its ATPase activity. (4) Packaging: Once the clathrin coat is fully removed, dopamine can be packaged into the nascent vesicle. (5) Vesicular dopamine: The dopamine-loaded vesicle is available for the next cycle of neurotransmitter release. Dopamine sequestration inside the vesicle also mitigates elevation of its cytosolic levels and prevents dopamine from becoming oxidized in the cytosol.
Other modes for synaptic vesicle retrieval have been well described, including kiss-and-run and several variations of bulk endocytosis such as ultrafast and activity-dependent bulk endocytosis [2,13,14]. These mechanisms are initially clathrin independent and occur on a much faster timescale. They have also been found to be regulated by proteins like dynamin, endophilin A1, and synaptojanin 1 [8,15]. In bulk endocytosis, following high neuronal stimulation a large membranous structure invaginates from the plasma membrane creating an endosome intermediary where cargo sorting and subsequent synaptic vesicle regeneration can occur [16]. Large-scale invagination of the plasma membrane allows the endosomal structure to retain high PtdsIns(4,5)P2 lipid levels, which can lead to subsequent clathrin-mediated synaptic vesicle budding from the endosome [14].
The regeneration of synaptic vesicles through SVE is essential to sustain neurotransmission and is therefore a highly regulated process. SVE is controlled by a large group of structurally distinct proteins termed dephosphins, which are regulated through phosphorylation–dephosphorylation events [10]. Classically these proteins include dynamin, synaptojanin 1, amphiphysin 1 and 2, and epsin among others [10]. Although clathrin, AP-2, endophilin A1, and auxilin have now been shown to be phosphorylated by several different kinases, whether calcineurin is the main regulatory phosphatase for these proteins remains unknown [12,17,18]. Under basal conditions, SVE proteins are constitutively phosphorylated, which inhibits them from associating with other proteins in the endocytic pathway [10]. When the neuron is stimulated, Ca2+ flow into the cell activates Ca2+-dependent calcineurin activity, which rapidly dephosphorylates endocytic proteins to enable their interaction and recruitment to endocytic sites [10,19,20]. SVE protein inactivation through rephosphorylation occurs on a much slower timescale in a stepwise progression. This process has been shown to be mediated in part by Cdk5 and Minibrain kinase [21,22]. However, there remains a significant gap in our knowledge of other possible protein kinases that are responsible for the proper control of SVE.
Mutations of Synaptic Genes in Parkinsonism
Multiple PD-linked genes involved in SVE have recently been identified, suggesting that defective SVE plays an important role in PD pathogenesis. These include mutations in DNAJC6 (auxilin) and SYNJ1 (synaptojanin 1), which were initially described in atypical-Parkinsonism patients [23–29]. Homozygosity mapping of two patients with juvenile Parkinsonism revealed a deleterious splice-site mutation, c.801–2 A>G, in DNAJC6, that led to a significant decrease in mRNA levels [23]. A separate study found a patient with a DNAJC6 homozygous truncating mutation, Q734X, leading to 20% loss of the C terminus, including its functional J domain responsible for binding hsc70 [24]. Thus far, these mutations have linked DNAJC6 to juvenile cases of atypical Parkinsonism. However, recent investigations reported additional DNAJC6 mutations, R927G and T741T, linked to early-onset PD cases [25]. These mutations resulted in lowered auxilin expression and are predicted to decrease its overall function [25].
In addition, R258Qand R459P mutations in SYNJ1were recently reported in several independent studies to be associated with juvenile or early-onset PD [26–29]. These mutations are located in the Sac1 domain of synaptojanin 1 and impair its phosphatase activity [26–28]. Interestingly, synaptojanin 1 haploinsufficiency led to delayed SVE in mouse midbrain dopaminergic but not cortical neurons suggesting that loss of synaptojanin 1 function could, in part, contribute to dopaminergic neuron vulnerability in PD pathogenesis [30]. Last, SH3GL2 (endophilin A1) was identified in a PD risk locus in a large-scale GWAS meta-analysis, linking yet another gene involved in SVE regulation to PD [31]. Collectively, these synaptic endocytic genes implicate defective SVE as a contributor to the degeneration of midbrain dopaminergic neurons in patients.
Animal knockout mouse models of DNAJC6, SYNJ1, and SH3GL2 have all exhibited endocytic defects at the synapse, highlighting the importance of proper SVE control in maintaining axon terminal integrity [9,32,33]. It was previously reported that the presynaptic compartment of auxilin knockout mice displayed characteristics of defective SVE including reduced synaptic vesicle density and increased CCVs and membraneless clathrin cages [32]. Interestingly, follow-up studies found that embryonic mouse lethality resulting from genetic ablation of both GAK, an auxilin homolog and PD risk gene, and DNAJC6 could be rescued by overexpression of the GAK C-terminal fragment carrying both the clathrin-binding and J domains [34–36]. Although GAK and auxilin have redundant clathrin-uncoating actions in the cell, these data suggest that GAK overexpression can potentially mitigate auxilin dysfunction in PD. Furthermore, lack of auxilin in Drosophila led to specific age-related locomotor deficits and accelerated αSynuclein (αSyn)-mediated dopaminergic neuron loss in this model [37]. This result implies that nigral neurons are more sensitive to loss of auxilin function. Consistent with previous reports, the synapses of SYNJ1 R258Q knock-in mice revealed drastic endocytic defects and higher levels of endocytic intermediates such as CCVs [11]. Additionally, dystrophic axon terminals were observed in the dorsal striatum of these mice, which is a primary site of SNc dopaminergic neuron projections in the brain [11]. Moreover, auxilin and parkin (PRKN) protein levels were reported to be elevated in synaptojanin 1-mutant mice [11]. Furthermore, endophilin A1 knockout mouse models also reported elevated parkin levels as well as accumulated CCVs, highlighting a potential functional connection between these SVE proteins and parkin [9,38].
The Role of αSyn in SVE
αSyn, encoded by SNCA, is a soluble protein located in the presynaptic terminal that is involved in the regulation of synaptic activity, plasticity, synaptic vesicle pool maintenance, and trafficking [39,40]. αSyn function has traditionally been linked to synaptic vesicle exocytosis, although whether it positively or negatively regulates this process remains controversial [39]. Recent studies have also pointed to a role for αSyn in the regulation of synaptic vesicle formation. Acute injection of human monomeric wild-type (WT) αSyn into lamprey synaptic terminals coupled with intense stimulation of neurons was shown to significantly reduce endocytic rates leading to accumulation of CCVs [41,42]. An additional study found that expression of dimeric WT αSyn led to the formation of structurally distinct clathrin-coated pits [43]. Taken together, these studies suggest that different αSyn conformations may have divergent effects on SVE, such that multimeric αSyn may affect the early stages of CCV formation and fission whereas monomeric αSyn may influence the final clathrin-uncoating step. Moreover, a triple-knockout mouse model of all synuclein isoforms (α, β, and γ) affected the kinetics of SVE leading to impaired endocytic capacity at steady states, whereas no marked changes were observed in the rates of exocytosis [44]. The reported deficiencies were rescued by separate overexpression of each isoform, implying that all three synucleins have redundant endocytic roles at the synapse. Further investigation revealed that synucleins are important mediators of presynaptic terminal size and organizers of distinct synaptic vesicle pools by specifically regulating synaptic vesicle tethers to the plasma membrane and to each other [45]. Together, these studies suggest that αSyn also has an important role in endocytosis by mediating synaptic vesicle regeneration following neuronal stimulation.
PD-Linked Genes as Regulators of SVE
While the discovery of these synaptic genes directly implicates synaptic dysfunction in PD pathogenesis, recent studies have also proposed that other PD genes, including LRRK2, VPS35, and PRKN (parkin), may also be potential regulators of SVE. Normal LRRK2 serine/threonine kinase activity is critical for proper SVE, as chemical inhibition of LRRK2 was shown to delay endocytosis [46]. In addition, LRRK2 mutant mice displayed an accumulation of CCVs and decreased synaptic vesicle density in dopaminergic terminals [47]. Many synaptic interacting partners and substrates have been described for LRRK2, but those specifically involved in SVE regulation have only recently been identified. Dynamin, which mediates the fission of CCVs from the plasma membrane, was identified as a LRRK2 interactor, highlighting a potential role for LRRK2 in the regulation of dynamin GTPase activity [7,48]. Moreover, LRRK2 was shown to phosphorylate endophilin A1 at positions T73 and S75 located in its BAR domain [17,18]. Subsequent investigations confirmed that LRRK2-mediated phosphorylation of endophilin A1 at S75 acted as a critical switch in mediating endophilin A1 function at the synapse [18]. Recent synaptic proteomic analysis of LRRK2 mutant knock-in Drosophila models has also identified synaptojanin 1 as a LRRK2 kinase substrate [49]. LRRK2 phosphorylates synaptojanin 1 at positions T1131 and T1205 located in its proline-rich sequence recognition domain resulting in defective synaptojanin 1–endophilin A1 interaction [30,49]. Interestingly, another study found that phosphorylation of synaptojanin 1 at S1029 mediates the regeneration of distinct synaptic vesicle pools, suggesting that phosphorylation–dephosphorylation events with different residues can result in protein involvement in separate parts of SVE [50]. Lastly, we recently identified auxilin as a novel substrate for LRRK2 kinase activity in iPSC-derived dopaminergic neurons [12]. We found that S627, located in auxilin’s clathrin-binding domain, is an LRRK2-mediated phosphorylation site and that dephosphorylation of this site led to increased auxilin association with clathrin [12]. Thus, LRRK2 kinase activity regulates several aspects of SVE, which could be exacerbated by LRRK2 disease-linked mutations, leading to downstream toxic effects that contribute to the degeneration of dopaminergic neurons.
LRRK2 has also been functionally linked to VPS35, a PD-linked gene that encodes a major component of the retromer complex involved in the recycling of proteins from the endosome/lysosome to the trans-Golgi network and from the endosome to the plasma membrane [51,52]. PD-associated mutations in VPS35, which display decreased function, functionally elevate WT LRRK2 kinase activity [51,53]. Additionally, loss of the Drosophila homolog VPS35 leads to deficits in synaptic vesicle recycling involving LRRK2 that could be rescued on VPS35 or LRRK2 overexpression [54]. Importantly, using unbiased approaches, LRRK2 has been shown to phosphorylate a subset of Rab GTPase proteins [55]. Although the specific function of each Rab protein remains to be elucidated, many of them may be involved in endosomal trafficking and sorting, which would ultimately allow the compartment to regulate the levels of regenerating synaptic vesicles and proteins in the synapse [16,52].
Another PD gene linked to SVE regulation encodes the E3 ubiquitin ligase parkin, which has been shown to ubiquitinate endophilin A1 as well as its major binding partners dynamin and synaptojanin 1 [38]. Ubiquitination by parkin may be responsible for either modulating the endophilin A1 expression level, as previously reported, or regulating its ability to bind, recruit, and engage its interaction partners at the plasma membrane or CCV interface [38]. Ubiquitination-interaction motifs were first mapped in the endocytic protein epsin, which was recently identified as an accessory factor in SVE, supporting the idea that ubiquitination is necessary to facilitate endocytic functions [56,57]. Furthermore, ubiquitination of epsin rendered the protein partially resistant to proteasomal degradation thereby allowing it to interact with other endocytic proteins [56]. Interestingly, parkin was recently shown to ubiquitinate VPS35, which did not result in proteasomal degradation of the protein [58]. Therefore, parkin may also alter SVE through retromer-dependent endosomal sorting. These data suggest that the parkin E3 ubiquitin ligase function is potentially an important regulator of synaptic vesicle recycling and that PRKN mutations that lead to loss of parkin function may negatively affect the stepwise progression of SVE.
While there have been no reports (to our knowledge) of parkin knockout models exhibiting endocytic defects at the synapse, one consequence of improper SVE would be impairments in dopamine (DA) neurotransmission and metabolism. Normally, DA is packaged into synaptic vesicles to prevent its accumulation and oxidation in the cytosol [59]. Interestingly, dopaminergic neurons derived from parkin patients demonstrate increased intracellular DA, suggesting that DA packaging or metabolism in parkin-mutant neurons may be impaired [60]. Parkin-deficient mice also revealed a decrease in evoked extracellular striatal DA [61,62]. However, conflicting studies reported an increased in spontaneous DA release and dampened DA uptake into the cell [63]. As these differences may be due to the experimentation methods employed in the studies, the role of parkin in SVE remains to be explored. Taken together, these studies reveal the existence of multiple layers of SVE regulation modulated by PD-linked genes and suggest that these pathways may functionally interact in the process of neurodegeneration.
SVE Dysfunction in Dopaminergic Neurodegeneration
As SVE is not unique to dopaminergic neurons of the ventral midbrain, the specific vulnerability of this neuronal population in PD to deficits in SVE has not been clear. PD-linked SYNJ1 R258Q mouse models revealed delays in SVE and marked changes in dopaminergic axon terminals in the dorsal striatum, highlighting a region-specific vulnerability of these neurons to synaptojanin 1 dysfunction [11]. Additionally, decreased synaptic densities and accumulation of CCVs, specifically in dopaminergic neurons, were also reported in SYNJ1 heterozygous knockout and LRRK2 G2019S transgenic mice leading to dystrophic axons and selective degeneration of these neurons [30].
One possibility may be the sensitivity of dopaminergic neurons to the accumulation of cytosolic DA. Acute overexpression of human αSyn, on of striatal terminals in the absence of nigral cell death [41,43,44,64]. This may be due in part to αSyn-induced DAleakage from synaptic vesicles [65,66]. DA is packaged into synaptic vesicles regenerated from SVE using a proton gradient created by vATPases located on the membrane surface of synaptic vesicles [67]. In contrast to previous work suggesting that synaptic vesicle acidification is needed for removal of the clathrin coat, a recent studyfoundthatacidification of the vesicle couldnotoccur while the clathrincoatwas retained [68]. Specifically, vATPase activity is sterically inhibited by theclathrin coat and is restoredonce the coat is removed through the function of auxilin [68]. This result suggests that a delay in SVE could lead to improper packaging of DA into vesicles leading to increased cytosolic DA, ultimately contributing to dopaminergic neurodegeneration beginning at the axon terminals.
Moreover, SNc dopaminergic neurons are subject to increased levels of oxidative stress resulting from the high metabolic activity that is required to support their extensive axonal arborization [69,70]. As a consequence of oxidative phosphorylation, toxic reactive oxygen species (ROS), including oxygen radicals, semiquinones, quinones, and H2O2, are produced and collectively contribute to oxidative stress [71]. These ROS can react with cytosolic DA, leading to the formation of highly reactive DA quinones [72,73]. In addition, cytosolic DA metabolized by monoamine oxidase (MAO) located in the outer mitochondrial membrane also produces H2O2 byproducts, thus further exacerbating accumulation of ROS and toxic DA quinones [74]. Nigral dopaminergic neurons also employ protective mechanisms such as the formation of neuromelanin and sequestration of DA into synaptic vesicles to minimize DA oxidation [59]. We recently showed that PD patient neurons carrying mutations in DJ-1 that lead to loss of mitochondrial antioxidant function exhibit increased levels of oxidized DA, which further result in downstream toxic effects including lysosomal dysfunction and aSyn accumulation [75]. Specifically, oxidized DA was also found to modify the lysosomal enzyme glucocerebrosidase on a critical cysteine residue leading to a decrease in its enzymatic activity [75]. We thus hypothesize that delays in SVE can lead to increased levels of unpackaged, cytosolic DA that is then subject to oxidation. Mutant LRRK2-mediated auxilin dysfunction resulted in increased levels of oxidized DA in patient-derived dopaminergic neurons that was partially rescued by expression of auxilin [12]. Taken together, these studies highlight a connection between synaptic, mitochondrial, and lysosomal function and suggest that defects in these pathways may synergize to contribute to PD pathogenesis (Figure 2, Key Figure).
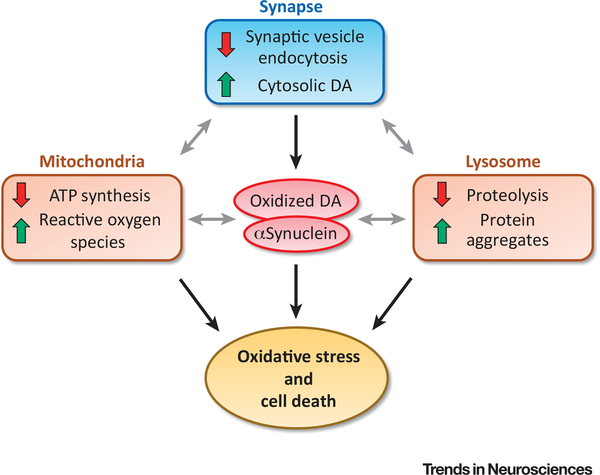
Figure 2.
Recent work has identified a pathway for cytosolic oxidized dopamine (DA) and αSynuclein accumulation due to dysfunction in synaptic vesicle endocytosis in human-derived dopaminergic neurons [12]. These byproducts can further inhibit mitochondrial function by impairing ATP production and increase reactive oxygen species production via mitochondrially mediated metabolism of cytosolic DA. In addition, they can contribute to lysosomal dysfunction, which may further involve defective proteolytic turnover of synaptic proteins and the accumulation of insoluble protein aggregates. It is conceivable that the convergence of synaptic, mitochondrial, and lysosomal dysfunction may exacerbate cytosolic DA and αSynuclein accumulation and ultimately result in cell death in Parkinson’s disease.
Key Figure Deficits in Synaptic Vesicle Endocytosis Potentially Mediate Dopaminergic Neurodegeneration through Intersections with Mitochondrial and Lysosomal Dysfunction
Another possibility for the selective vulnerability of nigral dopaminergic neurons to SVE deficits is their pacemaking function, which modulates the sustained release of DA to targeted brain regions such as the striatum [76]. Pacemaking activity leads to large influxes of Ca2+ into the neuron, which can stimulate the Ca2+-dependent calcineurin to initiate endocytosis of synaptic vesicles [10]. Mitochondria located at the synapse also contribute to the maintenance of pacemaker activity by buffering Ca2+ when cytosolic levels are high [71]. Of note, increased mitochondrial calcium stores have been shown to lead to mitochondrial dysfunction, suggesting that excessive cytosolic DA, ROS accumulation, and Ca2+ buffering by mitochondria may together compromise nigral dopaminergic function [71,74].
Dysfunction of Mitochondria and Autophagy at the Synapse
Mitochondria perform crucial energetic roles for active neurons by providing ATP to power SVE, which replenishes synaptic vesicles to sustain repeated release of neurotransmitters [77–79]. In agreement with this, oligomycin treatment of cells to block mitochondrial ATP synthesis led to complete cessation of SVE following sustained high-frequency stimulation [80]. Furthermore, inhibition of mitochondrial fission, which reduces mitochondrial mass in axon terminals, led to the preferential degeneration of nigral dopaminergic neurons in mice [81]. This degeneration was initiated at presynaptic terminals through loss of the striatal tyrosine hydroxylase (TH) signal, while ~65% of TH-positive neurons in the SNc remained [81].
Besides the regulation of SVE at the synapse, several endocytic genes have also been identified as critical modulators of synaptic autophagy, a pathway for the maintenance of synaptic protein homeostasis and turnover via the lysosome following neurotransmission [82]. Altered synaptojanin 1 function blocked autophagosome maturation in presynaptic terminals through the accumulation of Atg18a, an autophagy-related protein, in SYNJ1 R258Q patient-derived dopaminergic neurons [83]. This dysfunction ultimately led to dopaminergic neuron loss in the SYNJ1 R258Q Drosophila model [83]. Another study demonstrated that endophilin A1 function is required for synaptic autophagy through its recruitment of Atg3 to vesicular membranes following LRRK2-mediatedphosphorylation[84]. Wehaveshown thatoneconsequenceof defectiveSVE is the accumulation of oxidized DA in human-derived dopaminergic neurons [12,75]. Lysosomes may contribute to synaptic integrity by degrading oxidized DA adducts through the synaptic autophagy pathway, although the exact mechanisms are unclear [72,82]. Therefore, turnover of synaptic proteins and the elimination of damaged mitochondria and oxidized DA by lysosomes via synaptic autophagy are critical in maintaining dopaminergic synapses [82,85].
Last, we recently identified the dynamic formation of interorganelle mitochondria–lysosome contact sites, which were distinct from mitophagy or lysosomal engulfment of mitochondria [86]. These contact sites may further mediate mitochondrial and lysosomal dysfunction in PD, in addition to other previously identified contacts such as ER–mitochondria contacts [87,88]. Together, these contacts may regulate synaptic Ca2+ buffering and exchange and additionally regulate SVE by modulating Ca2+-dependent calcineurin activity during SVE [10,19,20,89]. Thus, synaptic, mitochondrial, and lysosomal dysfunction may synergize during PD pathogenesis.
Concluding Remarks
A major hurdle to the development of neuroprotective therapies for PD is an incomplete understanding of key pathways and targets for therapeutic development. The recent emergence of genetic forms of PDhas highlighted the importance of major molecular pathways in the pathogenesis of disease, including synaptic, mitochondrial, andlysosomaldysfunction.Despitethisnewevidence, there remain significant gaps in our understanding of the consequences of synaptic dysregulation andhowdeficitsinthispathwayareconnectedtootherpathogenicprocessessuchasmitochondrial and lysosomal dysfunction (see Outstanding Questions). In addition, there remains a need to better understand the link between deficits in synaptic, mitochondrial, and lysosomal dysfunction and the selective vulnerability of SNc dopaminergic neurons in PD. Ultimately, further investigation of these molecular pathways will be necessary to identify key targets for therapeutic intervention.
Outstanding Questions.
What are the key contributors to the intrinsic vulnerability of specific neuronal populations in Parkinson’s disease (PD)?
How does the contribution of mitochondrial and lysosomal dysfunction factor into the vulnerability of nigral dopaminergic neurons?
Does SVE dysfunction affect non-dopaminergic neurons in PD?
Which of these cellular pathways are first affected in PD pathogenesis and what is the mechanism of disease progression at the molecular level?
LRRK2 and PRKN mutations have been recently linked to synaptic dysfunction through their roles in the regulation of synaptic vesicle endocytosis (SVE) proteins. Do other PD-linked genes, such as DJ-1, PINK1, VPS35, and GBA1 directly regulate SVE or perhaps indirectly, for instance through perturbation of mitochondrial (DJ-1 and PINK1) or lysosomal (VPS35 and GBA1) function, which are necessary to maintain synaptic integrity?
Recent studies have suggested that classical SVE genes also have endocytosis-independent functions at the synapse (i.e., synaptic autophagy). Besides the regeneration of synaptic vesicles, are these independent mechanisms important in maintaining protein concentrations in specific compartments of the synapse?
Many cellular processes, particularly some at the synapse, involve membranes that depend on highly regulated lipid concentrations (i.e., PtdsIns). What are the mechanisms that control lipid concentrations at the synapse? Are PD-linked genes and endocytosis-independent functions (i.e., synaptic autophagy) involved in this process?
Highlights.
Emerging genetic and mechanistic studies link synaptic, mitochondrial, and lysosomal dysfunction as major contributors to the degeneration of midbrain dopaminergic neurons.
Recently, endocytic genes (DNAJC6, SYNJ1, and SH3GL2) have been linked to Parkinson’s disease pathogenesis or identified as a risk factor for the disease, implicating a role for impaired synaptic vesicle endocytosis (SVE) in neurodegeneration.
Among the modifiers of proteins involved in SVE are LRRK2 and parkin. LRRK2’s and parkin’s involvement in this context is through their phosphorylation and ubiquitination actions, respectively.
Dysfunction in SVE in dopaminergic neurons can lead to increased levels of unpackaged, cytosolic DA that is subject to oxidation and pathogenic downstream effects
Acknowledgments
The authors were supported by NIH grants as follows: M.N. by 2T32AG020506-16, Y.C.W. by K99 NS109252, D.Y. by T32 NS041234, and D.K. by R01 NS076054 and R37 NS096241.
References
- 1.Poewe W et al. (2017) Parkinson disease. Nat. Rev. Dis. Primers 3, 17013. [DOI] [PubMed] [Google Scholar]
- 2.Saheki Y and De Camilli P (2012) Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol 4, a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly BT et al. (2014) Clathrin adaptors. AP2 controls clathrin polymerization with a membrane-activated switch. Science 345, 459–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pechstein A et al. (2015) Vesicle uncoating regulated by SH3–SH3 domain-mediated complex formation between endophilin and intersectin at synapses. EMBO Rep. 16, 232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstreken P et al. (2003) Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40, 733–748 [DOI] [PubMed] [Google Scholar]
- 6.Schuske KR et al. (2003)Endophilin is requiredforsynapticvesicle endocytosis by localizing synaptojanin. Neuron 40, 749–762 [DOI] [PubMed] [Google Scholar]
- 7.Sundborger A et al. (2011) An endophilin–dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J. Cell Sci 124, 133–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe S et al. (2018) Synaptojanin and endophilin mediate neck formation during ultrafast endocytosis. Neuron 98, 1184–1197.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milosevic I et al. (2011) Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72, 587–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousin MA and Robinson PJ (2001) The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 24, 659–665 [DOI] [PubMed] [Google Scholar]
- 11.Cao M et al. (2017) Parkinson Sac domain mutation in synaptojanin 1 impairs clathrin uncoating at synapses and triggers dystrophic changes in dopaminergic axons. Neuron 93, 882–896.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen M and Krainc D (2018) LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A 115, 5576–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousin MA (2017) Integration of synaptic vesicle cargo retrieval with endocytosis at central nerve terminals. Front. Cell. Neurosci 11, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milosevic I (2018) Revisiting the role of clathrin-mediated endoytosis in synaptic vesicle recycling. Front. Cell. Neurosci. 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y et al. (2014) A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis. eLife 3, e01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoopmann P et al. (2010) Endosomal sorting of readily releasable synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A 107, 19055–19060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matta S et al. (2012) LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75, 1008–1021 [DOI] [PubMed] [Google Scholar]
- 18.Ambroso MR et al. (2014) Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation. Proc. Natl. Acad. Sci. U. S. A 111, 6982–6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XS et al. (2014) Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 7, 982–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottrell JR et al. (2016) Calcineurin A(is a functional phosphatase that modulates synaptic vesicle endocytosis. J. Biol. Chem 291, 1948–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CK et al. (2014) Activity-dependent facilitation of synaptojanin and synaptic vesicle recycling by the Minibrain kinase. Nat. Commun 5, 4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan TC et al. (2003) Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol 5, 701–710 [DOI] [PubMed] [Google Scholar]
- 23.Edvardson S et al. (2012) A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile Parkinsonism. PLoS One 7, e36458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koroglu C et al. (2013) DNAJC6 is responsible for juvenile Parkinsonism with phenotypic variability. Parkinsonism Relat. Disord 19, 320–324 [DOI] [PubMed] [Google Scholar]
- 25.Olgiati S et al. (2016) DNAJC6 mutations associated with early-onset Parkinson’s disease. Ann. Neurol. 79, 244–256 [DOI] [PubMed] [Google Scholar]
- 26.Quadri M et al. (2013) Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum. Mutat 34, 1208–1215 [DOI] [PubMed] [Google Scholar]
- 27.Krebs CE et al. (2013) The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum. Mutat 34, 1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olgiati S et al. (2014) PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics 15, 183–188 [DOI] [PubMed] [Google Scholar]
- 29.Kirola L et al. (2016) Identification of a novel homozygous mutation Arg459ProinSYNJ1geneofanIndianfamilywithautosomalrecessive juvenile Parkinsonism. Parkinsonism Relat. Disord 31, 124–128 [DOI] [PubMed] [Google Scholar]
- 30.Pan PY et al. (2017) Parkinson’s disease-associated LRRK2 hyperactive kinase mutant disrupts synaptic vesicle trafficking in ventral midbrain neurons. J. Neurosci 37, 11366–11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang D et al. (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet 49, 1511–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yim YI et al. (2010) Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc. Natl. Acad. Sci. U. S. A 107, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim WT et al. (2002) Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc. Natl. Acad. Sci. U. S. A 99, 17143–17148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalls MA et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46, 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park BC et al. (2015) The clathrin-binding and J-domains of GAK support the uncoating and chaperoning of clathrin by Hsc70 in the brain. J. Cell Sci 128, 3811–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beilina A et al. (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl. Acad. Sci. U. S. A 111, 2626–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song L et al. (2017) Auxilin underlies progressive locomotor deficits and dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Cell Rep. 18, 1132–1143 [DOI] [PubMed] [Google Scholar]
- 38.Cao M et al. (2014) Upregulation of parkin in endophilin mutant mice. J. Neurosci 34, 16544–16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lautenschlager J et al. (2017) α-Synuclein – regulator of exocytosis, endocytosis, or both? Trends Cell Biol 27, 468–479 [DOI] [PubMed] [Google Scholar]
- 40.Lautenschlager J et al. (2018) C-terminal calcium binding of α-Synuclein modulates synaptic vesicle interaction. Nat. Commun 9, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busch DJ et al. (2014) Acute increase of α-Synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. Mol. Biol. Cell 25, 3926–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eguchi K et al. (2017) Wild-type monomeric α-Synuclein can impair vesicle endocytosis and synaptic fidelity via tubulin polymerization at the calyx of Held. J. Neurosci. 37, 6043–6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros AT et al. (2017) α-Synuclein dimers impair vesicle fission during clathrin-mediated synaptic vesicle recycling. Front. Cell. Neurosci 11, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas KJ et al. (2014) Synucleins regulate the kinetics of synaptic vesicle endocytosis. J. Neurosci 34, 9364–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vargas KJ et al. (2017) Synucleins have multiple effects on presynaptic architecture. Cell Rep. 18, 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arranz AM et al. (2015) LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. J. Cell Sci 128, 541–552 [DOI] [PubMed] [Google Scholar]
- 47.Xiong Y et al. (2018) Robust kinase- and age-dependent dopaminergic and norepinephrine neurodegeneration in LRRK2 G2019S transgenic mice. Proc. Natl. Acad. Sci. U. S. A 115, 1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stafa K et al. (2014) Functional interaction of Parkinson’s disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum. Mol. Genet 23, 2055–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam MS et al. (2016) Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson’s disease. Hum. Mol. Genet 25, 5365–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng J et al. (2016) Phosphorylation of synaptojanin differentially regulates endocytosis of functionally distinct synaptic vesicle pools. J. Neurosci 36, 8882–8894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Follett J et al. (2014) The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic 15, 230–244 [DOI] [PubMed] [Google Scholar]
- 52.Burd C and Cullen PJ (2014) Retromer: a master conductor of endosome sorting. Cold Spring Harb. Perspect. Biol 6, a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mir R et al. (2018) The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J 475, 1861–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoshita T et al. (2017) Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum. Mol. Genet. 26, 2933–2948 [DOI] [PubMed] [Google Scholar]
- 55.Steger M et al. (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5, e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oldham CE et al. (2002) The ubiquitin-interacting motifs target the endocytic adaptor protein epsin for ubiquitination. Curr. Biol 12, 1112–1116 [DOI] [PubMed] [Google Scholar]
- 57.Kyung JW et al. (2016) Epsin1 modulates synaptic vesicle retrieval capacity at CNS synapses. Sci. Rep 6, 31997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams ET et al. (2018) Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum. Mol. Genet 27, 3189–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sulzer D et al. (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 97, 11869–11874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang H et al. (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat. Commun. 3, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itier JM et al. (2003) Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum. Mol. Genet 12, 2277–2291 [DOI] [PubMed] [Google Scholar]
- 62.Oyama G et al. (2010) Impaired in vivo dopamine release in parkin knockout mice. Brain Res. 1352, 214–222 [DOI] [PubMed] [Google Scholar]
- 63.Chung SY et al. (2016) Parkin and PINK1 patient iPSC-derived midbrain dopamine neurons exhibit mitochondrial dysfunction and α-Synuclein accumulation. Stem Cell Rep. 7, 664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phan JA et al. (2017) Early synaptic dysfunction induced by α-Synuclein in a rat model of Parkinson’s disease. Sci. Rep 7, 6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plotegher N et al. (2017) DOPAL derived α-Synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep 7, 40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ysselstein D et al. (2015) Effects of impaired membrane interactions on α-Synuclein aggregation and neurotoxicity. Neurobiol. Dis 79, 150–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eiden LE et al. (2004) The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 447, 636–640 [DOI] [PubMed] [Google Scholar]
- 68.Farsi Z et al. (2018) Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity. eLife 7, e32569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pacelli C et al. (2015) Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr. Biol 25, 2349–2360 [DOI] [PubMed] [Google Scholar]
- 70.Giguere N et al. (2018) Comparative analysis of Parkinson’s disease-associated genes in mice reveals altered survival and bioenergetics of parkin-deficient dopamine neurons. J. Biol. Chem 293, 9580–9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaichick SV et al. (2017) The role of Ca2+ signaling in Parkinson’s disease. Dis. Model. Mech 10, 519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dias V et al. (2013) The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis 3, 461–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jana S et al. (2011) Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta 1812, 663–673 [DOI] [PubMed] [Google Scholar]
- 74.Meiser J et al. (2013) Complexity of dopamine metabolism. Cell Commun. Signal 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burbulla LF et al. (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357, 1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzman JN et al. (2009) Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci 29, 11011–11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sobieski C et al. (2017) Differential presynaptic ATP supply for basal and high-demand transmission. J. Neurosci. 37, 1888–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reeve AK et al. (2018) Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson’s disease. NPJ Parkinsons Dis. 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rangaraju V et al. (2014) Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pathak D et al. (2015) The role of mitochondrially derived ATP in synaptic vesicle recycling. J. Biol. Chem. 290, 22325–22336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berthet A et al. (2014) Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J. Neurosci 34, 14304–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soukup SF et al. (2018) Parkinson’s disease: convergence on synaptic homeostasis. EMBO J. 37, e98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vanhauwaert R et al. (2017) The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J. 36, 1392–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soukup SF et al. (2016) A LRRK2-dependent endophilinA phosphoswitch is critical for macroautophagy at presynaptic terminals. Neuron 92, 829–844 [DOI] [PubMed] [Google Scholar]
- 85.Ashrafi G et al. (2014) Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and parkin. J. Cell Biol 206, 655–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong YC et al. (2018) Mitochondria–lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirabayashi Y et al. (2017) ER–mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science 358, 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y et al. (2017) Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. U. S. A 114, E4859–E4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marland JR et al. (2016) Mitochondrial calcium uptake modulates synaptic vesicle endocytosis in central nerve terminals. J. Biol. Chem 291, 2080–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]