Abstract
Acute ischemic stroke is inadequately treated in the USA and worldwide due to a lengthy history of neuroprotective drug failures in clinical trials. The majority of victims must endure life-long disabilities that not only affect their livelihood, but also have an enormous societal economic impact. The rapid development of a neuroprotective or cytoprotective compound would allow future stroke victims to receive a treatment to reduce disabilities and further promote recovery of function. This opinion article reviews in detail the enormous costs associated with developing a small molecule to treat stroke, as well as providing a timely overview of the cell-death time-course and relationship to the ischemic cascade. Distinct temporal patterns of cell-death of neurovascular unit components provide opportunities to intervene and optimize new cytoprotective strategies. However, adequate research funding is mandatory to allow stroke researchers to develop and test their novel therapeutic approach to treat stroke victims.
Keywords: Translational, Cytoprotection, Neuroprotection, Neuroprotective, Cytoprotective, Brain, Stroke, Hemorrhage, SAH, ICH, Clinical trial, NIHSS, STAIR, RIGOR, Transparency, Cost, Animal research, Drug discovery, Cell-death cascade, rt-PA
Introduction
Stroke, in particular acute ischemic stroke is a major problem worldwide that is escalating as the worldwide population dramatically shifts to an aged state. With a global stroke incidence of 10.3–16.9 million annually, at least 5.9 million stroke-related deaths, and 25.7–33 million survivors that require some form of therapy or novel treatment, the former has still not been achieved and may not be achieved for decades if the current pace of research and level of funding are maintained.
There are two ways to address the societal problem related to stroke, either prevent the occurrence of stroke, or design practical efficacious therapies to treat stroke. The discovery and development of the elusive neuroprotective therapy, which will herein be called “cytoprotective” for stroke is a time intensive process that is high cost and it can be high reward when a therapy is translated into the target patient population. There are no short cuts, no inexpensive drug screens, and no inexpensive model efficacy testing if the development process is to be transparent and adheres to current elevated research standards required for publication in Translational stroke research, stroke, Journal of Cerebral Blood Flow and Metabolism, and other high impact stroke-related journals.
The recent success of endovascular procedures with an extended therapeutic window has re-energized stroke cytoprotection research and increased optimism that we can now make advances, consolidate efforts, and develop an effective cytoprotective therapy to be used in combination with endovascular procedures, or with recombinant tissue plasminogen activator (rt-PA), the only Food and Drug administration (FDA)-approved drug, a biologic for stroke. If we can demonstrate additional and significant clinical improvement with a cytoprotective compound in standardized translational embolic stroke models, in patients undergoing thrombolytic/endovascular procedures, then it should also be proposed to determine the clinical efficacy of the therapy. Another scenario is that the cytoprotective compound may reduce the side-effects of thrombolysis and make the treatment safer, thus reducing complications in stroke victims.
Stroke Is a High Cost Burden to Society
Stroke, cerebral infarction, and hemorrhagic stroke, have been recognized as a disease in man throughout the ages; various descriptions can be found in Hippocratic transcripts [1], and through the ages [2–8], most recently in the form of American Heart Association (AHA) updates [9–16]. Stroke is the fifth leading cause of mortality and leading cause of adult morbidity in the United States, and it is estimated that annually 800,000 people suffer a stroke in the USA [16], an incidence rate of 146–228 per 100,000. In Canada, there is an annual estimate of 62,000 strokes, an incidence rate of 92–197 per 100,000. The cost of stroke in North America range from $3.6 billion (Canada) to $34 billion (US) [17]. Table 1 is populated with stroke incidence and estimated cost data for selected locations worldwide. Throughout the world, stroke is still a leading cause of mortality and morbidity. In the United Kingdom (UK), stroke incidence is similar to both Canada and the USA, an incidence of 115–150 per 100,000, with 152,000 reported strokes in 2016. In two major Asian countries, stroke is more prevalent than in the USA, UK, or Canada with an incidence rate of 301–517 per 100,000 in China and an annual incidence as high as 3200 per 100,000 in Japan [18, 19].
Table 1.
Stroke incidence
| Country (data year) | Total strokes | Stroke incidence (per 100,000) | Website or reference |
|---|---|---|---|
| Australia (2009) | 381,400 | 84–122 | http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4429.0main+features100262009 |
| Canada (2009) | 62,000 | 92–197 | http://www.phac-aspc.gc.ca/cd-mc/cvd-mcv/sh-fs-2011/index-eng.php; [18] |
| China (2010) | 1.3 million | 301–517 | http://www.world-heart-federation.org/cardiovascular-health/stroke/ [18, 19] |
| India (2010) | NA | 153–251 | [18, 19] |
| Japan (2010–2012) | NA | 156–235 [192.47–3200] | [18, 19] |
| United Kingdom (2010–2016) | 100–132 | https://www.stroke.org.uk/sites/default/files/state_of_the_nation_2016_110116_0.pdf; [18] | |
| USA (2015) | 795,000 (610,000 new) | 146–228 | http://www.cdc.gov/stroke/facts.htm; [16] |
| Worldwide (2013) | 10.3–15 million First strokes appx. 16.9 million | http://www.world-heart-federation.org/cardiovascular-health/stroke/; [18] |
NA Not available
The most recent Global Burden of Disease (GBD Stroke) Atlas and Demographic and Epidemiologic Drivers documents from Roth, Mensah and colleagues [20] conclude that the burden of stroke continues to increase, and this is threatening worldwide sustainability. While 10.3 million strokes occur annually, the GBD estimated that there were 25.7 million stroke survivors in 2013 [18]; 67% ischemic/33% hemorrhagic. The document clearly demonstrates a significant increase in ischemic-stroke related deaths measured between 1990 and 2013; a 50.2% increase globally [18].
Endovascular Procedures
Efficacy
The 2015–2016 endovascular trials, Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR. CLEAN) [21], Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) [22], Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT) [23], Solitaire With the Intention For Thrombectomy as PRIMary Endovascular Treatment (SWIFT PRIME) Trial [24], and Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial (EXTEND-IA) [25] demonstrated that a well-defined, but heterogeneous population of acute ischemic stroke patient with an NIHSS score range of 13–21 upon admission, can be successfully treated by thrombectomy in combination with the application of a thrombolytic.
In the five endovascular procedure trials, rt-PA (Alteplase) or urokinase were administered IVat least 120 min before the thrombectomy procedure. In the trials, thrombolytic administration was 85–145 min after enrollment, and endovascualr procedures were conducted in the embolectomy arm and within 87–145 min in the thrombolysis arm, both well within current FDA-approved guidelines. Moreover, in the embolectomy arm, the initiation of “thrombolysis” occurred well before the procedure. The studies used a range of endovascular times from 190 to 340 min and thrombolytic administration times of 65–180 min. Efficacy was demonstrated by increased functional independence at 90 days, and a corresponding shift in modified Rankin Scale score (mRS) 0–2 (common odds ratio range of 1.7–3.1) in 13.5–31% of patients undergoing the endovascular procedure. Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY) [26] is an unpublished endovascular trial, and Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke (THRACE) [27], which was positive, is pending final publication of the study results. Thrombectomy in patients ineligible for IV rt-PA (THRILL) was terminated early, in November of 2014, after other clinical trials demonstrated efficacy of thrombectomy [28]. Moreover, and Medical Management Versus Medical Management Alone in Wake Up and Late Presenting Strokes (DAWN) is an ongoing trial [29] as is POSITIVE, a trial to include patients ineligible for or refractory to treatment with IV rt-PA [30]. The trial is designed to include appropriate image selection (ASPECTS of >7) and patient treatment with mechanical thrombectomy within 6–12 h of symptom onset.
In summary, thrombectomy has now been shown to be safe in patients with large vessel occlusions, salvageable brain tissue (i.e., large penumbra) with small infarct areas Alberta stroke program early CT score (ASPECTS) score 7–10, and median National Institute of Neurological Disorders and Stroke (NINDS) score of 16–17. Moreover, meta-analysis published by the Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration (Goyal et al. 31) also reveals that optimal reperfusion outcome is achieved when ASPECTS was 6–8 or 9–10 indicating a significant amount of penumbra, when the embolus was located in either the internal carotid artery (ICA) or M1 segment of the middle cerebral artery (MCA), and when intervention was initiated ≤5 h. There were no significant gender differences, but age-dependent improvement was observed. There was benefit in patients 50–80 years of age, but less benefit between 18 and 49 years of age. In Table 2, mRS shift analysis for each of the published embolectomy trials is presented.
Table 2.
Endovascular procedures–cumulative results
| Clinical trial designation | mRS (% per tier) | |||||||
|---|---|---|---|---|---|---|---|---|
| No symptoms -----------------------------------------------►death | ||||||||
| Study | Treatment | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| MR CLEAN | Control (267) | 0 | 6 | 13 | 16 | 30 | 12 | 22 |
| Intervention (233) | 3 | 9 | 21 | 18 | 22 | 6 | 21 | |
| ESCAPE | Control (150) | 7 | 10 | 12 | 15 | 24 | 12 | 19 |
| Intervention (165) | 15 | 21 | 18 | 16 | 13 | 7 | 10 | |
| REVASCAT | Control (103) | 5.8 | 6.8 | 15.5 | 19.4 | 16.5 | 20.4 | 15.5 |
| Intervention (103) | 6.8 | 17.5 | 19.4 | 18.4 | 7.8 | 11.7 | 18.4 | |
| SWIFT PRIME | Control (98) | 9 | 11 | 16 | 17 | 22 | 26 | |
| Intervention (98) | 17 | 26 | 17 | 12 | 15 | 12 | ||
| EXTEND –IA | Control (35) | 17 | 11 | 11 | 11 | 17 | 11 | 20 |
| Intervention (35) | 26 | 26 | 20 | 17 | 3 | 0 | 9 | |
| CUMULATIVE ENDOVASCULAR STUDIES | Control (645 ) | 5.0 | 7.9 | 13.6 | 16.4 | 24.7 | 13.5 | 18.9 |
| Intervention (633 ) | 10.0 | 16.9 | 19.1 | 16.9 | 15.6 | 6.2 | 15.3 | |
| r t-PA eligible | Control (565 ) | 5.1 | 8.1 | 13.8 | 17.5 | 23.7 | 13.3 | 18.4 |
| Intervention (525 ) | 9.9 | 17.1 | 19.4 | 16.6 | 17.3 | 5.9 | 13.7 | |
| rt -PA ineligible | Control (8 0) | 3.6 | 6.2 | 12.5 | 8.7 | 31.2 | 15.0 | 22.5 |
| Intervention (108 ) | 10.2 | 15.7 | 17.6 | 18.5 | 7.4 | 7.4 | 23.1 | |
mRS modified Rankin scale (%)
Highlighted boxes (red) indicate mRS 0–2 functional independence
Highlighted boxes (blue) indicate rt-PA ineligible patients with mechanical embolectomy (mRS 0–2 functional independence and significant improvement)
There is now important and compelling evidence resulting from retrospective analysis of the embolectomy trial database [32–36], demonstrating that embolectomy alone in patients ineligible for rt-PA is beneficial [37] based upon mRS scores, and reperfusion measures (See Table 2). Notably, benefit was observed in patients with ASPECTS scores of 8–9 [37] indicative of large penumbral areas as a physical “substrate” for therapy. In rt-PA ineligible patients, 43.5% of the patients were mRS 0–2 in the intervention arm compared to 22.3% in the control arm.
Cost-Effectiveness
The recent AHA/ASA guidelines now state that patients eligible for IV rt.-PA should receive the thrombolytic whether or not endovascular procedures can be performed because of demonstrated efficacy [38]. The cost-effectiveness of thrombectomy procedures in the United Kingdom, United States and Canada has been documented in a series of recent articles. Xie et al. Canadian-based authors reported that there was a calculated incremental cost-effectiveness ratio (ICER) of $11,990 per quality-adjusted life-years (QALYs) for thrombectomy plus IV thrombolytic [39]. Likewise, Aronsson and colleagues [40] used a Markov model to conclude that thrombectomy with thrombolysis increased QALY by 0.99 years and with a cost saving of $221 per patient, benefiting the health care payer as well as the patient. The third cost-analysis study, using a real world dollar analysis found that endovascular procedures over IV rt.-PA alone was more than $163,000 amounting to more than $8 billion for every 50,000 patients treated [41].
The report by Lobotesis et al. [42], which is written from the healthcare provider perspective suggests that the high cost of endovascular procedures with thrombolysis (SWIFT PRIME patients) can be offset because the stroke patient has improved quality of life and health status. Numerically, the benefit per patient is £79,402 (appx. $103,530 USD). In the Canada assessment using a Markov model, the cost effectiveness of thrombectomy was compared to IV thrombolysis. The analysis showed that thrombectomy was more expensive than thrombolysis by $2520, and thrombectomy was associated with a cost-effective ratio of $11,990 per QALY gained by the patient. Thrombectomy was cost-effective since it significantly improved independence [43]. The same conclusion was reached by Ganesalingam et al. [44] in an earlier study.
Thrombolysis
Efficacy
The thrombolytic, rt.-PA (Activase) was first approved by the FDA in 1996 and is now widely accepted, yet underutilized as a standard-of-care treatment for ischemic stroke. rt.-PA use remains controversial according to a recent publication [45]. Over the last 20 years, the cost of a single use vial of rt.-PA has escalated from $2000 (per dose/100 mg vial) in 1995 [46], reported cost of $2746 CDN ($2470 USD) in 2006 [47] to the current list price market value of $9954.22 for a 100 mg vial of drug, which is to be administered IV at a dose of 0.6–0.9 mg/kg [48–51]. Activase has been shown to be effective up to 4.5 h after a stroke [ 52, 53], is beneficial with thrombectomy up to 6 h after a stroke [31], but it is currently FDA-approved for use within a 3-h therapeutic window. It has been difficult to estimate the actual use and application of rt.-PA in eligible stroke victims, but it has been suggested that less than 7–10% of stroke patients are being treated with rt.-PA in the United States [54–56] despite the fact that rt.-PA may be beneficial in up to 50% of patients provided the drug as a treatment option [51]. A recent hospital census showed that rt.-PA use is between 6.5 and 7.2% in the 18–64 and ≥90 year old population, respectively [57, 58].
Cost-Effectiveness
Early articles by Taylor et al. [59, 60] indicated that an ischemic stroke has a financial burden of $90,981 (unadjusted 1990 value) and the lifetime cost associated with all stroke occurring in 1990 (i.e., estimated 392,344 stroke patients) was $29.0 billion. By 2010, Boudreau et al. [61] estimated that the financial burden due to stroke escalated to $74 billion in the USA. Importantly, cost analysis from the ECASSIII trial showed an age-dependent, incremental cost benefit of $6255 per QALY for victims less than 65 years old and $35,813 per QALY for victims above 65 years old. The cost benefit was also dependent upon NIHSS scores, in patients with NIHSS 0 to 9; the benefit was $16,322 per QALY; NIHSS 10 to 19 increased to $37,462 per QALY, and high NIHSS scores ≥20 corresponded to a very low cost benefit ($2432 per QALY). In a subsequent analysis by Boudreau et al. [62], they reported that rt-PA use is associated with a lifetime cost-saving of $25,000.
In summary, as reviewed in sections 2.0 and 3.0, reperfusion therapy procedures (thrombolysis and endovascular procedures), when provided as a monotherapy or when a thrombolytic was provided prior to an endovascular procedure, improved the health and well-being of stroke patients. However, as indicated above, there is substantial room for additional improvement over either reperfusion technique, and there may be an opportunity to expand the therapeutic window to enroll additional patients. This can all be achieved with a cytoprotective agent add-on to achieve increased clinical improvement, enhance the safety profile of thrombolysis, or extend the therapeutic window for current treatments [63–67]. The goal of the next section is to develop the path forward for the development of neuroprotective therapies.
The Rigorous Path to Demonstrate Neuroprotection: Animal Models
To develop new neuroprotective stroke therapies, high quality translational studies that incorporate STAIR [68], RIGOR [69–71] and CAMARADES [72, 73] guidelines are mandatory. The limited “therapeutic window” for thrombolytic and embolectomy efficacy described herein should be recognized as critical to the success of future therapies. Arguments have been made for rapid administration of therapy [74–76], and the recommended DTNT for thrombolytic therapy administration is less than 1 h [75, 77–79], within the golden hour window. Nevertheless, all embolectomy trials described in section 2 used a treatment window 5 or more fold in excess of that recommendation, and efficacy was still demonstrated, either with or without rt-PA. The fact that enhanced efficacy and safety were measured when a thrombolytic was pre-administered to patients may be an important factor, but it is not a critical component to demonstrate significant clinical efficacy as reviewed above and in HERMES [37] and by Mokin et al. [80].
Caveats
The progression of an ischemic stroke occurs over a non-linear timeline, affecting as many as seven different cell types in brain [81], with neurons being the most vulnerable. The spatial and temporal profile of cell damage and cell death following a stroke is highly dependent upon location (i.e., stroke core, penumbra, vascular); core cells cannot be saved, but penumbral cells can be saved. The temporal profile of differential cell death of neurovascular unit components should be viewed as an opportunity to intervene at different targets using pharmacological therapeutics directed toward specific pathways and processes, with the possibility of intervention being initiated at different time post-stroke. For instance, based upon in vitro cell profiling, it appears that neurons are most sensitive to insult, and that neuronal death is an irreversible process, thus rapid intervention for “neuroprotection” is required. Let us recall the hypothesis of Saver regarding the “golden hour” [74] and propose that the window should be no longer than 1 h in order to target neurons. Thereafter, targeting endothelial cells, pericytes, microglia, and neuroglia with a cytoprotective will be most important since they are the next cells recruited by death pathways.
Because of the differential sensitivity of brain cells to ischemia, it may be feasible to intervene at multiple stages post-stroke to provide optimal cellular protection and survival, if the tacit assumption, that there is a similar time-line in vitro in lissencephalic and gyrencephalic animals and then in humans. Taken together, collectively or individually (second phase, third phase, and so forth), each cell type may represent additional therapeutic targets. While this hypothesis is highly sensible, the approach to multiple targets may be problematic from a drug development efficacy and safety point of view, as well as regulatory standpoint.
Translational Stroke Research
There are still no guidelines documenting the “optimized” drug development path to achieve success with a cytoprotective therapy, whether the therapy be a small molecule or protein. This is evidenced by the diversity of studies published in the literature, and extreme diversity of critiques from special emphasis panels at National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS), American Heart Association (AHA), and a variety of international funding agencies and foundations. As an effective first approach to aid stroke researchers with therapy development challenges, this section will deal with proposed guidelines that should be considered when designing translational studies.
Drug Development Considerations
Standard industry drug development guidelines should be considered by researchers interested in applying their research to developing a drug through to fruition, a clinical trial end-point. The development of a CNS-active drug to treat stroke requires special attention since they must be able to cross the blood-brain barrier (BBB) to penetrate into the penumbra. This can be taken into consideration when developing molecules using the Lipinski rules as well as utilizing BBB penetration assays (in vitro) for primary candidate selection and then in vivo for drug development [82–84]. Moreover, during the initial stages of drug development, rapid and cost-effective toxicity screens (CeeTox and micronucleus assays) can help eliminate compounds with excessive unwanted “side effects”.
Table 3 provides a guide and references for many useful drug-development profiling tools alongside current market costs for the assays from North American and international sources cited in the table legend. This information is provided so that the reader of this article will understand standard costs associated with the drug development process; the authors do not endorse any specific contract research organization (CRO) to conduct the assays.
Table 3.
Drug development
| Drug development profile | Selected references | Estimated assay cost |
|---|---|---|
| 1. Chemical profile for CNS active drugs blood brain barrier penetrating drugs (pKa, logP, logD, solubility, chemical stability) | [83–85] | $1200–1800 per compound |
| 2. BBB penetration profile in vitro (MDCK analysis) | [86] | $550–750 per compound |
| 3. Microsome and plasma stability | [87] | $2100–18,000 per compound per species |
| 4. hERG inhibitory activity (patch clamp) | [88–92] | $1250–29,000 |
| 5. CP450 inhibition analysis (Standard IC50 analysis: 1A2, 2D6, 2C9, 2C19, 3A4) | [93–95] | $500–32,000 per compound |
| 6. Toxicity screen (in vitro CeeTox analysis) | [96, 97] | $6400 per compound |
| 7. Genetic screen (Ames Test and In vitro micronucleus test) | [98] | $4950–11,350 per compound |
| 8. Mouse lymphoma assay | [99] | $36,000 |
| 9. Blood chemistry (CBC) and comprehensive chemistry | [100] | $589 Rodent (n = 3) $845 Rabbit (n = 3) |
| 10. PK and BBB penetration profile in vivo (multiple species in normal animals—recommendation for animals with embolic stroke or ischemic damage) | [82, 101] | $4500–5500 Rodent (n = 3) $540–12,000 Rabbit (n = 3) $17,500–40,000 Primate (n = 3) |
| 11. Regulatory submission documentation | $200,000–275,000 |
GLP drug preparation, benchmarking and characterization is in the range of $155,000–275,000 [146]
As can be gleaned from Table 3, the cost per compound for basic profiling (Steps 1–8) can be in the range of $52,000–135,000 excluding in vivo PK, BBB penetration, and blood chemistry analysis (Steps 9–10), which should only be performed after a comprehensive in vitro chemistry profile is documented. For screening of libraries of just a few small molecules, profiling costs can easily amount to $1–5 million during the compound selection process. Nevertheless, the funnel approach at initial stages is cost-effective and highly recommended.
Steps subsequent to molecule characterization, include animal-based testing and development for both efficacy and safety in appropriate stroke models [69, 71, 102–105]. It is essential to include multiple species in a drug development plan, and incorporate gender [106], aging, and comorbidities normally associated with the aged stroke patient [32–37, 107–110]. With two current standards-of-care therapy for ischemic stroke, both of which target the blood clot, which is causal for ischemic stroke, it would be pragmatic to use embolic stroke models during the drug development process to accurately model the target population [see [111–115]], and not rely solely upon similarities between ischemia and embolism-induced stroke [111].
Moreover, although not commonly utilized in stroke research, in vivo analysis of blood chemistry, CBC, PK, and BBB penetration profiles should be conducted using animal models of embolic or ischemic stroke where there is known BBB breakdown [116–120]. This would better reflect drug administration in the stroke patient population, and accumulation in the penumbral target.
Table 4 presents FDA-recommended [121–123] drug-development and testing scheme inclusive of standardized efficacy and toxicity testing in two species with partial or full dose-response analysis, which would allow for selection of a maximum recommended starting dose (MRSD) in patients. The information in this Table pertains to a single drug development scheme, with a fixed administration time following a stroke.
Table 4.
In vivo pharmacology and toxicology
| Drug development profiles | Selected references | Estimated assay cost |
|---|---|---|
| Dose-response analysis in multiple species (required for estimating dosing in clinical trials—human equivalent dosing) | [124] | Three dose-rodent intraluminal suture + vehicle |
| • $1000–1500 per animal enrolled in study | ||
| Three dose-rabbit embolic + vehicle + tPA positive control | ||
| • $3000–6000 per animal enrolled in study | ||
| Therapeutic window analysis (clinically relevant in humans) (See NINDS rt-PA and endovascular procedure trials: “golden hour” analysis and extended window) | [32–36, 51] | Three time-point rodent |
| • $1000–1500 per animal enrolled in study | ||
| Three time-point rabbit | ||
| • $1000–1500 per animal enrolled in study | ||
| Combination analysis with tPA single time-point (models incorporating embolism-induced stroke) models for efficacy and safety |
[113, 116, 125, 126] | Four group analysis using a rodent embolism model |
| • $1000–1500 per animal enrolled in study | ||
| Four group analysis—rabbit embolic stroke model | ||
| • $3000–6000 per animal enrolled in study plus tPA costs | ||
| Toxicity screen (in vivo- two species) 28-day GLP toxicity IV administration (3–5 dose escalation- single injection) |
[127, 128] | Rat |
| • $80,000–260,000 per compound | ||
| Rabbit | ||
| • $125,000–325,000 per compound |
However, there are now caveats that must be considered when developing a cytoprotective agent.
First, the term “neuroprotective” or “neuroprotectant” has the inherent implication that only nerves or neurons are being saved. We now have a better understanding that neurons are not the only target of importance. For example, as early as 2004, a series of articles dedicated to the neurovascular unit were seeded in the literature [129–137]. Over time, our understanding of the neurovascular unit has evolved to a point where we now believe that the unit is integrated, and that cytoprotection of all components may be necessary to achieve significant and optimal improvement following a stroke.
Secondly, recent published information suggests that a variety of cell types within the neurovascular unit are affected by ischemia on very different time-courses. For example, it appears that neurons are most vulnerable to ischemia, followed by brain endothelial cells, pericytes, microglia, and then astrocytes [138, 139]. In rodent models of ischemia, it has also been established that oligodendrocytes survive the insult longer than neurons, and astrocytes are least sensitive to ischemia [81]. The damage and death time-course of different cellular populations should not be overlooked, and in fact, should be used as an advantage (See Fig. 1).
Fig. 1.
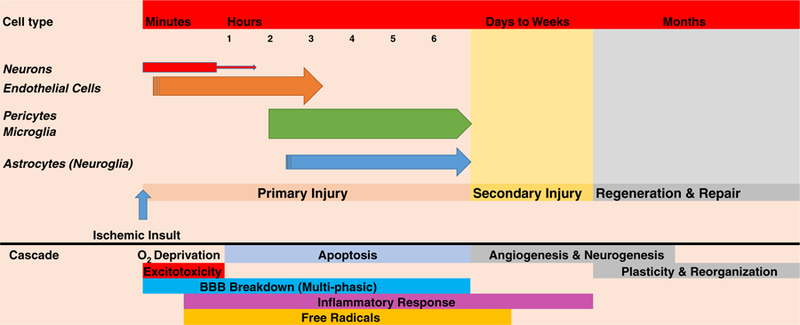
Sequence of cell death following ischemic stroke. This figure is a comprehensive composite constructed from literature data [81, 111, 138, 140–142] mined from various primary and secondary sources. It shows the apparent time-course of cell death for neurons, endothelial cells, pericytes, microglia, and astrocytes (in order) derived from in vitro oxygen-glucose deprivation studies detailed by Redzic et al. [138] and discussed by Carmichael [81]. Moreover, the cell-death time-course is compared to our current understanding of the ischemic stroke cascade constructed from data described in the literature [81, 140–145]. Since cells die with different time-courses, there may be multiple opportunities for cytoprotective strategies to be administered to promote cell survival and improve clinical function, but the rapid death of neurons will limit neuroprotective strategies
In Fig. 1, we present the time-course for cell death extrapolated from in vitro cell culture models and directly compare cell death with our current understanding of the ischemic cascade. There are many opportunities for pharmacological intervention to attenuate the evolution of the cell death cascade.
-
2.1.
If the sequence of cell vulnerability and death in vivo in man and animals is the same as described for cells in vitro in a culture dish, then we can attempt to target different cell types using specific molecules after the initial stroke insult and prior to or following intervention with thrombolysis and/or endovascular procedures. Of course, this innovative treatment schedule proposal comes with its own caveats.
-
2.1.1.
The development process for multiple drugs used in sequential combination is unknown and has not been established.
-
2.1.2.
In stroke patients, it is often difficult to ascertain the exact time of the stroke event. Thus, the therapeutic window will not be well-defined for each individual.
-
2.1.3.
Success will depend upon administration of drugs during critical therapeutic windows for each cellular component.
-
2.1.4.
Initial demonstration of success may depend on the presence of the physical substrate utilized in endovascular procedure trials (ASPECTS >8).
-
2.1.5.
Efficacy testing in multiple species will require extensive funding.
-
2.1.6.
Animal testing for toxicity profiles and tolerated doses will require extensive funding.
-
2.1.1.
Conclusion
rt-PA is cost-effective. Endovascular procedures are cost-effective. Can efficacious and cost-effective cytoprotection for stroke be achieved within the next 5 or 10 years? There are many promising therapeutic intervention opportunities available that should be tested in stroke victims in combination with thrombolysis and endovascular procedures, since both interventions provide significant reperfusion benefit in patients. Randomized, blinded, controlled clinical trials should not be initiated for any compound or device until the cytoprotective strategies are thoroughly investigated in multiple species, including a rodent, and one or more thoroughly validated large animal models representative of the target stroke population. This will provide the heightened level of de-risking of the development process, to reduce the unending trend for failure in stroke victims.
Funding agencies worldwide should be cognizant of the inherent costs to develop cytoprotectives, in particular small molecules that must undergo a lengthy series of profiling and screening assays for efficacy and toxicity.
Acknowledgments and Funding
This article was written without direct financial support from government sources (PAL). JHZ was supported by NIH (NS081740 and NS084921).
Footnotes
Compliance with Ethical Standards
Conflict of Interest PAL is Editor-in-Chief, Journal of Neurology & Neurophysiology and Associate Editor, Translational Stroke Research; JHZ is Editor-in-Chief, Translational Stroke Research and Editor-in-Chief, Medical Gas Research.
References
- 1.Poirier J, Derouesné D. VIII.9: Apoplexy and Stroke The Cambridge World History of Human Disease 1993. p. 10.1017/CHOL9780521332866.071. [DOI] [Google Scholar]
- 2.Wepfer J Observationes anatomicae ex cadaveribus eorum quos sustulit apoplexia (cum exercitatione de eious loco affecto). Schaffussii: J C Suteri 1658:670. [Google Scholar]
- 3.Gurdjian ES, Gurdjian ES. History of occlusive cerebrovascular disease I. From Wepfer to Moniz. Arch Neurol 1979;36(6):340–3. [DOI] [PubMed] [Google Scholar]
- 4.Willis T Cerebri Anatome: cui accessit nervorum descriptio et usus London; 1664. [Google Scholar]
- 5.Virchow R Thrombose und Embolie: Gefässenzýndung und Septische Infektion, in Gesammelte Abhandlungen zur wissen schaftlichen Medicin: Frankfurt, a. M., Meidinger, Sohn und Co, 1856. [Google Scholar]
- 6.Dechambre A Dictionnaire Encyclopédique des Sciences Médicales Paris; 1866. [Google Scholar]
- 7.Bramwell B Spontaneous meningeal haemorrhage. Edinburgh Medical Journal 1886;32:101. [Google Scholar]
- 8.Symonds CP. Spontaneous sub-arachnoid haemorrhage. Proceedings of the Royal Society of Medicine. Neurol Sect 1924;17:39–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 2009;119(3):480–6. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121(7):948–54. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121(7):e46–e215. [DOI] [PubMed] [Google Scholar]
- 12.Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep 2002;2(1):38–43. [DOI] [PubMed] [Google Scholar]
- 13.Lyden PD, Zivin JA. Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc Brain Metab Rev 1993;5(1):1–16. [PubMed] [Google Scholar]
- 14.Bernstein RA, Del-Signore M. Recent advances in the management of acute intracerebral hemorrhage. Curr Neurol Neurosci Rep 2005;5(6):483–7. [DOI] [PubMed] [Google Scholar]
- 15.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369(9558):306–18. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 17.CDC. http://www.cdc.gov/Stroke/faqs.htm.2016.
- 18.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet 2014;383(9913):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suwanwela NC, Poungvarin N. Asian stroke advisory P. Stroke burden and stroke care system in Asia. Neurol India 2016;64(Suppl):S46–51. [DOI] [PubMed] [Google Scholar]
- 20.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372(14):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CLEAN M. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1804.
- 22.ESCAPE. https://clinicaltrials.gov/ct2/show/NCT01778335.
- 23.REVASCAT. https://clinicaltrials.gov/ct2/show/NCT01692379.
- 24.PRIME S. https://clinicaltrials.gov/ct2/show/NCT01657461.
- 25.EXTEND-IA. https://clinicaltrials.gov/ct2/show/NCT01492725.
- 26.THERAPY. https://clinicaltrials.gov/ct2/show/NCT01429350.
- 27.THRACE. https://clinicaltrials.gov/ct2/show/NCT01062698.
- 28.Bendszus M, Thomalla G, Hacke W, Knauth M, Gerloff C, Bonekamp S, et al. Early termination of THRILL, a prospective study of mechanical thrombectomy in patients with acute ischemic stroke ineligible for i.V. Thrombolysis. Clin Neuroradiol 2016; doi: 10.1007/s00062-016-0538-8. [DOI] [PubMed] [Google Scholar]
- 29.DAWN. https://www.clinicaltrialsgov/ct2/show/NCT02142283?term=DAWN&rank=10.
- 30.POSITIVE. https://www.clinicaltrialsgov/ct2/show/NCT01852201?term=nct01852201&rank=1.
- 31.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723–31. [DOI] [PubMed] [Google Scholar]
- 32.Berkhemer OAFP, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372(1):11–20. [DOI] [PubMed] [Google Scholar]
- 33.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372(11):1019–30. [DOI] [PubMed] [Google Scholar]
- 34.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372(11):1009–18. [DOI] [PubMed] [Google Scholar]
- 35.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372(24):2296–306. [DOI] [PubMed] [Google Scholar]
- 36.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372(24):2285–95. [DOI] [PubMed] [Google Scholar]
- 37.Rebello LC, Haussen DC, Grossberg JA, Belagaje S, Lima A, Anderson A, et al. Early endovascular treatment in intravenous tissue plasminogen activator-ineligible patients. Stroke 2016;47(4):1131–4. [DOI] [PubMed] [Google Scholar]
- 38.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46(10):3020–35. [DOI] [PubMed] [Google Scholar]
- 39.Xie X, Lambrinos A, Chan B, Dhalla IA, Krings T, Casaubon LK, et al. Mechanical thrombectomy in patients with acute ischemic stroke: a cost-utility analysis. CMAJ Open 2016;4(2):E316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aronsson M, Persson J, Blomstrand C, Wester P, Levin LA. Cost-effectiveness of endovascular thrombectomy in patients with acute ischemic stroke. Neurology 2016;86(11):1053–9. [DOI] [PubMed] [Google Scholar]
- 41.Mangla S, O’Connell K, Kumari D, Shahrzad M. Novel model of direct and indirect cost-benefit analysis of mechanical embolectomy over IV tPA for large vessel occlusions: a real-world dollar analysis based on improvements in mRS. J Neurointerv Surg 2016; doi: 10.1136/neurintsurg-2015-012152. [DOI] [PubMed] [Google Scholar]
- 42.Lobotesis K, Veltkamp R, Carpenter IH, Claxton LM, Saver JL, Hodgson R. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischemic stroke in the UK. J Med Econ 2016;19(8):785–94. [DOI] [PubMed] [Google Scholar]
- 43.Health Quality Ontario. Mechanical thrombectomy in patients with acute ischemic stroke: a health technology assessment. Ont Health Technol Assess Ser 2016;16(4):1–79. [PMC free article] [PubMed] [Google Scholar]
- 44.Ganesalingam J, Pizzo E, Morris S, Sunderland T, Ames D, Lobotesis K. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke 2015;46(9):2591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zivin JA, Simmons J. tPA for stroke : the story of a controversial drug New York: Oxford University Press; 2011. xiv, 191 p. p. [Google Scholar]
- 46.Genentech. http://www.nytimes.com/1995/04/13/business/company-reports-genentech-earnings-exceed-expectations.html
- 47.TNKase. https://www.scribd.com/document/9700876/Government-of-Canada-PMPRB-report-on-pricing-of-TNKase-tenecteplase
- 48.Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan alteplase clinical trial (J-ACT). Stroke 2006;37(7):1810–5. [DOI] [PubMed] [Google Scholar]
- 49.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375(9727):1695–703. [DOI] [PubMed] [Google Scholar]
- 50.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 2009;8(12):1095–102. [DOI] [PubMed] [Google Scholar]
- 51.NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995;333(24):1581–7. [DOI] [PubMed] [Google Scholar]
- 52.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359(13):1317–29. [DOI] [PubMed] [Google Scholar]
- 53.Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke 2009;40(7):2438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med 2010;5(7):406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke 2005;36(6):1232–40. [DOI] [PubMed] [Google Scholar]
- 56.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6(5):543–9. [DOI] [PubMed] [Google Scholar]
- 57.Joo H, Wang G, George MG. Use of intravenous tissue plasminogen activator and hospital costs for patients with acute ischaemic stroke aged 18–64 years in the USA. Stroke Vasc Neurol 2016;1(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arora R, Salamon E, Katz JM, Cox M, Saver JL, Bhatt DL, et al. Use and outcomes of intravenous thrombolysis for acute ischemic stroke in patients >/=90 Years of age. Stroke 2016;47(9):2347–54. [DOI] [PubMed] [Google Scholar]
- 59.Taylor TN. The medical economics of stroke. Drugs 1997;54(Suppl 3):51–7. discussion 7–8 [DOI] [PubMed] [Google Scholar]
- 60.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke 1996;27(9):1459–66. [DOI] [PubMed] [Google Scholar]
- 61.Boudreau DM, Guzauskas G, Villa KF, Fagan SC, Veenstra DL. A model of cost-effectiveness of tissue plasminogen activator in patient subgroups 3 to 4.5 hours after onset of acute ischemic stroke. Ann Emerg Med 2013;61(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boudreau DM, Guzauskas GF, Chen E, Lalla D, Tayama D, Fagan SC, et al. Cost-effectiveness of recombinant tissue-type plasminogen activator within 3 hours of acute ischemic stroke: current evidence. Stroke 2014;45(10):3032–9. [DOI] [PubMed] [Google Scholar]
- 63.Henninger N, Fisher M. Extending the time window for endovascular and pharmacological reperfusion. Transl Stroke Res 2016;7(4):284–93. [DOI] [PubMed] [Google Scholar]
- 64.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res 2016; doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res 2016;7(4):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyden P, Weymer S, Coffey C, Cudkowicz M, Berg S, O’Brien S, et al. Selecting patients for intra-arterial therapy in the context of a clinical trial for neuroprotection. Stroke 2016;47(12):2979–85. doi: 10.1161/STROKEAHA.116.013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lapchak PA. Critical early Thrombolytic & Endovascular Reperfusion Therapy for Acute Ischemic Stroke Victims: a call for adjunct neuroprotection. Translational Stroke Research 2015;6(5):345–54. doi: 10.1007/s12975-015-0419-5(6):345-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30(12): 2752–8. [DOI] [PubMed] [Google Scholar]
- 69.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012;490(7419): 187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapchak PA. Scientific rigor recommendations for optimizing the clinical applicability of translational research. J Neurol Neurophysiol 2012;3:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapchak PA. Recommendations and practices to optimize stroke therapy: developing effective translational research programs. Stroke 2013;44(3):841–3. [DOI] [PubMed] [Google Scholar]
- 72.Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PM, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke 2008;39(3): 929–34. [DOI] [PubMed] [Google Scholar]
- 73.Hirst JA, Howick J, Aronson JK, Roberts N, Perera R, Koshiaris C, et al. The need for randomization in animal trials: an overview of systematic reviews. PLoS One 2014;9(6):e98856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saver JL. Time is brain—quantified. Stroke 2006;37(1):263–6. [DOI] [PubMed] [Google Scholar]
- 75.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke 2010;41(7):1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lapchak PA. Fast neuroprotection (fast-NPRX) for acute ischemic stroke victims: the time for treatment is now. Transl Stroke Res 2013;4(6):704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desai JA, Smith EE. Prenotification and other factors involved in rapid tPA administration. Curr Atheroscler Rep 2013;15(7):337. [DOI] [PubMed] [Google Scholar]
- 78.Olson DM, Constable M, Britz GW, Lin CB, Zimmer LO, Schwamm LH, et al. A qualitative assessment of practices associated with shorter door-to-needle time for thrombolytic therapy in acute ischemic stroke. J Neurosci Nurs 2011;43(6):329–36. [DOI] [PubMed] [Google Scholar]
- 79.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association’s target: stroke initiative. Stroke 2011;42(10):2983–9. [DOI] [PubMed] [Google Scholar]
- 80.Mokin M, Snyder KV, Siddiqui AH, Levy EI, Hopkins LN. Recent endovascular stroke trials and their impact on stroke Systems of Care. J Am Coll Cardiol 2016;67(22):2645–55. [DOI] [PubMed] [Google Scholar]
- 81.Carmichael ST. The 3 Rs of stroke biology: radial, relayed, and regenerative. Neurotherapeutics 2016;13(2):348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lapchak PA. Drug-like property profiling of novel neuroprotective compounds to treat acute ischemic stroke: guidelines to develop pleiotropic molecules. Transl Stroke Res 2013;4(3):328–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods 2000;44(1):235–49. [DOI] [PubMed] [Google Scholar]
- 84.Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 2004;1(4):337–41. [DOI] [PubMed] [Google Scholar]
- 85.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001;46(1–3):3–26. [DOI] [PubMed] [Google Scholar]
- 86.Hellinger E, Veszelka S, Toth AE, Walter F, Kittel A, Bakk ML, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur J Pharm Biopharm 2012;82(2):340–51. [DOI] [PubMed] [Google Scholar]
- 87.Laine R Metabolic stability: main enzymes involved and best tools to assess it. Curr Drug Metab 2008;9(9):921–7. [DOI] [PubMed] [Google Scholar]
- 88.Brimecombe JC, Kirsch GE, Brown AM. Test article concentrations in the hERG assay: losses through the perfusion, solubility and stability. J Pharmacol Toxicol Methods 2009;59(1):29–34. [DOI] [PubMed] [Google Scholar]
- 89.Cheng CS, Alderman D, Kwash J, Dessaint J, Patel R, Lescoe MK, et al. A high-throughput HERG potassium channel function assay: an old assay with a new look. Drug Dev Ind Pharm 2002;28(2):177–91. [DOI] [PubMed] [Google Scholar]
- 90.Gintant G An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011;129(2):109–19. [DOI] [PubMed] [Google Scholar]
- 91.Goineau S, Legrand C, Froget G. Whole-cell configuration of the patch-clamp technique in the hERG channel assay to predict the ability of a compound to prolong QT interval. Curr Protoc Pharmacol 2012; Chapter 10: Unit 10 5. [DOI] [PubMed] [Google Scholar]
- 92.Yu HB, Zou BY, Wang XL, Li M. Investigation of miscellaneous hERG inhibition in large diverse compound collection using automated patch-clamp assay. Acta Pharmacol Sin 2016;37(1):111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McDonnell AM, Dang CH. Basic review of the cytochrome p450 system. J Adv Pract Oncol 2013;4(4):263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hedlund E, Gustafsson JA, Warner M. Cytochrome P450 in the brain: a review. Curr Drug Metab 2001;2(3):245–63. [DOI] [PubMed] [Google Scholar]
- 95.Evers R, Dallas S, Dickmann LJ, Fahmi OA, Kenny JR, Kraynov E, et al. Critical review of preclinical approaches to investigate cytochrome p450-mediated therapeutic protein drug-drug interactions and recommendations for best practices: a white paper. Drug Metab Dispos 2013;41(9):1598–609. [DOI] [PubMed] [Google Scholar]
- 96.Lapchak PA, Bombien R, Rajput PS. J-147 a Novel Hydrazide Lead Compound to Treat Neurodegeneration: CeeTox Safety and Genotoxicity Analysis. J Neurol Neurophysiol 2013; 4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lapchak PA, Schubert DR, Maher PA. Delayed treatment with a novel neurotrophic compound reduces behavioral deficits in rabbit ischemic stroke. J Neurochem 2011;116(1):122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirsch-Volders M, Decordier I, Elhajouji A, Plas G, Aardema MJ, Fenech M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis 2011;26(1):177–84. [DOI] [PubMed] [Google Scholar]
- 99.Wiesner J, Ziemann C, Hintz M, Reichenberg A, Ortmann R, Schlitzer M, et al. FR-900098, an antimalarial development candidate that inhibits the non-mevalonate isoprenoid biosynthesis pathway, shows no evidence of acute toxicity and genotoxicity. Virulence 2016;7(6):718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steinmetz KL, Spack EG. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol 2009;9(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pellegatti M Preclinical in vivo ADME studies in drug development: a critical review. Expert Opin Drug Metab Toxicol 2012;8(2):161–72. [DOI] [PubMed] [Google Scholar]
- 102.Fisher M Recommendations for advancing development of acute stroke therapies: stroke therapy academic industry roundtable 3. Stroke 2003;34(6):1539–46. [DOI] [PubMed] [Google Scholar]
- 103.Fisher M, Hanley DF, Howard G, Jauch EC, Warach S. Recommendations from the STAIR V meeting on acute stroke trials, technology and outcomes. Stroke 2007;38(2):245–8. [DOI] [PubMed] [Google Scholar]
- 104.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M, Consortium SV. Stroke therapy academic industry roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke 2009;40(7):2594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke treatment academic industry roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 2011;42(9):2645–50. [DOI] [PubMed] [Google Scholar]
- 106.Ahnstedt H, McCullough LD, Cipolla MJ. The importance of considering sex differences in translational stroke research. Transl Stroke Res 2016;7(4):261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ergul A, Hafez S, Fouda A, Fagan SC. Impact of comorbidities on acute injury and recovery in preclinical stroke research: focus on hypertension and diabetes. Transl Stroke Res 2016;7(4):248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.King AJ. The use of animal models in diabetes research. Br J Pharmacol 2012;166(3):877–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sasase T, Ohta T, Masuyama T, Yokoi N, Kakehashi A, Shinohara M. The spontaneously diabetic torii rat: an animal model of nonobese type 2 diabetes with severe diabetic complications. J Diabetes Res 2013;2013:976209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kemmochi Y, Fukui K, Maki M, Kimura S, Ishii Y, Sasase T, et al. Metabolic disorders and diabetic complications in spontaneously diabetic Torii Lepr (fa) rat: a new obese type 2 diabetic model. J Diabetes Res 2013;2013:948257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab 2012;32(7):1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lapchak PA. A clinically relevant rabbit embolic stroke model for acute ischemic stroke therapy development: mechanisms & targets. In: Lapchak PA, Zhang JH, editors.: Translational Stroke Research: From Target Selection to Clinical Trials, Springer, USA; 2011. Chapter 27, p. 541–84 [Google Scholar]
- 113.Lapchak PA. A cost-effective rabbit embolic stroke bioassay: insight into the development of acute ischemic stroke therapy. Transl Stroke Res 2015;6(2):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turner R, Jickling G. Sharp, F are underlying assumptions of current animal models of human stroke correct: from STAIRS to high hurdles? Translational Stroke Research 2011;2(2):138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kent TA, Mandava P. Embracing biological and methodological variance in a new approach to pre-clinical stroke testing. Transl Stroke Res 2016;7(4):274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 2000;31(12):3034–40. [DOI] [PubMed] [Google Scholar]
- 117.Liu DZ, Sharp FR. Excitatory and Mitogenic signaling in cell death, blood-brain barrier breakdown, and BBB repair after intracerebral hemorrhage. Transl Stroke Res 2012;3(Suppl 1):62–9. [DOI] [PubMed] [Google Scholar]
- 118.Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 2016;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kassner A, Merali Z. Assessment of blood-brain barrier disruption in stroke. Stroke 2015;46(11):3310–5. [DOI] [PubMed] [Google Scholar]
- 120.Borlongan CV. The blood brain barrier in stroke. Curr Pharm Des 2012;18(25):3613–4. [DOI] [PubMed] [Google Scholar]
- 121.Demaerschalk BM. Alteplase treatment in acute stroke: incorporating Food and Drug Administration prescribing information into existing acute stroke management guide. Curr Atheroscler Rep 2016;18(8):53. [DOI] [PubMed] [Google Scholar]
- 122.Benz RD. Toxicological and clinical computational analysis and the US FDA/CDER. Expert Opin Drug Metab Toxicol 2007;3(1): 109–24. [DOI] [PubMed] [Google Scholar]
- 123.Muller PY, Milton M, Lloyd P, Sims J, Brennan FR. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr Opin Biotechnol 2009;20(6):722–9. [DOI] [PubMed] [Google Scholar]
- 124.Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 2009;157(6):907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lapchak PA, Araujo DM, Zivin JA. Comparison of tenecteplase with alteplase on clinical rating scores following small clot embolic strokes in rabbits. Exp Neurol 2004;185(1):154–9. [DOI] [PubMed] [Google Scholar]
- 126.Lapchak PA, Daley JT, Boitano PD. A blinded, randomized study of L-arginine in small clot embolized rabbits. Exp Neurol 2015;266:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meador V, Jordan W, Zimmermann J. Increasing throughput in lead optimization in vivo toxicity screens. Curr Opin Drug Discov Devel 2002;5(1):72–8. [PubMed] [Google Scholar]
- 128.Yoon M, Campbell JL, Andersen ME, Clewell HJ. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol 2012;42(8):633–52. [DOI] [PubMed] [Google Scholar]
- 129.Lee SR, Wang X, Tsuji K, Lo EH. Extracellular proteolytic pathophysiology in the neurovascular unit after stroke. Neurol Res 2004;26(8):854–61. [DOI] [PubMed] [Google Scholar]
- 130.Richardson J, Murray D, House CK, Lowenkopf T. Successful implementation of the National Institutes of Health stroke scale on a stroke/neurovascular unit. J Neurosci Nurs 2006;38(4 Suppl):309–15. [DOI] [PubMed] [Google Scholar]
- 131.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med 2010;267(2):156–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ago T The neurovascular unit in health and ischemic stroke. Nihon Rinsho 2016;74(4):583–8. [PubMed] [Google Scholar]
- 133.Cai W, Zhang K, Li P, Zhu L, Xu J, Yang B, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.ElAli A The implication of neurovascular unit signaling in controlling the subtle balance between injury and repair following ischemic stroke. Neural Regen Res 2016;11(6):914–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lake EM, Bazzigaluppi P, Mester J, Thomason LA, Janik R, Brown M, et al. Neurovascular unit remodelling in the subacute stage of stroke recovery. Neuroimage 2016. S10538119(16)30480–3. doi: 10.1016/j.neuroimage.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 136.Hermann DM, Buga AM, Popa-Wagner A. Neurovascular remodeling in the aged ischemic brain. J Neural Transm (Vienna) 2015;122(Suppl 1):S25–33. [DOI] [PubMed] [Google Scholar]
- 137.del Zoppo GJ. Aging and the neurovascular unit. Ann N Y Acad Sci 2012;1268:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Redzic ZB, Rabie T, Sutherland BA, Buchan AM. Differential effects of paracrine factors on the survival of cells of the neurovascular unit during oxygen glucose deprivation. Int J Stroke 2015;10(3):407–14. [DOI] [PubMed] [Google Scholar]
- 139.Barakat R, Redzic Z. Differential cytokine expression by brain microglia/macrophages in primary culture after oxygen glucose deprivation and their protective effects on astrocytes during anoxia. Fluids Barriers CNS 2015;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dirnagl U Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 2012;1268:21–5. [DOI] [PubMed] [Google Scholar]
- 141.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999;22(9):391–7. [DOI] [PubMed] [Google Scholar]
- 142.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010;67(2):181–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xing C, Hayakawa K, Lo EH. Mechanisms, Imaging, and Therapy in Stroke Recovery. Transl Stroke Res 2016. doi: 10.1007/s12975-016-0503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jolkkonen J, Kwakkel G. Translational hurdles in stroke recovery studies. Transl Stroke Res 2016;7(4):331–42. [DOI] [PubMed] [Google Scholar]
- 145.Koh SH, Park HH. Neurogenesis in Stroke Recovery. Transl Stroke Res 2016. doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- 146.Commercial sources were utilized to estimate assay costs Sources include Cedars-Sinai Medical Center Department of Comparative Medicine, Pharmaron Inc, Absorption Systems Inc, Ricera inc., Cyprotex Inc, and Oxygen BioResearch PVT Ltd. [Google Scholar]


