Abstract
Background:
The outlook for ambulatory patients with advanced heart failure (HF) and the appropriate timing for left ventricular assist device (LVAD) or transplant remain uncertain. The aim of this study was to better understand disease trajectory and rates of progression to subsequent LVAD therapy and transplant in ambulatory advanced HF.
Methods:
Patients with advanced HF who were NYHA class III-IV and Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Profiles 4–7 despite optimal medical therapy (without inotropic therapy) were enrolled across 11 centers and followed for the end-points of survival, transplantation, LVAD placement, and health-related quality of life. A secondary intention-to-treat survival analysis compared outcomes for MedaMACS patients to a matched group of Profile 4–7 patients with LVADs from the INTERMACS registry.
Results:
Between May 2013 to October 2015 161 patients were enrolled with Profile 4 (12%), Profile 5 (32%), Profile 6 (49%), and Profile 7 (7%). By 2 years after enrollment, 75 (47%) patients had experienced a primary endpoint with 39 (24%) deaths, 17 (11%) patients undergoing LVAD implantation, and 19 (12%) receiving a transplant. Compared to 1753 patients with Profiles 4–7 receiving LVAD therapy there was no overall difference in intention-to-treat survival between medical and LVAD therapy, but survival with LVAD therapy was better than medical therapy among Profile 4–5 patients (p=0.0092). Baseline health related quality of life was lower among patients receiving LVAD than those enrolled on continuing oral medical therapy, but increased after 1 year for survivors in both cohorts.
Conclusions:
Ambulatory patients with advanced HF are at high risk for poor outcomes, with only 53% alive on medical therapy after 2 years of follow up. Survival was similar with medical versus LVAD therapy in the overall cohort which included the lower severity Profiles 6 and 7, but survival was better with LVAD therapy among patients in Profiles 4 and 5. Given the poor outcomes in this group of advanced HF patients, timely consideration of transplant and LVAD is of critical importance.
Keywords: mechanical circulatory support, ventricular assist device, cardiac transplantation, advanced heart failure, patient decision making
Introduction
Despite widespread use of evidence-based therapies, morbidity and mortality from systolic heart failure (HF) remain high. Over the last decade, breakthroughs in mechanical circulatory support technology have extended survival and improved quality of life in selected patients with advanced HF, as chronicled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report.1 Most patients receiving left ventricular assist devices have cardiogenic shock (INTERMACS Patient Profiles 1 and 2) or dependence on intravenous inotropic therapy (Profile 3), in whom LVAD therapy has clearly been shown to extend survival currently classified as compared to ongoing medical treatment.2,3 However, decisions surrounding appropriate use and timing of LVAD therapy in “less-sick” ambulatory patients with advanced HF on oral therapy without inotropic support (Profiles 4–7) are challenging, in part because of the information gap surrounding the trajectory of disease for this group of patients on oral HF therapies.
It is also important to appreciate this disease trajectory in advanced HF to better inform discussions with patients. Given the complexities inherent in treating advanced HF patients with LVAD or cardiac transplantation, patient-centered care requires that we understand the myriad of possible outcomes, including adverse events.4–6 Balancing the risk and benefits of continued medical management against those associated with surgical interventions such as LVAD or transplant remains the central decision in advanced HF management.
Thus, the two primary aims of the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) study were to identify and study a cohort of ambulatory patients with advanced HF on oral medical and electrical therapies to better characterize prognosis and disease trajectory during follow-up at experienced centers offering LVAD therapy, and to facilitate real-world contemporary comparison of optimal medical versus LVAD therapy, utilizing the INTERMACS registry.1 We hypothesized that the event rate would be high in patients with advanced HF even if ambulatory without need for chronic inotropic infusion. We further hypothesized that it may be possible to identify a subgroup of these patients likely to have better outcomes with LVAD therapy.
Methods
Patient Selection
Ambulatory patients with advanced HF (New York Heart Association class III-IV, INTERMACS Patient Profiles 4–7) were enrolled in the prospective, observational MedaMACS Study from May 2013 to October 2015 across 11 United States centers offering advanced HF management including LVAD and cardiac transplantation programs. Study entry criteria have been published and are provided in (Supplemental Figure 1) and included patients with chronic advanced HF (diagnosis for at least 1 year and on evidence-based medications for at least 3 months unless documented contraindication or intolerance) and one prior HF hospitalization in the preceding year. Inclusion required at least one additional high risk feature including (1) an additional HF related hospitalization in the prior year for a total of at least 2, (2) high natriuretic peptide level (BNP>1000 pg/ml or NT-proBNP>4000 pg/ml), (3) poor functional status as assessed by cardiopulmonary exercise testing or 6-minute walk, or (4) a high predicted mortality according to the Seattle HF model.7,8 Key exclusion criteria were current intravenous inotrope therapy, active listing for heart transplant, a congenital heart defect, a diagnosis of cardiac amyloidosis, or a non-cardiac diagnosis anticipated to limit survival or functional status. All participating institutions were required to comply with local regularity and privacy guidelines and to submit the MedaMACS protocol for review and approval by their institutional review boards. This MedaMACS study from 2013–2015 is distinct from the screening pilot feasibility study that enrolled patients in a smaller group of centers between October 2010 and April 2011.9–11
Study Design and Outcome Assessment
Baseline demographics, clinical characteristics, laboratory, echocardiography, hemodynamic, and functional status data were collected at enrollment. The primary endpoints of the MedaMACS study were mortality on medical therapy or progression to LVAD placement or cardiac transplantation. Secondary endpoints included hospitalization, inotrope utilization, and measures of generic health-related quality of life as assessed using the EuroQoL visual analog scale (VAS). These measures and interval events were assessed at 1 month, 1 year, and 2 years after entry into the study. Additional phone calls to capture interval events were made at 6 and 18 months after enrollment. Originally, data were to be collected prospectively for patients up to a study endpoint or the pre-specified 2 year follow up period; however due to discontinuation of funding for ongoing data collection, the study ended on December 2016 and this report represents analysis of all available data collected up to this date.
Registry Based Comparison of Medical versus LVAD Therapy
In order to compare real-world, contemporary outcomes of medical therapy vs. LVAD therapy, patients in MedaMACS were compared to patients enrolled in the INTERMACS registry who were Profile 4–7 at the time of LVAD implantation.1 Specifically, the INTERMACS registry was queried during a similar time period from January 2012 to December 2016. We chose to focus on only those patients in INTERMACS who received a primary durable LVAD implant and excluded patients receiving a total artificial heart or biventricular assist devices. An intention-to-treat actuarial survival analysis was performed comparing the MedaMACS and INTERMACS outcomes for the entire group of Profile 4–7 patients and then after pre-specified stratification by INTERMACS Patient Profile. In addition, baseline and changes in health-related quality of life were determined for survivors in both MedaMACS and INTERMACS registries. In order to address potential biases with any missing quality of life data, comparisons between medical versus LVAD therapy were also made using a combined end-point of survival with good quality of life among those patients who completed the quality of life assessments.
Statistical Analysis
All statistical analyses were performed centrally at the University of Alabama at Birmingham Data and Clinical Coordinating Center. Numerical data were reported as mean values ± standard deviations or count (percentage). Univariate comparisons between the cohorts of patients based on INTERMACS Profile at enrollment were performed using the chi-square test of Fisher’s exact test for categorical variables and the one-way ANOVA test for continuous variables. For the entire MedaMACS study population, Kaplan-Meier survival curves were used to demonstrate (1) overall unadjusted actuarial survival (with patients censored at the time of transplant, LVAD placement, or last follow-up), (2) survival without LVAD (with patients censored at the time of transplant or last follow-up), and (3) survival without LVAD or transplant (with patients censored at the time of last follow-up). Within the MedaMACS study population, Kaplan-Meier survival curves and log rank tests were used to demonstrate unadjusted survival differences based on INTERMACS Profile at enrollment, with comparisons made between individual Profiles as well as Profiles 4–5 versus Profiles 6–7.
In addition, Kaplan-Meier survival curves and log rank tests were used to compare intention-to-treat actuarial survival among medical therapy patients in MedaMACS and LVAD therapy patients in INTERMACS. For medical therapy, patients were censored at the time of transplant, LVAD placement, or last follow-up. For LVAD therapy, patients were censored at the time of LVAD explant for recovery, transplant, or last follow-up. Pre-specified comparisons between medical and LVAD therapy were also made based on Patient Profile at the time of enrollment. Comparisons were made among the individual Patient Profiles (Profiles 4, 5, 6, and 7) as well as by combining Patient Profiles 4–5 and 6–7 due to the small number of patients in each of these individual groups. SAS 9.4 statistical software (Cary, NC) was used for all statistical analysis.
Results
Baseline Characteristics of the MedaMACS Cohort
A total of 161 patients were enrolled between May 2013 and October 2015. This cohort had a mean age of 59 years, LVEF of 21%, and NT-proBNP of 5365 pg/ml with 32% patients of non-Caucasian race and 34% female gender (Table 1). Reflective of an advanced HF population, INTERMACS Patient Profiles at enrollment were: Profile 4 (12%), Profile 5 (32%) Profile 6 (49%), and Profile 7 (7%). INTERMACS Profiles assigned by treating physicians at enrollment tracked with functional status and quality of life, with Profile 4 patients having worse measures of functional status and lower quality of life scores than other ambulatory patients on oral therapy (Table 2).
Table 1.
Baseline patient characteristics at enrollment into the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Study compared to pre-implant characteristics of Profile 4–7 patients who received a LVAD and were enrolled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).
| MedaMACS Study N=161 | INTERMACS N=1753 | |
|---|---|---|
| Demographics | ||
| Age (years) | 59 ± 11 | 61 ± 12 |
| Female gender | 34% | 18%* |
| Non-Caucasian race | 32% | N/A |
| Married or domestic partnership | 60% | 71%* |
| Heart Failure Characteristics | ||
| Ischemic etiology of cardiomyopathy | 39% | 52%* |
| Idiopathic dilated cardiomyopathy | 39% | 28%* |
| Other etiology of cardiomyopathy | 22% | 20% |
| Implantable Cardioverter Defibrillator | 85% | 89% |
| Cardiac Resynchronization Therapy | 31% | N/A |
| Inotrope therapy required in preceding 6 months | 18% | N/A |
| Number of cardiac hospitalizations in preceding year | ||
| One | 29% | 32% |
| Two | 38% | 34% |
| Three | 16% | 15% |
| Four or more | 17% | N/A |
| Unknown | 0% | 19% |
| INTERMACS Patient Profile | ||
| Profile 4 | 12% | 80%* |
| Profile 5 | 32% | 13%* |
| Profile 6 | 49% | 4%* |
| Profile 7 | 7% | 3%* |
| Medication Usage at Enrollment | ||
| ACE inhibitor/Angiotensin Receptor Blocker | 60% | 38%* |
| β-blockers | 90% | 77%* |
| Aldosterone antagonist | 64% | 50%* |
| Loop diuretics | 93% | 88% |
| Digoxin | 49% | N/A |
| Laboratory Values | ||
| NT-pro BNP (pg/ml) | 5365 ± 5065 | 829 ± 893* |
| Sodium (mmol/L) | 137 ± 4 | 137 ± 4 |
| Blood urea nitrogen (mg/dL) | 35 ± 20 | 27 ± 15* |
| Creatinine (mg/dL) | 1.5 ± 0.6 | 1.4 ± 0.7* |
| Alanine aminotransferase (u/L) | 34 ± 43 | 40 ± 80 |
| Aspartate aminotransferase (u/L) | 33 ± 24 | 34 ± 53 |
| Total bilirubin (mg/dL) | 1.2 ± 0.8 | 1.0 ± 0.8 |
| Total cholesterol (mg/dL) | 131 ± 43 | 153 ± 266 |
| Uric acid (mg/dl) | 9.6 ± 5.9 | 7.9 ± 2.6 |
| Hemoglobin (g/dl) | 12.7 ± 2.4 | 12.2 ± 2.0* |
| Echocardiographic and Hemodynamic Data | ||
| Left ventricular ejection fraction (%) | 21 ± 7 | 10 ± 7* |
| Left ventricular dimension diastole (cm) | 6.5 ± 0.9 | 6.8 ± 1.0* |
| Heart rate (beats per minute) | 79 ± 14 | 79 ± 15 |
| Systolic blood pressure (mmHg) | 111 ± 15 | 109 ± 16 |
| Diastolic blood pressure (mmHg) | 68 ± 11 | 66 ± 12* |
| Right atrial pressure (mmHg) | 11 ± 6 | 12 ± 8 |
| Pulmonary artery systolic pressure (mmHg) | 52 ± 13 | 48 ± 15* |
| Pulmonary artery diastolic pressure (mmHg) | 25 ± 8 | 22 ± 9* |
| Pulmonary wedge pressure (mmHg) | 21 ± 8 | 22 ± 9 |
| Cardiac output (L/min) | 4.5 ± 1.4 | 4.3 ± 1.4 |
| Cardiac index (L/min/m2) | 2.2 ± 0.7 | 2.3 ± 1.0 |
| Baseline Functional and Quality of Life Status | ||
| Six minute walk (m) | 196 ± 156 | 304 ± 1125 |
| Gait speed (m/s) | 1.0 ± 0.4 | 1.1 ± 0.5 |
| Peak oxygen consumption (mL/kg/min) | 12.1 ± 4.6 | 19.0 ± 8.2 |
| EuroQol 5D Index Score | 0.69 ± 0.19 | 0.68 ± 0.21 |
| EuroQol Visual Analog Scale | 56 ± 21 | 43 ± 24* |
| Kansas City Cardiomyopathy Questionnaire score | 51 ± 10 | 36 ± 20* |
P<0.01 for comparison of MedaMACS vs. INTERMACS.
N/A=Data Not Available, as these variables were not collected in INTERMACS
Table 2.
INTERMACS patient profiles at enrollment into the MedaMACS study track with functional status and quality of life.
| Profile 4 (N=21) | Profile 5 (N=49) | Profile 6 (N=76) | Profile 7 (N=15) | |
|---|---|---|---|---|
| Age (years) | 58 ± 10 | 60 ± 11 | 59 ± 11 | 57 ± 9 |
| Ejection fraction (%) | 21 ± 8 | 21 ± 7 | 21 ± 7 | 22 ± 6 |
| NT-pro BNP (pg/ml) | 6496 ± 4617 | 6407 ± 6952 | 4997 ± 3908 | 3564 ± 5946 |
| Six minute walk (m)* | 166 ± 143 | 143 ± 140 | 239 ± 154 | 212 ± 192 |
| Gait speed (m/s)* | 0.8 ± 0.3 | 0.9 ± 0.5 | 1.1 ± 0.4 | 1.4 ± 0.3 |
| Peak VO2 (mL/kg/min)* | 9.8 ± 4.1 | 10.9 ± 3.1 | 13.2 ± 5.5 | 12.9 ± 1.0 |
| EuroQol 5D Index* | 0.52 ± 0.21 | 0.70 ± 0.15 | 0.72 ± 0.19 | 0.73 ± 0.19 |
| EuroQol VAS* | 45 ± 20 | 54 ± 21 | 59 ± 21 | 60 ± 17 |
| KCCQ score | 52 ± 10 | 52 ± 12 | 50 ± 8 | 48 ± 14 |
P<0.05 for comparison across INTERMACS Patient Profiles
VO2=Oxygen consumption, VAS=Visual Analog Scale, KCCQ=Kansas City Cardiomyopathy Questionnaire
Primary and Secondary Endpoints of the Entire MedaMACS Study population
One-year primary and secondary endpoint data were available on 143 (89%) of the original 161 patients. Of the 18 (11%) patients without one-year outcomes data, 8 withdrew consent for the study and 10 transferred their care to a center not participating in the MedaMACS study. Of the 161 total participants, 55 (34%) experienced a primary endpoint after one year. This included 27 (17%) deaths, 13 (8%) LVAD implants, and 15 (9%) transplants (Table 3). This cohort of advanced HF patients also experienced substantial morbidity with an average of 1.7 additional hospitalizations per patient, 57% of patients requiring at least one rehospitalization for HF, and many requiring use of continuous intravenous inotropes (Table 3).
Table 3.
Primary and secondary endpoints of the entire MedaMACS Study population at 1 year.
| MedaMACS Study (N=161) | |
|---|---|
| Primary Endpoints | |
| Mortality | 27 (17%) |
| Left Ventricular Assist Device (LVAD) received | 13 (8%) |
| Transplant received | 15 (9%) |
| Alive without LVAD or Transplant* | 88 (55%) |
| Unknown* | 18 (11%) |
| Secondary Endpoints | |
| Inotropes required | 22 (14%) |
| At least one rehospitalization | 92 (57%) |
| Total Number of Rehospitalizations | 1.7 ± 2.2 |
8 patients withdrew consent; 10 patients transferred to centers not participating in MedaMACS
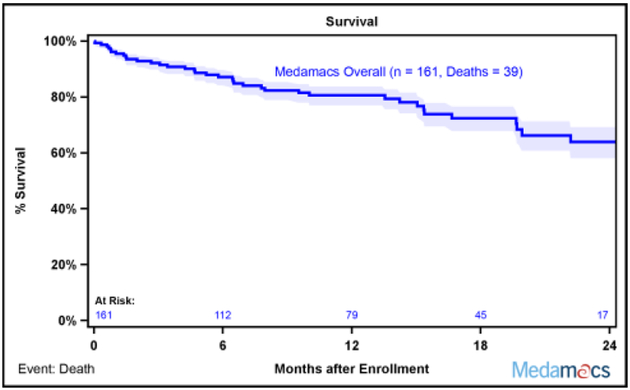
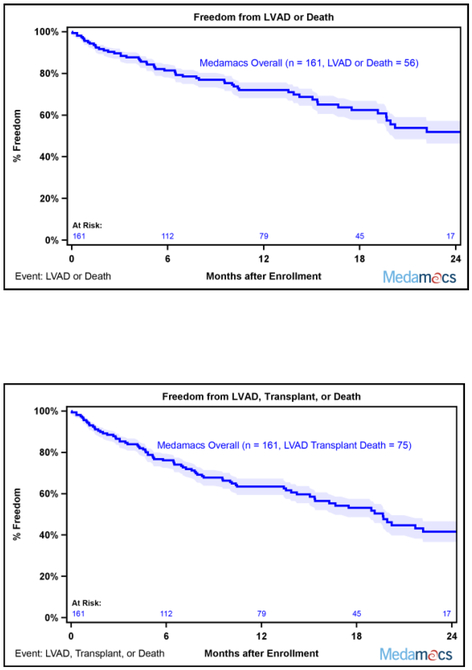
Additional follow up data were collected for patients until a study endpoint, a pre-specified 2 year follow up period, or the end of the study on December 2016. As enrollment continued until October of 2015, 2 year follow up data were not available for every enrolled patient. Of the initial 161 patients, 75 patients experienced a study endpoint (39 deaths, 17 LVAD implantations, and 18 transplants), 18 were lost to follow-up (as noted above, 8 withdrew consent and 10 transferred care), and 42 did not reach the full 2 year mark due to end of study funding on December 2016. Survival curves based on the maximal duration of follow up for each patient revealed that this ambulatory advanced HF patient population continued a downward trajectory with longer follow up, with 24% mortality after two years (Figure 1A). In addition, 35% of patients had either died or undergone LVAD implantation by 2 years (Figure 1B). Similarly, the rate of death, LVAD placement, and transplant remained high, as only 53% of patients were alive without a LVAD or transplant 2 years after enrollment (Figure 1C).
Fig. 1.
MedaMACS Two Year Survival. (A) Kaplan-Meier survival of the entire MedaMACS cohort through 24 months of follow-up. The mortality rate was 17% at 1 year and 24% at 2 years following enrollment. Patients were censored at time of transplant, left ventricular assist device (LVAD) placement, or last follow-up. (B) Kaplan-Meier survival free of LVAD. The rate of death or LVAD placement was 35% 2 years after enrollment. Patients were censored at time of transplant or last follow-up. (C) Kaplan-Meier survival free of LVAD or Transplant. The rate of death, LVAD placement, or transplant was 47% 2 years after enrollment. Patients were censored at time of last follow-up. Shaded areas represent 70% confidence intervals.
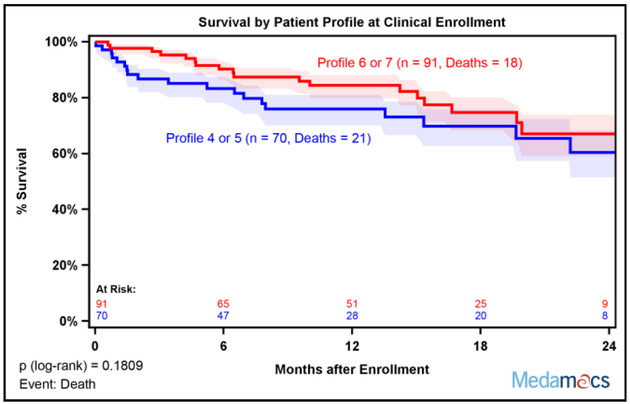
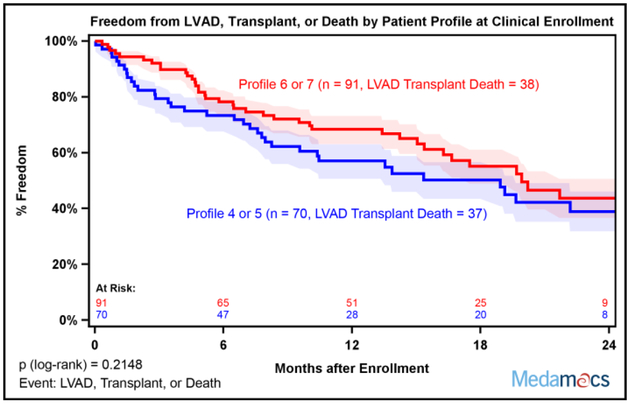
Despite the differences in baseline measures of functional status among the different INTERMACS Patient Profiles, there were not clear statistical differences in two year survival stratified by Patient Profile at the time of enrollment, which may have been related to small patient numbers among individual Profiles (Supplemental Figure 2). When comparisons were made between Profiles 4–5 versus 6–7, there was a trend for poorer outcomes among lower Patient Profiles that did not reach statistical significance (Figure 2).
Fig. 2:
Two Year Survival Stratified by Baseline INTERMACS Patient Profile. (A) Kaplan-Meier survival stratified by baseline Patient Profile was not statistically different. Patients were censored at time of transplant, left ventricular assist device (LVAD) placement, or last follow-up. (B) Kaplan-Meier survival free of LVAD or Transplant stratified by baseline Patient Profile was not statistically different. Patients were censored at time of last follow-up. Patient Profiles 4–5 and 6–7 were combined for analysis. Shaded areas represent 70% confidence intervals.
Survival with Medical vs. LVAD Therapy
A total of 10,139 patients received a primary durable LVAD between January 2012 and December 2016 (excluding total artificial hearts and biventricular assist devices). The majority (8386 patients) were INTERMACS Patient Profiles 1–3 at the time of LVAD implant and 1753 patients were Profiles 4–7 (Supplemental Figure 3). These 1753 LVAD patients from INTERMACS were compared to the 161 medical therapy patients from MedaMACS, all whom were Profiles 4–7 at enrollment. There were some differences in baseline characteristics between patients enrolled in MedaMACS and patients enrolled in INTERMACS (Table 1). Most notably, there was a smaller proportion of women, greater proportion of Profile 4 patients, lower HF medical therapy utilization, and worse baseline quality of life among INTERMACS patients. Interestingly, there were some markers of HF disease severity including natriuretic peptide levels and renal function that were actually worse among MedaMACS patients.
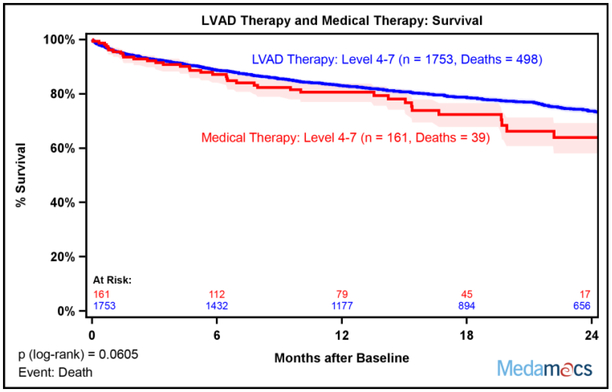
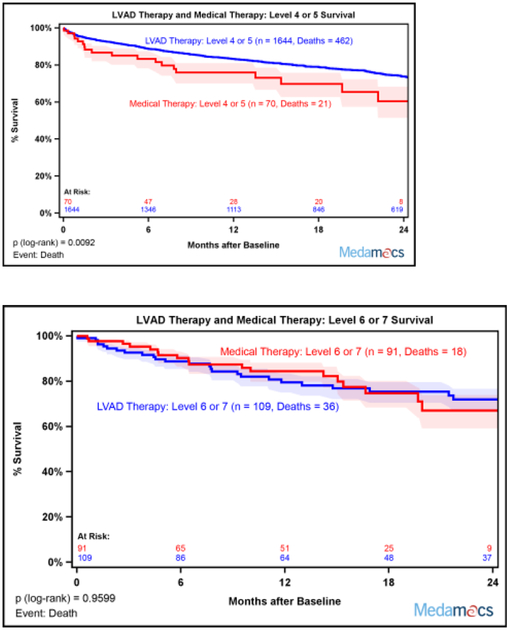
Actuarial survival by intention-to-treat was not different between the medical therapy cohort from the MedaMACS study and the real world LVAD therapy cohort from INTERMACS at two years (Figure 3). There was a numeric trend toward less favorable outcomes in medical therapy 24 months after enrollment in the overall cohort, p=0.0605. Analysis stratified by individual INTERMACS Profiles at enrollment showed a strong trend for better survival with LVAD therapy for Profile 4 and Profile 5 that did not reach statistical significance with no differences in Profile 6 and Profile 7 (Supplemental Figure 4). Analysis stratified by combining Patient Profiles 4 and 5 showed that LVAD therapy was associated with better survival compared to medical therapy, p=0.0092 (Figure 4A). However, survival curves for survival with medical versus LVAD therapy were superimposable for Profiles 6 and 7 (Figure 4B).
Fig. 3.
Intention to Treat Two Year Survival with Medical versus LVAD therapy. Kaplan-Meier survival was equivalent with medical therapy (MedaMACS cohort) compared to LVAD therapy (INTERMACS cohort). Medical therapy patients were censored at time of transplant, ventricular assist device placement, or last follow-up. LVAD therapy patients were censored at recovery, transplant, or last follow up. Shaded areas represent 70% confidence intervals.
Fig. 4.
Two Year Survival with Medical versus LVAD therapy stratified by Patient Profile at Enrollment. (A) Kaplan-Meier survival suggested improved actuarial survival with LVAD versus medical therapy among Profile 4–5 patients. (B) Kaplan-Meier survival was equivalent with medical versus LVAD therapy among Profile 6–7 patients. Medical therapy patients were censored at time of transplant, ventricular assist device placement, or last follow-up. LVAD therapy patients were censored at recovery, transplant, or last follow up. Shaded areas represent 70% confidence intervals.
Quality of Life with Medical vs. LVAD Therapy
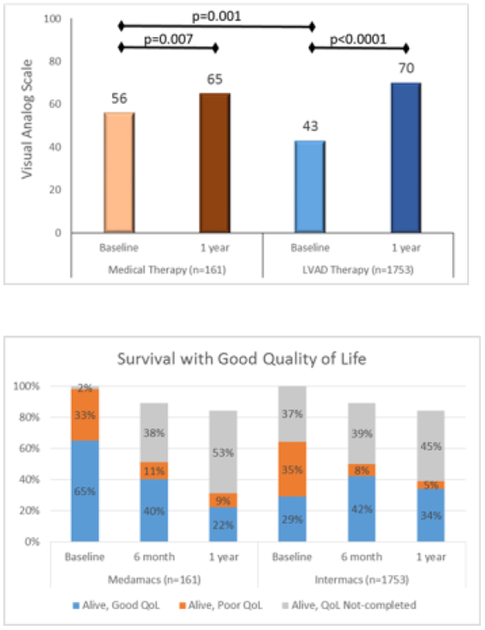
Health related quality of life, as assessed by the EuroQol VAS, was lower at baseline among patients receiving LVAD than those patients in whom medical therapy was continued, but increased after 1 year for the survivors in both cohorts (Figure 5A). Specifically, the VAS increased from 56 at baseline to 65 (p=0.007) at 1 year in the medical therapy cohort. There was a larger increase in quality of life among LVAD therapy patients as the mean EuroQol VAS increased from 43 at baseline to 70 (p<0.0001) after 1 year of follow up.
Fig. 5:
Quality of life and survival with medical versus LVAD therapy. (A) Health related quality of life scores as measured by the EuroQol Visual Analog Scale increased with both medical and LVAD therapy. (B) The proportion of patients alive with good quality of life decreased in the medical therapy arm, but increased in the LVAD therapy arm.
Survival with a good quality of life was defined as one-year survival with EuroQol VAS ≥50 and compared between the medical and LVAD cohorts. In order to account for bias that may result from patients who did not complete quality of life surveys, the results are presented as 3 groups for each cohort (Alive with Good QoL, Alive with Poor QoL, and Alive-QoL info not completed) at 3 time points (Baseline, 6 months, and 1 year) as shown in Figure 5B. There was a decrease in the proportion of patients who are Alive with Good QoL from baseline (65%) to 1 year (22%) among medical therapy patients. By contrast, the proportion of patients Alive with Good QoL increased from 29% at baseline to 34% at 1 year among LVAD therapy patients.
Discussion
Ambulatory Advanced HF Patients are at High Risk for Poor Outcomes
The MedaMACS study is the largest prospective study to date of the trajectory for ambulatory patients on oral therapy with advanced systolic HF followed at centers also offering LVADs. The 161 total patients enrolled represented a real-world, diverse population of patients with advanced HF who were receiving contemporary medical and electrical therapies. This is the largest population also of these patients with predominantly INTERMACS Profiles 5 and 6, usually considered “less sick” or “too well” for mechanical circulatory support. In this entire ambulatory advanced HF cohort, two year event rates were striking, with 24% mortality, 11% VAD, 12% transplant, and only 53% alive on medical therapy. Given that only approximately half of the patients were alive on medical therapy without a transplant or VAD at two years, this suggests that HF patients similar to those enrolled in the MedaMACS study are at substantial risk for poor outcomes on continued medical therapy. The morbidity in this population was also high as over half required another hospitalization, and many required multiple hospitalizations for HF. Given the high risk of failing oral medical therapies, ambulatory advanced HF patients as described by the inclusion criteria for this study should be considered for life-saving treatmentfor advanced heart failure such as cardiac transplantation and LVAD placement.
Medical vs. LVAD Therapy in Ambulatory Advanced Heart Failure
The comparison of medical therapy from the 161 patients enrolled in MedaMACS to LVAD therapy from the 1753 Profile 4–7 patients enrolled in the INTERMACS registry represents the single largest comparison of medical vs. LVAD therapy in ambulatory advanced HF. There was no difference in intention-to-treat survival between medical vs. LVAD therapy. However, the medical therapy patients who were Profiles 4 and 5 appeared to be at exceptionally high risk for poor outcomes, and had better survival with LVAD therapy. This suggests that Profile 4 and 5 symptoms are a robust predictor of poor outcomes, and as the event rate with medical therapy is so high in this cohort, strong consideration should be given to not-delaying LVAD therapy in eligible patients who may benefit from current mechanical support devices under existing indications. In fact, as this was an intention-to-treat analysis where patients did cross over and receive transplants and LVADs, this analysis suggests there may be a mortality cost to delaying LVAD therapy among Profile 4 and 5 patients.
Among Profile 6 and 7 patients, given the equivalent survival with medical and LVAD therapy, additional considerations may be needed to guide whether a patient gets a LVAD or continues with medical therapy.12 The adverse event profile and rates of contemporary LVADs may influence patient decision-making, but could change with advancements in pump technology. Furthermore, the patient reported outcome of health-related quality of life as measured by EuroQoL VAS improved with both medical and LVAD therapy, with a greater net increase following LVAD therapy related to a lower baseline score. Future studies comparing medical with LVAD in this less sick advanced HF population should emphasize patient-reported outcomes to enhance shared decision making about when to proceed with mechanical support.6,8,13
MedaMACS Confirms and Extends the Results of ROADMAP
The use of LVADs in non-inotrope dependent advanced HF was studied recently in the Risk Assessment and Comparative Effectiveness of LVAD and Medical Management in Ambulatory HF Patients (ROADMAP) study, where intention-to-treat survival was identical for LVAD and medical therapy at 1 or 2 years of follow-up.14–16 The results of the MedaMACS study not only confirm some of the findings from ROADMAP, but extend the findings to a larger real world population (Table 4). In addition, the MedaMACS study included a higher proportion of INTERMACs Profiles 5–7 which were previously not as well studied. As expected with this shift toward less sick patients with an emphasis on Profiles 5 and 6, the overall mortality rate at 1 and 2 years is lower in the MedaMACS study than in ROADMAP. While there was no intention-to-treat survival benefit for LVAD therapy in ROADMAP, the larger MedaMACS study for the first time suggests a mortality benefit for proceeding with LVAD therapy among Profile 4–5 patients and not waiting for clinical deterioration prior to implantation. An important caveat in the interpretation of both the MedaMACS and ROADMAP studies is that these non-randomized studies did not specifically require eligibility for LVAD, and thus included some patients with contraindications to LVAD therapy in the medical arm. The conditions which render patients less likely to be LVAD candidates may also render them at higher risk on medical therapy.7
Table 4.
Medical versus LVAD therapy in ambulatory non-inotrope dependent advanced heart failure. A comparison of Patient Profiles at enrollment and raw mortality with medical therapy (MedaMACS), LVAD therapy (INTERMACS), and the medical and LVAD therapy groups of the ROADMAP study.14,15.
| MedaMACS N=161 | INTERMACS N=1753 | ROADMAP Medical Therapy N=103 | ROADMAP LVAD Therapy N=97 | |
|---|---|---|---|---|
| Patient Profile | ||||
| Profile 4 | 12% | 80% | 34% | 65% |
| Profile 5 | 32% | 13% | 28% | 22% |
| Profile 6 | 49% | 4% | 34% | 10% |
| Profile 7 | 7% | 3% | 2% | 0% |
| Outcome | ||||
| Death at 1 year | 17% | 16% | 22% | 20% |
| Death at 2 year | 24% | 28% | 30% | 26% |
Furthermore, similar patterns for improvements in quality of life scores in both the medical and LVAD therapy arms were observed in both MedaMACS and ROADMAP. Such similar patterns of survival and quality of life with medical and LVAD therapy highlight the need for endpoints that integrate survival and robust assessments of patient-reported outcomes such as quality of life, particularly among Profile 6 and 7 patients. Survival endpoints alone may not be adequate to distinguish meaningful outcomes between medical and LVAD therapy in an ambulatory population of advanced HF.17
Limitations
The results of this study should be interpreted in light of several limitations. First, this was a registry-based comparison of medical vs. LVAD therapy, and not a randomized controlled trial. This introduces unmeasured confounding in the comparison of the two cohorts, which are different as depicted by the baseline quality of life data. The patients included in the LVAD therapy arm were not propensity matched but rather included based on INTERMACS Patient Profile 4–7 at enrollment which has some subjectivity. Also, not all medical therapy patients completed 2 years of follow-up and these patients were censored at the time of last follow-up in Kaplan-Meier analysis. Despite this reduction in statistical power, there were still differences detected between medical versus LVAD therapy. Furthermore, the survival analysis was intention-to-treat, and many of the medical therapy patients received a transplant or underwent LVAD implantation to prevent death during the study, so the benefit of these life-saving therapies was not fully captured in this analysis. In addition, LVAD recipients were inclusive of all INTERMACS centers, while medical participants were restricted to just 11 centers which could limit the generalizability, but this was necessary as restriction to ambulatory patients with Profiles 4–7 at the time of LVAD implantation would have resulted in too few patients had the analysis been restricted to just 11 centers. Event rates in the different INTERMACS Profiles in the medical cohort should be interpreted within the context of study entry criteria, which mandated a recent HF hospitalization and other features defining an advanced stage of HF. Data regarding the reason for hospitalization or characteristics at the time of a study endpoint was also not captured. Finally, quality of life assessments were not completed by all patients. Despite these limitations, the MedaMACS study is the single largest published prospective outcome study of advanced HF patients on oral medical therapy and reflects real-world contemporary practice.
Conclusions
Ambulatory patients with advanced HF in INTERMACS Profiles 4–7 who are receiving an initial strategy of oral medical therapy are at high risk for poor outcomes, including mortality and the need for LVAD or transplantation. Only 53% of MedaMACS subjects were alive with ongoing medical therapy after 2 years of follow up. As there are life-saving treatment options available for patients within this high risk group—earlier referral to LVAD-transplant centers for consideration of life-saving treatment options is needed so that patients may make well-informed decisions. Although overall two year survival for the entire cohort is similar with LVAD or medical therapy, Profiles 4 and 5 identify patients with poor outcomes with medical therapy who may have better survival with LVAD therapy. Future studies should include a combined endpoint that includes survival with a patient-centered outcome such as quality of life to better inform decisions about the timing of mechanical support prior to inotrope dependence.
Supplementary Material
Acknowledgements
The authors are indebted to Lynne Warner Stevenson, MD, for the encouragement and formation of the MedaMACS Registry collaboration. The authors also wish to acknowledge Marissa A. Miller, DVM, MPH of the Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute for support of the MedaMACS Registry.
Funding/Support
This project has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C and with funds received from the Board of Trustees of the University of Alabama for the University of Alabama at Birmingham. Dr. Ambardekar was supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program. Dr. Kirklin received support as Director of the STS Intermacs Registry, which is paid to his institution. Dr. Stevenson received support from NHLBI HHSN268201100025C as co-investigator for INTERMACS. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
Dr. DeVore reports receiving research support from the American Heart Association, Amgen, the NIH, and Novartis and consulting with Novartis. Dr. Teuteberg reports receiving advertising board and speaking honoraria from Medtronic, CareDx, and Abiomed as well as receiving support from Abbott for the HeartMate 3 Clinical Events Committee. The remaining authors have no disclosures.
References
- 1.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43. [DOI] [PubMed] [Google Scholar]
- 3.Hashim T, Sanam K, Revilla-Martinez M, et al. Clinical Characteristics and Outcomes of Intravenous Inotropic Therapy in Advanced Heart Failure. Circ Heart Fail 2015;8:880–6. [DOI] [PubMed] [Google Scholar]
- 4.McIlvennan CK, Magid KH, Ambardekar AV, Thompson JS, Matlock DD, Allen LA. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail 2014;7:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breathett K, Allen LA, Ambardekar AV. Patient-centered care for left ventricular assist device therapy: current challenges and future directions. Curr Opin Cardiol 2016;31:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012;125:1928–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambardekar AV, Forde-McLean RC, Kittleson MM, et al. High early event rates in patients with questionable eligibility for advanced heart failure therapies: Results from the Medical Arm of Mechanically Assisted Circulatory Support (Medamacs) Registry. J Heart Lung Transplant 2016;35:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambardekar AV, Thibodeau JT, DeVore AD, et al. Discordant Perceptions of Prognosis and Treatment Options Between Physicians and Patients With Advanced Heart Failure. JACC Heart Fail 2017;5:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart GC, Kittleson MM, Cowger JA, et al. Who wants a left ventricular assist device for ambulatory heart failure? Early insights from the MEDAMACS screening pilot. J Heart Lung Transplant 2015;34:1630–3. [DOI] [PubMed] [Google Scholar]
- 10.Stewart GC, Kittleson MM, Patel PC, et al. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) Profiling Identifies Ambulatory Patients at High Risk on Medical Therapy After Hospitalizations for Heart Failure. Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 11.Stewart GC, Ambardekar AV, Kittleson MM. Defining Ambulatory Advanced Heart Failure: MedaMACS and Beyond. Curr Heart Fail Rep 2017;14:498–506. [DOI] [PubMed] [Google Scholar]
- 12.Bruce CR, Blumenthal-Barby JS, Meyers D. Benefits and Challenges of Early Introduction of Left Ventricular Assist Device Placement: A Patient-Centered Perspective. J Am Coll Cardiol 2015;66:1762–5. [DOI] [PubMed] [Google Scholar]
- 13.McIlvennan CK, Thompson JS, Matlock DD, et al. A Multicenter Trial of a Shared Decision Support Intervention for Patients and Their Caregivers Offered Destination Therapy for Advanced Heart Failure: DECIDE-LVAD: Rationale, Design, and Pilot Data. J Cardiovasc Nurs 2016;31:E8–E20. [DOI] [PubMed] [Google Scholar]
- 14.Estep JD, Starling RC, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol 2015;66:1747–61. [DOI] [PubMed] [Google Scholar]
- 15.Starling RC, Estep JD, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail 2017;5:518–27. [DOI] [PubMed] [Google Scholar]
- 16.Shah KB, Starling RC, Rogers JG, et al. Left ventricular assist devices versus medical management in ambulatory heart failure patients: An analysis of INTERMACS Profiles 4 and 5 to 7 from the ROADMAP study. J Heart Lung Transplant 2018;37:706–14. [DOI] [PubMed] [Google Scholar]
- 17.Stehlik J, Estep JD, Selzman CH, et al. Patient-Reported Health-Related Quality of Life Is a Predictor of Outcomes in Ambulatory Heart Failure Patients Treated With Left Ventricular Assist Device Compared With Medical Management: Results From the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.