Abstract
Following acute infection of mucosal surfaces by bovine herpesvirus 1 (BoHV-1), sensory neurons are a primary site for life-long latency. Stress, as mimicked by the synthetic corticosteroid dexamethasone, consistently induces reactivation from latency. Two viral regulatory proteins (VP16 and bICP0) are expressed within 1 hour after calves latently infected with BHV-1 are treated with dexamethasone. Since the immediate early transcription unit 1 (IEtu1) promoter regulates both bICP0 and bICP4 expression, we hypothesized that the bICP4 protein is also expressed during early stages of reactivation from latency. In this study, we tested whether infected cell protein 4 (bICP4) and bICP22, the only other BoHV-1 protein known to be encoded by an immediate early gene, were expressed during reactivation from latency by generating peptide specific antiserum to each protein. bICP4 and bICP22 protein expression were detected in trigeminal ganglionic (TG) neurons during early phases of dexamethasone induced reactivation from latency, operationally defined as the escape from latency. Conversely, bICP4 and bICP22 were not readily detected in TG neurons of latently infected calves. In summary, it seems clear that all proteins encoded by known BoHV-1 IE genes (bICP4, bICP22, and bICP0) were expressed during early stages of dexamethasone-induced reactivation from latency.
Keywords: bovine herpevirus 1, stress-induced reactivation from latency, ICP4, ICP22
INTRODUCTION
Acute infection of cattle by bovine herpesvirus 1 (BoHV-1) can result in clinical disease in the upper respiratory tract, nasal cavity, and ocular cavity (Jones and Chowdhury, 2007). Furthermore, BoHV-1 can cause reproductive failure in cattle following infection of the ovary and/or fetus (Chase et al., 2017), making it the most frequently diagnosed cause of viral abortion in North America. Following acute infection, a primary site of BoHV-1 latency is sensory neurons in trigeminal ganglia (TG). Periodically, reactivation from latency occurs: consequently, the virus is widespread in cattle (Jones, 1998, 2003, Jones et al., 2006, Jones, 2009). The incidence of BoHV-1 reactivation from latency is increased following stressful stimuli that increase corticosteroid levels, reviewed in (Jones et al., 2011; Jones and Chowdhury, 2007; Perng and Jones, 2010). Administration of the synthetic corticosteroid dexamethasone (DEX) to latently infected calves or rabbits consistently induces BoHV-1 reactivation from latency (Inman et al., 2002; Jones, 1998, 2003, Jones et al. 2006; Jones et al., 2000; Rock et al., 1992). Six hours after DEX treatment lytic cycle viral RNA expression is readily detected in a subset of trigeminal ganglionic neurons of latently infected calves (Winkler et al., 2002; Winkler et al., 2000). Within 1–3 hours after dexamethasone treatment of latently infected calves, which is operationally defined as the escape from latency, two viral regulatory proteins are detected (VP16 and bICP0) (Frizzo da Silva et al., 2013; Kook et al., 2015a). Interestingly, bICP0 and VP16+ neurons detected during reactivation from latency, also frequently express the glucocorticoid receptor (GR). In contrast to VP16, other late viral proteins (gC and gD) were not detected until later after dexamethasone-induced reactivation from latency.
In contrast to herpes simplex virus 1 (HSV-1), BoHV-1 expresses only 3 proteins encoded by IE genes: bICP0, bICP4, and bICP22 (Jones, 2003). Furthermore, expression of bICP0 is complex because two distinct promoters regulate its expression. For example, the IE transcription unit 1 (IEtu1) promoter drives expression of bICP0 and bICP4 because a single IE transcript is differentially spliced and then translated into bICP0 or bICP4 (Wirth et al., 1992; Wirth et al., 1989; Wirth et al., 1991) and (Figure 1A and B). The bICP0 protein is also translated from an E mRNA (E2.6) because a separate E promoter drives expression of the bICP0 E transcript (Fraefel et al., 1994; Wirth et al., 1992; Wirth et al., 1989; Wirth et al., 1991), Figure 1A and B. IEtu1 promoter activity is stimulated by the GR and the synthetic corticosteroid dexamethasone because two consensus GR response elements (GREs) are located in the promoter (El-Mayet et al., 2017; Kook et al., 2015b) suggesting this promoter is activated by stress induced transcription factors during reactivation from latency. If the IEtu1 promoter is activated by cellular factors during reactivation from latency, bICP4 should also be expressed during the escape from latency.
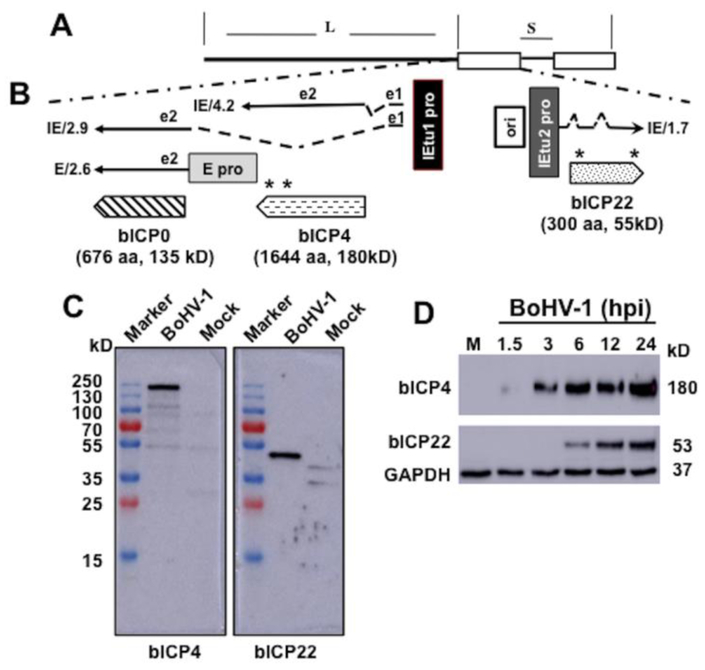
Figure 1: Schematic of IE region and location of the bICP0, bICP4, and bICP22 genes.
Panel A: Structure of BoHV-1 genome and location of unique long (L) region, direct repeats (open rectangles), and unique long region (S).
Panel B: The IE/4.2 mRNA encodes the bICP4 protein and IE/2.9 mRNA encodes the bICP0 protein. A single IE promoter activates expression of IE/4.2 and IE/2.9 (IEtu1; black rectangle). E/2.6 is the early bICP0 mRNA and is regulated by the bICP0 early promoter (E pro; gray rectangle). bICP0 protein coding sequences are in Exon 2 (e2). Origin of replication (ORI) separates IEtu1 from IEtu2. IEtu2 promoter (IEtu2 pro) regulates IE1.7 mRNA expression, which is translated into the bICP22 protein. Solid lines in IE/2.9, IE/4.2, and IE/1.7 are exons (e1, e2, or e3) and dashed lines introns. Location and size of the three proteins encoded by IEtu1 and IEtu2 are shown: arrows denote directionality of the proteins. The bICP4 peptides synthesized for producing the peptide specific antibody are: amino acids 1461–1478 (RRAGQAPGREAREGRGRG) and amino acids 1604–1621 (GVSPWGSRGVRAFRRPPG). The peptides synthesized for producing the bICP22 antibody are: amino acids 26–40 (GPAPADEHARRGPGA) and amino acids 281–296 (GSPSGRARARPAPAKR). An asterisk denotes the location of the peptides within the bICP4 and bICP22 ORFs.
Panel C: Monolayers of CRIB cells were mock infected or infected with BoHV-1 (MOI=2) and whole-cell lysate prepared at 12 hours after infection. Proteins (50 ug protein in each lane) were separated in a 10% SDS-PAGE and detected by Western blotting using the rabbit anti-bICP4 or bICP22 peptide antibody as described in Materials and Methods. The marker lane is a Thermo Scientific Page Ruler pre-stained protein marker and the size of these proteins is denoted on the left.
Panel D: As described above, CRIB cells were mock infected (lane M) or infected with BoHV-1 (MOI=2) for the denoted time (hours after infection). Whole-cell lysate (50 ug protein in each lane) was separated in a 10% SDS-PAGE and detected by Western blotting using the rabbit anti-bICP4 or bICP22 peptide antibody. GAPDH protein levels were analyzed in the respective samples as a loading control. Approximate size of the respective proteins is denoted on the right of the Western Blots.
Although we previously developed antiserum that recognizes bICP4 during productive infection by Western blot studies, this serum yielded high levels of background when formalin fixed and paraffin embedded TG sections prepared from latently infected calves or during reactivation from latency were incubated with the same antiserum (data not shown). Consequently, we developed new antiserum directed against two bICP4 peptides to test whether its expression was induced during reactivation from latency. We also developed antiserum directed against the other IE protein (bICP22) to compare its expression during the latency-reactivation cycle. bICP04+ and bICP22+ TG neurons were detected at 3 hours after dexamethasone treatment, but were not readily detected in TG prepared from latently infected calves prior to DEX treatment or uninfected TG. Consecutive sections suggested that a subset of bICP4+ or bICP22+ TG neurons were also GR+. In summary, similar to bICP0, these studies suggested bICP4 and bICP22 were expressed during the escape from latency.
MATERIALS AND METHODS
Cells, virus, and antibodies:
Bovine kidney (CRIB) cells, which are unable to be infected by bovine viral diarrhea virus (BVDV), (Flores et al., 1996) were obtained from ATCC (Manassas, VA). CRIB cells were maintained in Minimal Essential Medium (MEM) supplemented with 10% fetal calf serum, penicillin (10 U/ml), and streptomycin (100 μg/ml). The Cooper strain of BoHV-1 (wt virus) was obtained from the National Veterinary Services Laboratory, Animal and Plant Health Inspection Services (Ames, IA) and was grown in CRIB cells. Pierce Custom Services, Thermo Scientific (Rockford, IL) identified potential antigenic peptides and then generated peptide-specific rabbit polyclonal antibodies directed against bICP4 and bICP22 (Program No. TA2422X and TA2422X). For details of the peptides used and the location of these peptides, see Figure 1B and legend of Figure 1.
Bovine TG samples:
Unvaccinated male calves (primarily Holsteins) from dairy farms in Nebraska were bled and the presence of BoHV-1 antibodies examined by an enzyme-linked immunosorbent assay (ELISA) performed by the University of Nebraska Diagnostic Center. Calves (~200 kg) that lacked BoHV-1 specific antibodies were inoculated with 107 PFU of BoHV-1 into ocular and nasal cavities as described previously (Inman et al., 2001, 2002; Lovato et al., 2003; Perez et al., 2005; Perez et al., 2006; Perez et al., 2008). Calves were housed under strict isolation and given antibiotics to prevent bacterial pneumonia. At 60 days post-inoculation (dpi), calves were considered to be latently infected, and were injected intravenously (jugular vein) with 100 mg of water-soluble DEX (Sigma, D2915, St Louis, MO) to initiate reactivation from latency. The latently infected and DEX treated calves were then anesthetized with XylaMed (Bimeda, Inc, LE Sueur, MN) followed by electrocution. After decapitation, TG were collected, and samples from each TG were formalin fixed and paraffin embedded. The remainder of both TG were minced into small pieces were then stored at −80 °C. TG used in this study were derived from earlier studies (Kook et al., 2015; Workman et al., 2012). Experiments were performed in accordance with the American Association of Laboratory Animal Care guidelines and the University of Nebraska IACUC committee.
Immunohistochemistry:
Immunohistochemistry was performed using the Ultra-Sensitive ABC Rabbit IgG Staining Kit (32054, Pierce; Thermo Scientific; Rockford, IL) essentially as previously described (Frizzo da Silva et al., 2013; Kook et al., 2015; Liu et al., 2016; Workman et al., 2018; Zhu et al., 2017). Briefly, thin sections (4 to 5 μm) were cut from each TG and mounted onto slides and processed as described previously (Liu et al., 2016; Sinani et al., 2013). After blocked with the animal-free blocking solution (15019L, Cell Signaling; Danvers, MA) for 1 h at room temperature, tissue sections were subsequently incubated with the bICP4 (1:250 dilution; TA2422X; Pierce Custom Services, Thermo Scientific, Rockford, IL) or bICP22 peptide-specific rabbit polyclonal antibody (1:100 dilution; TA2424X; Pierce Custom Services, Thermo Scientific, Rockford, IL), or GR-specific rabbit monoclonal antibody (1:250 dilution; 12041; Cell Signaling; Danvers, MA) overnight in a humidified chamber at 4 °C. Three washes in 1×TBS (pH 7.6) containing 0.025% Triton X 100 (TBST) were performed between each step. The next day, slides were incubated with biotinylated goat anti-rabbit IgG (32054, Pierce Custom Services, Thermo Scientific, Rockford, IL) for 30 min at room temperature in a humidified chamber followed by Avidin-biotinylated enzyme complex (32054, Pierce Custom Services, Thermo Scientific, Rockford, IL) incubation for 30 min at room temperature. After washing in 1×TBS, slides were incubated with freshly prepared substrate (SK-4800; Vector Laboratories; Cole-Parmer; Vernon Hills, IL), rinsed with distilled water, and lightly counterstained with hematoxylin (51275; Sigma-Aldrich, St Louis, MO). Thin sections from latently infected calves were used as a negative control.
Western blot analysis:
Confluent CRIB cells grown in 60-mm dishes were infected with a multiplicity of infection (MOI) of 2 PFU/cell of BoHV-1. At the designated times after infection, cells were washed with PBS and lysed with 0.2 ml of radioimmunoprecipitation assay (RIPA) buffer (1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with complete protease inhibitor (Roche Molecular Biochemicals; Indianapolis, IN) in 10 ml buffer). The respective samples were boiled in Laemmli sample buffer for 5 min, and all samples were separated on a 10% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore-Sigma; St Louis, MO) and blocked for 2 h in 5% nonfat dry milk with Tris-buffered saline-0.1% Tween 20 (TBS-T). Membranes were then incubated with bICP4 or bICP22 peptide-specific rabbit polyclonal antibody (1:1000 dilution) overnight at 4°C. An antibody directed against Glyceraldehyde 3-phosphate dehydrogenase (GAPHD) (1:20,000 dilution; sc-365062; Santa Cruz Biotechnology; Santa Cruz, CA) was used as a loading control. After washing with TBS-T (5 times for 5 minutes each), blots were incubated with anti-rabbit IgG, HRP-linked antibody (7074S; Cell Signaling; Danvers, MA) or ECL™ anti-mouse IgG horseradish peroxidase linked whole antibody (from sheep) GPR (NXA931V; GE Healthcare UK Limited), which was diluted 1:2,000 in 5% nonfat milk in TBS-T. Blots were then washed with TBS-T as above, exposed to Clarity™ Western ECL Substrate (1705061; Bio-Rad; Hercules, CA), and autoradiography performed.
RESULTS
Expression of bICP4 and bICP22 in productively infected bovine cells
Peptide specific antibodies directed against bICP4 and bICP22 were generated in rabbits using two peptides for each ORF (see Figure 1B for location of the individual peptides and legend of Figure 1 for amino acids used for each peptide). To test the specificity of the bICP4 and bICP22 antibodies, CRIB cells were infected with BoHV-1 (MOI of 2) for 12 hours and then viral protein expression examined by Western blot analysis (Figure 1C). bICP4 protein expression (approximately 180 kD protein) was detected in infected cells, but not in mock-infected cells. The bICP22 anti-serum in infected, but not uninfected cell lysate specifically recognized a 53 kD protein. Based on the size of the bICP4 and bICP22 open reading frames, the proteins migrated at the expected size. Additional studies compared bICP4 and bICP22 expression at 1.5, 3, 6, 12, and 24 hours post-infection (hpi) by Western blots analysis. As shown in Fig. 1D, bICP4 was readily detected in infected cells as early as 3 hpi and at 6, 12, and 24 hpi. In contrast to bICP4, bICP22 was not readily detected until 6 hpi and also at 12 and 24 hpi. As expected, bICP4 and bICP22 were not detected in uninfected cells: conversely, similar levels of GAPDH were detected in uninfected and infected cells. In summary, these results indicated that both bICP4-and bICP22-peptide specific antibodies recognized the expected protein during productive infection.
Detection of bICP4 and bICP22 during reactivation from latency
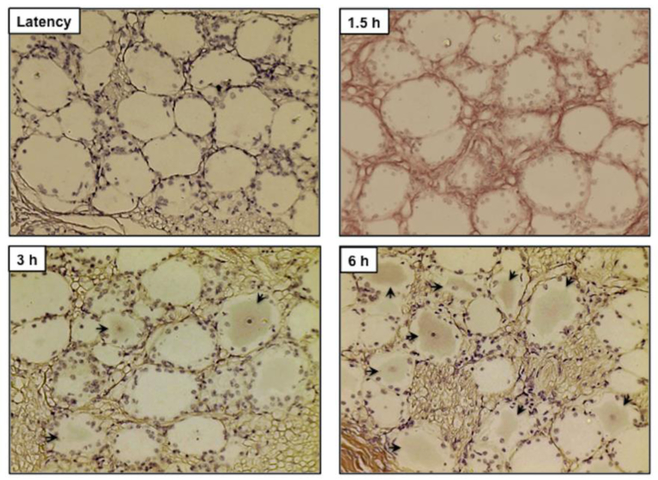
To test whether bICP4 and bICP22 protein expression was detected during reactivation from latency, immunohistochemistry (IHC) studies were performed using TG sections from latently infected calves, and calves treated with DEX to induce reactivation from latency. As expected, bICP4-positive (bICP4+) TG neurons were not readily detected in calves latently infected with wt BoHV-1 (Figure 2) or uninfected calves (data not shown). In contrast, bICP4+ TG neurons were detected in the latently infected TG neurons that were treated with DEX for 3 and 6 h (arrows denote TG neurons that were specifically stained): however, bICP4+ TG neurons were not detected at 1.5 hours after DEX treatment.
Figure 2. bICP4 is expressed in TG neurons following DEX treatment to induce reactivation from latency.
Thin sections were cut from formalin-fixed, paraffin-embedded TG sections of the latently infected claves and those treated with DEX for the designated times (1.5 h, 3 h and 6 h) after infection. IHC was performed using rabbit anti-bICP4 peptide antibody (1:250) and Biotinylated goat anti-rabbit IgG (Vector Laboratories) were used as the primary and secondary antibodies as described in Materials and Methods. Arrows denote neurons that were recognized by the bICP4 antibody. Magnification is approximately 400X.
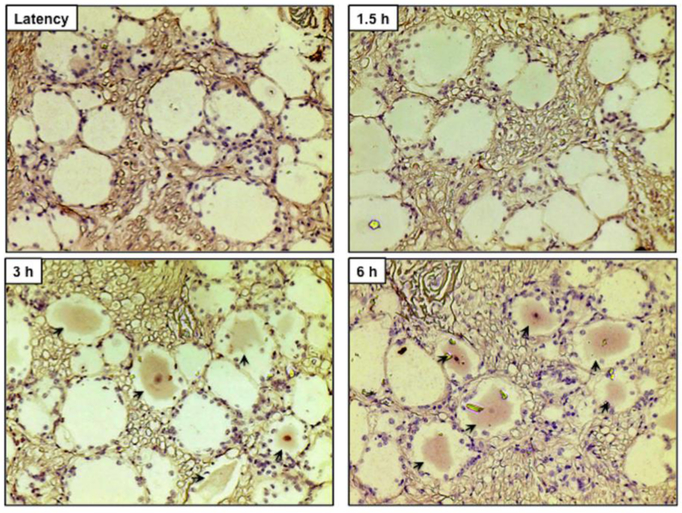
As with the bICP4 anti-serum, the bICP22 specific anti-serum did not readily detect TG neurons from latently infected (Figure 3) or uninfected calves (data not shown). Conversely, bICP22+ TG neurons were readily detected in latently infected TG that had been treated with DEX for 3 and 6 h. In some TG neurons, the nucleus was heavily stained with the bICP22 antibody. bICP22+ TG neurons were also not readily detected at 1.5 hours after DEX treatment.
Figure 3. bICP22 is expressed in TG neurons following DEX treatment to induce reactivation from latency.
Thin sections were cut from formalin-fixed, paraffin-embedded TG sections of the latently infected claves and these treated with DEX for the designated times (1.5 h, 3 h and 6 h) after infection. IHC was performed using rabbit anti-bICP22 peptide antibody (1:100) and Biotinylated goat anti-rabbit IgG (Vector Laboratories) were used as the primary and secondary antibodies as described in Materials and Methods. Arrows denote neurons recognized by the bICP22 antibody. Magnification is approximately 400X.
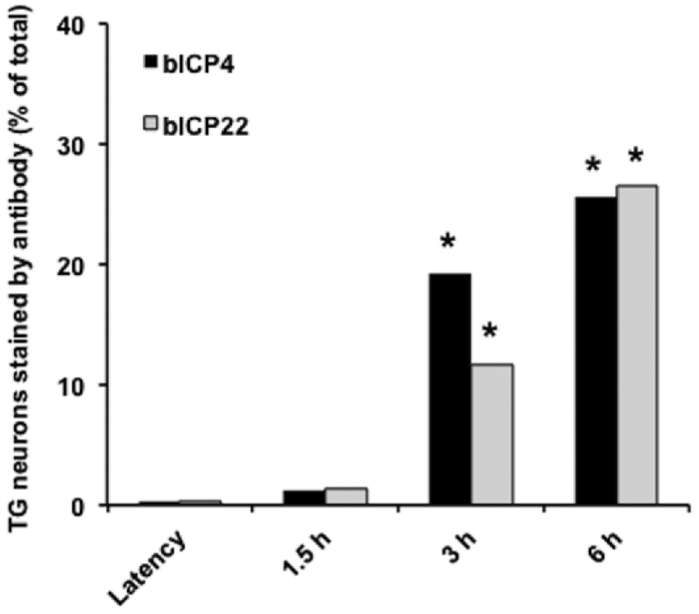
The percentage of bICP4+ and bICP22+ TG neurons was estimated by counting neurons in many sections (Figure 4). At 3 and 6 h after DEX treatment, approximately 19 and 25% TG neurons were bICP4. For bICP22, approximately 11% of TG neurons were bICP22+ and 26% at 6 h after DEX treatment. Since it is impossible to section the entire bovine TG and perform IHC on all sections, these numbers are only an estimation of TG neurons expressing bICP4 or bICP22. Furthermore, bICP4+ or bICP22+ TG neurons were not uniformly expressed in each section. With that said, it was clear there were significantly more bICP4+ or bICP22+ TG neurons at 3 and 6 h after DEX treatment relative to latently infected TG or at 1.5 h after DEX treatment.
Figure 4. Estimating the number of neurons that express bICP4 and bICP22 during reactivation from latency.
The % of bICP4+ TG neurons was estimated by counting the following number of TG neurons: latency (538), 1.5 h after DEX treatment (515), 3 h after DEX treatment (668), and 6 hours after DEX treatment (546). The % of bICP22+ TG neurons was estimated by counting the following numbers of TG neurons: latency (582), 1.5 h after DEX (497), 3 h after DEX treatment (734), and 6 h after DEX treatment (597). An asterisk denotes a significant increase in bICP4+ or bICP22+ TG neurons relative to latency. The asterisks denote significant differences (P<0.001) in the numbers of bICP4+ neurons as determined by a Student t test.
Certain TG neurons that express bICP4 or bICP22 also express the GR.
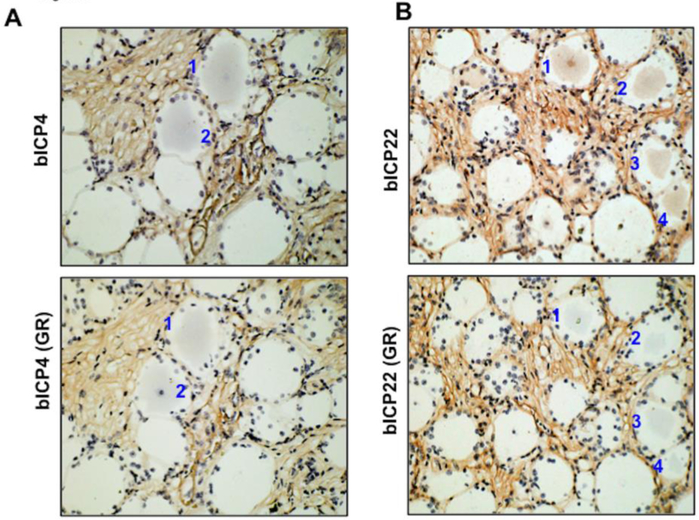
Since glucocorticoid receptor positive (GR+) TG neurons increase during reactivation from latency, and TG neurons that express bICP0 during early stages of reactivation frequently express the GR (Frizzo da Silva et al., 2013; Kook et al., 2015a), we further tested whether GR and bICP4 or bICP22 were expressed in the same neuron following DEX treatment. Consecutive sections were prepared from TG samples at 6 h after DEX treatment, and each section was stained with the GR, bICP4 or bICP22 specific antibody. GR+ and bICP4+ (Figure 5A) or bICP22+ (Figure 5B) TG neurons were detected in a subset of TG neurons at 6 h after DEX treatment. We found that 14 out of 15 bICP4+ TG neurons were also GR+. Seven out of 10 bICP22+ TG neurons were also GR+. These studies suggested that most neurons expressing bICP4 or bICP22 also expressed the GR.
Figure 5. Identification of GR+ TG neurons that express bICP4 or bICP2 in the same TG neuron.
IHC was performed using the bICP4 or bICP22 antibodies as described in Figures 2 and 3 using sections cut from TG of latently infected calves at 6 h after DEX treatment to initiate reactivation from latency. IHC was also performed using a GR-specific antibody on consecutive sections. Numbers denote TG neurons that were bICP4+ or bICP22+ and GR+.
DISCUSSION
In this study, we provided evidence that bIC4 and bICP22 were expressed during the escape from latency. We have now provided evidence that all three BoHV-1 proteins encoded by IE genes are expressed during the escape from latency. Since bICP0 and bICP4 expression are regulated by the same IE promoter (Figure 1A), we further suggest that the IEtu1 promoter is active during the escape from latency. Interestingly, the IEtu1 promoter contains two functional GREs that can be activated by the synthetic corticosteroid DEX (El-Mayet et al., 2017; Kook et al., 2015b; Sawant et al., 2018) suggesting activation of this promoter is an early response to a stressful stimulus. bICP0 and bICP4 are crucial for activating early and late viral gene expression and productive infection (Boutell and Everett, 2013; Parkinson and Everett, 2000, 2001; Saira et al., 2008), functions crucial for reactivation from latency. Although the exact function of bICP22 is unknown, ICP22 encoded by HSV-1 is a general transcriptional regulator of cellular and viral mRNAs, in part by mediating changes on the host RNA polymerase II (Frase and Rice, 2007; Orlando et al., 2006). Thus, bICP22 expression during reactivation may also stimulate lytic cycle viral gene expression.
A previous study demonstrated that bICP0 and VP16 was detected in TG neurons of latently infected calves at 30 and 90 minutes after DEX treatment (Kook et al., 2015a). Conversely, we were unable to detect bICP4 and bICP22 at 90 minutes after DEX-induced reactivation from latency. Although this could be because the bICP0 and VP16 antiserum we used had greater avidity for their targets relative to the antiserum developed against the bICP4 or the bICP22 protein, we cannot exclude the possibility that the bICP0 E promoter initially stimulates bICP0 protein expression during the escape from latency. Thus, it is possible that bICP0 and/or VP16 are expressed earlier than bICP4 during reactivation from latency. Studies designed to test whether the bICP0 E promoter is stimulated by stress-induced transcription factors are currently in progress.
In contrast to two late viral proteins (gC and gD) that are not readily detected during BoHV-1 reactivation from latency (Frizzo da Silva et al., 2013), VP16 was readily detected in TG neurons during the escape from latency, as early as 30 or 90 minutes after DEX treatment of latently infected calves (Frizzo da Silva et al., 2013; Kook et al., 2015a). With respect to HSV-1, VP16 was reported to play a crucial role during reactivation from latency (Camarena et al., 2010; Kim et al., 2012; Sawtell and Thompson, 2016; Thompson et al., 2009). Considering VP16 is a tegument protein that stimulates IE gene expression (Misra et al., 1994; Misra et al., 1995), VP16 may initiate IE gene expression in a subset of TG neurons during reactivation in latency. Conversely, in a distinct subset of TG neurons, the IEtu1 or bICP0 E promoter may be initially activated by the GR and stress-induced transcription factors. Regardless of which scenario is correct, production of infectious virus particles during reactivation from latency will likely require all of these cellular and viral transcription factors. In addition to stimulating viral gene expression, the ability of corticosteroids to suppress inflammation and immune responses (Rhen and Cidlowski, 2005; Smoak and Cidlowski, 2004) would be expected to enhance the efficiency of virus shedding and spread to peripheral sites.
ACKNOWLEDGEMENTS
This research was supported by grants from the USDA-NIFA Competitive Grants Program (13–01041 and 16–09370), National Institute Of Neurological Disorders And Stroke of the NIH (R21NS102290), support from the Oklahoma Center for Respiratory and Infectious Diseases (National Institutes of Health Centers for Biomedical Research Excellence Grant # P20GM103648), and funds derived from the Sitlington Endowment.
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest.
REFERENCES
- Boutell C and Everett RD, 2013. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J Gen Virol 94, 465–481. [DOI] [PubMed] [Google Scholar]
- Camarena V, Kobayashi M, Kim JK, Roehm P, Perez R, Gardner J, Wilson AC, Mohr I, and Chao MV, 2010. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe 8, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase C, Fulton RW, O’Toole D, Gillette B, Daly RF, Perry G, and Clement T, 2017. Bovine herpesvirus 1 modified live vaccines for cattle reproduction: balancing protection with undesired effects. Vet Microbiol 206, 69–77. [DOI] [PubMed] [Google Scholar]
- El-Mayet FS, Sawant L, Thungunutla P, and Jones C, 2017. Combinatorial effects of the glucocorticoid receptor and Krüppel-like transcription factor 15 on bovine herpesvirus 1 transcription and productive infection. J Virol 91, 91:e00904–00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EF, Kreutz LC, and Donis RO., 1996. Swine and ruminant pestiviruses require the same cellular factor to enter bovine cells. J Gen Virol 77, 1295–1303. [DOI] [PubMed] [Google Scholar]
- Fraefel C, Zeng J, Choffat Y, Engels M, Schwyzer M, and Ackermann M, 1994. Identification and zinc dependence of the bovine herpesvirus 1 transactivator protein BICP0. J Virol 68, 3154–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frase KA and Rice SA, 2007. Herpes simplex virus immediate-early protein ICP22 triggers loss of serine 2-phosphorylated RNA polymerase II. J Virol 81, 5091–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzo da Silva L, Kook I, Doster A, Jones C, 2013. Bovine herpesvirus 1 regulatory proteins bICP0 and VP16 are readily detected in trigeminal ganglionic neurons expressing the glucocorticoid receptor during the early stages of reactivation from latency. Journal of virology 87, 11214–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzo da Silva L, Kook I, Doster A, and Jones C, 2013. Bovine herpesvirus 1 regulatory proteins, bICP0 and VP16, are readily detected in trigeminal ganglionic neurons expressing the glucocorticoid receptor during the early stages of reactivation from latency. J Virol 87, 11214–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman M, Lovato L, Doster A, and Jones C, 2002. A mutation in the latency related gene of bovine herpesvirus 1 interferes with the latency-reactivation cycle of latency in calves. J Virol 76, 6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman M, Lovato L, Doster A, and Jones C, 2001. A mutation in the latency-related gene of bovine herpesvirus 1 leads to impaired ocular shedding in acutely infected calves. J Virol 75, 8507–8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman M, Lovato L, Doster A, and Jones C, 2002. A mutation in the latency-related gene of bovine herpesvirus 1 disrupts the latency reactivation cycle in calves. J Virol 76, 6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv Virus Res 51, 81–133. [DOI] [PubMed] [Google Scholar]
- Jones C, 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin Micro Rev 16, 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, 2009. Regulation of innate immune responses by bovine herpesvirus 1 and infected cell protein 0. Viruses 1, 255–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, da Silva LF, and Sinani D, 2011. Regulation of the latency-reactivation cycle by products encoded by the bovine herpesvirus 1 (BHV-1) latency-related gene. J Neurovirol 17, 535–545. [DOI] [PubMed] [Google Scholar]
- Jones C, Newby TJ, Holt T, Doster A, Stone M, Ciacci-Zanella J, Webster CJ, and Jackwood MW, 2000. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine 18, 3185–3195. [DOI] [PubMed] [Google Scholar]
- Jones C, Geiser V, Henderson G, Jiang Y, Meyer F, Perez S, and Zhang Y., 2006. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet Micro 113, 199–210. [DOI] [PubMed] [Google Scholar]
- Jones C and Chowdhury S, 2007. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex, and development of improved vaccines. Adv in Anim Health 8, 187–205. [DOI] [PubMed] [Google Scholar]
- Kim JY, Mandarino A, Chao MV, Mohr I, and Wilson AC, 2012. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of HSV-1 in neurons. PloS Pathogens 8, e1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook I, Doster A, and Jones C, 2015a. Bovine herpesvirus 1 regulatory proteins are detected in trigeminal ganglionic neurons during the early stages of stress-induced escape from latency. J Neurovirology 21, 585–591. [DOI] [PubMed] [Google Scholar]
- Kook I, Doster A, and Jones C, 2015. Bovine herpesvirus 1 regulatory proteins are detected in trigeminal ganglionic neurons during the early stages of stress-induced escape from latency. Journal of neurovirology 21, 585–591. [DOI] [PubMed] [Google Scholar]
- Kook I, Henley C, Meyer F, Hoffmann F, and Jones C, 2015b. Bovine herpesvirus 1 productive infection and the immediate early transcription unit 1 are stimulated by the synthetic corticosteroid dexamethasone. Virology 484, 377–385. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hancock M, Workman A, Doster A, and Jones C, 2016. beta-Catenin, a Transcription Factor Activated by Canonical Wnt Signaling, Is Expressed in Sensory Neurons of Calves Latently Infected with Bovine Herpesvirus 1. Journal of virology 90, 3148–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato L, Inman M, Henderson G, Doster A, and Jones C, 2003. Infection of cattle with a bovine herpesvirus 1 strain that contains a mutation in the latency-related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. Journal of virology 77, 4848–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V, Bratanich AC, Carpenter D, and O’Hare P, 1994. Protein and DNA elements involved in transactivation of the promoter of the bovine herpesvirus (BHV) 1 IE-1 transcription unit by the BHV alpha gene trans-inducing factor. J Virol 68, 4898–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V, Walker S, Hayes S, and O’Hare P, 1995. The bovine herpesvirus alpha gene trans-inducing factor activates transcription by mechanisms different from those of its herpes simplex virus type 1 counterpart VP16. J Virol 69, 5209–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando JS, Astor TL, Rundle SA, and Schaffer PA, 2006. The products of the herpes simplex virus type 1 immediate-early US1/US1.5 genes downregulate levels of S-phase-specific cyclins and facilitate virus replication in S-phase Vero cells. J Virol 80, 4005–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J and Everett RD, 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J Virol 74, 10006–10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J and Everett RD, 2001. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 induce the formation of colocalizing, conjugated ubiquitin. J Virol 75, 5357–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S, Inman M, Doster A, and Jones C, 2005. Latency-related gene encoded by bovine herpesvirus 1 promotes virus growth and reactivation from latency in tonsils of infected calves. Journal of clinical microbiology 43, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S, Lovato L, Zhou J, Doster A, and Jones C, 2006. Comparison of inflammatory infiltrates in trigeminal ganglia of cattle infected with wild-type Bovine herpesvirus 1 versus a virus strain containing a mutation in the LR (latency-related) gene. Journal of neurovirology 12, 392–397. [DOI] [PubMed] [Google Scholar]
- Perez S, Meyer F, Saira K, Doster A, and Jones C, 2008. Premature expression of the latency-related RNA encoded by bovine herpesvirus type 1 correlates with higher levels of beta interferon RNA expression in productively infected cells. J Gen Virol 89, 1338–1345. [DOI] [PubMed] [Google Scholar]
- Perng G-C and Jones C, 2010. Towards an understanding of the Herpes Simplex Virus Type 1 latency-reactivation cycle. Interdisciplinary Perspectives on Infectious Diseases. 2010, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, and Cidlowski JA, 2005. Antiinflammatory action of glucocorticoids - new mechanisms of old drugs. New England J of Medicine 353, 1711–1723. [DOI] [PubMed] [Google Scholar]
- Rock D, Lokensgard J, Lewis T, and Kutish G, 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J Virol 66, 2484–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saira S, Chowdhury S, Gaudreault N, Henderson G, Doster A, and Jones C, 2008. The zinc RING finger of the bovine herpesvirus 1 encoded bICP0 protein is crucial for viral replication and virulence. J Virol 82, 12060–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant L, Kook I, Vogel JL, Kristie TM, and Jones C, 2018. The cellular coactivator HCF-1 is required for glucocorticoid receptor-mediated transcription of bovine herpesvirus 1 immediate early genes. J Virol 92, e00987–00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell N and Thompson RL, 2016. De Novo Herpes Simplex Virus VP16 Expression Gates a Dynamic Programmatic Transition and Sets the Latent/Lytic Balance during Acute Infection in Trigeminal Ganglia. PloS Pathogens 12, e1005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinani D, Frizzo da Silva L, and Jones C, 2013. A bovine herpesvirus 1 protein expressed in latently infected neurons (ORF2) promotes neurite sprouting in the presence of activated Notch1 or Notch3. Journal of virology 87, 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoak KL and Cidlowski JA, 2004. Mechanisms of glucocorticoid receptor signaling during inflammation. Mechanisms of Aging and Development 125, 697–706. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Preston CM, and Sawtell NM, 2009. De novo synthesis of VP16 corrdinates the exit form HSV latency in vivo. Plos Pathogens 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MT, Doster A, Sur JH, and Jones C, 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet Microbiol 86, 139–155. [DOI] [PubMed] [Google Scholar]
- Winkler MT, Doster A, and Jones C, 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J Virol 74, 5337–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth UV, Fraefel C, Vogt B, Vlcek C, Paces V, and Schwyzer M, 1992. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3’ coterminal and encode a putative zinc finger transactivator protein. J Virol 66, 2763–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth UV, Gunkel K, Engels M, and Schwyzer M, 1989. Spatial and temporal distribution of bovine herpesvirus 1 transcripts. J Virol 63, 4882–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth UV, Vogt B, and Schwyzer M, 1991. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J Virol 65, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman A, Eudy J, Smith L, da Silva LF, Sinani D, Bricker H, Cook E, Doster A, and Jones C, 2012. Cellular transcription factors induced in trigeminal ganglia during dexamethasone-induced reactivation from latency stimulate bovine herpesvirus 1 productive infection and certain viral promoters. Journal of virology 86, 2459–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman A, Zhu L, Keel BN, Smith TPL, and Jones C, 2018. The Wnt Signaling Pathway Is Differentially Expressed during the Bovine Herpesvirus 1 Latency-Reactivation Cycle: Evidence That Two Protein Kinases Associated with Neuronal Survival, Akt3 and BMPR2, Are Expressed at Higher Levels during Latency. Journal of virology 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Workman A, and Jones C, 2017. Potential Role for a beta-Catenin Coactivator (High-Mobility Group AT-Hook 1 Protein) during the Latency-Reactivation Cycle of Bovine Herpesvirus 1. Journal of virology 91. [DOI] [PMC free article] [PubMed] [Google Scholar]