Abstract
Osteocytes experience plasma membrane disruptions (PMD) that initiate mechanotransduction both in vitro and in vivo in response to mechanical loading, suggesting that osteocytes use PMD to sense and adapt to mechanical stimuli. PMD repair is crucial for cell survival; antioxidants (e.g., alpha-tocopherol, also known as Vitamin E) promote repair while reactive oxygen species (ROS), which can accumulate during exercise, inhibit repair. The goal of this study was to determine whether depleting Vitamin E in the diet would impact osteocyte survival and bone adaptation with loading. Male CD-1 mice (3 wk old) were fed either a regular diet (RD) or Vitamin E-deficient diet (VEDD) for up to 11 weeks. Mice from each dietary group either served as sedentary controls with normal cage activity, or were subjected to treadmill exercise (one bout of exercise or daily exercise for 5 weeks). VEDD-fed mice showed more PMD-affected osteocytes (+50%) after a single exercise bout suggesting impaired PMD repair following Vitamin E deprivation. After 5 weeks of daily exercise, VEDD mice failed to show an exercise-induced increase in osteocyte PMD formation, and showed signs of increased osteocytic oxidative stress and impaired osteocyte survival. Surprisingly, exercise-induced increases in cortical bone formation rate were only significant for VEDD-fed mice. This result may be consistent with previous studies in skeletal muscle, where myocyte PMD repair failure (e.g., with muscular dystrophy) initially triggers hypertrophy but later leads to widespread degeneration. In vitro, mechanically wounded MLO-Y4 cells displayed increased post-wounding necrosis (+40 fold) in the presence of H2O2, which could be prevented by Vitamin E pre-treatment. Taken together, our data support the idea that antioxidant-influenced osteocyte membrane repair is a vital aspect of bone mechanosensation in the osteocytic control of PMD-driven bone adaptation.
Keywords: bone, skeleton, osteocyte, mechanotransduction, vitamin E, alpha-tocopherol, mechanical loading
Introduction
Antioxidants are crucial to cell health and survival because they scavenge and neutralize free radicals, protecting the body against oxidative damage. One antioxidant of interest in the musculoskeletal system is Vitamin E. This lipid-soluble antioxidant has two main classes, tocopherols and tocotrienols, both of which possess four isoforms (α, β, γ, δ). Alpha-tocopherol demonstrates the highest bioavailability and is therefore the most common form of Vitamin E in the body [1–3]. Alpha-tocopherol preferentially binds to the alpha-tocopherol transport protein (αTTP), the transporter responsible for loading Vitamin E into lipoproteins which can then be transported into the bloodstream and distributed throughout the body. Previous studies on the role of Vitamin E in skeletal health have yielded conflicting results. For example, one study reported that young mice deficient in αTTP had high bone mass, suggesting a negative effect of Vitamin E in bone [4]. However, subsequent studies demonstrated that 1) aged αTTP knockout mice had reduced cortical bone mass, 2) low dietary Vitamin E intake did not increase bone mass in mature rats, 3) high dietary Vitamin E intake did not reduce cortical bone mass in mature rats, and 4) high Vitamin E intake did not affect cortical bone mass in maturing rats [5,6]. Human studies, likewise, have shown conflicting results regarding the beneficial or detrimental effects of Vitamin E on bone health [7–13]. Thus, the role of Vitamin E in bone remains unclear and somewhat controversial.
Free radicals are generated during the mechanical loading of exercise [14]. In many cell types, mechanical loading also produces small (nanometer to micron sized) tears in the cell membrane called plasma membrane disruptions (PMD), and Vitamin E can be a vital component of PMD repair in this context [1,3]. Membrane disruptions in eukaryotic cells that are on the order of nanometers may heal spontaneously, but larger disruptions require active repair mechanisms that are largely dependent on calcium signaling [15,16]. Repair of membrane disruptions is slowed or even prevented by the presence of reactive oxygen species like hydrogen peroxide [17,3]. In skeletal muscle myocytes, Vitamin E deficiency leads to muscle atrophy caused by death of myocytes that are unable to properly repair PMD formed in their sarcolemma [17]. Bone, like muscle, is a mechanically responsive tissue; mechanical loading promotes an increase in skeletal mass and strength because matrix-embedded osteocytes transduce mechanical signals to direct skeletal adaptation. Our laboratory recently demonstrated that osteocytes develop PMD during in vitro and in vivo mechanical loading, and that these PMD initiate osteocyte mechanotransduction mechanisms including calcium signaling and up-regulation of the mechanoresponsive protein cfos [18]. These data suggest that PMD help osteocytes detect and respond to mechanical loading. However, repair of these membrane tears is necessary for cell survival [16,3], and the mechanisms by which osteocytes repair their cell membrane after a PMD are not well understood. In skeletal muscle, Vitamin E supplementation promoted myocyte membrane repair rate [17]. Myocytes from Vitamin E-deprived rats had an inhibited ability to repair PMD, but this phenotype could be reversed with Vitamin E supplementation [3]. In addition, Vitamin E-deprived rats subjected to downhill treadmill running demonstrated increased muscle damage as compared to rats fed a regular diet, but supplementation with Vitamin E ameliorated the effects of exercised-induced muscle damage [3].
In our previous study, we showed that the antioxidants Vitamin C (ascorbic acid) and Vitamin E (alpha-tocopherol) increased PMD repair rate in osteocytes, similar to earlier reports for myocytes [3,17]. These data suggest that osteocytes are capable of rapidly repairing membrane tears when they occur, and that repair rate can be modulated pharmacologically via antioxidants. However, this relationship between antioxidants and osteocyte membrane repair rate has only been studied in vitro thus far. To test the role of the antioxidant Vitamin E in bone adaptation and osteocyte membrane repair in vivo, we subjected mice to a Vitamin E-deficient diet and examined the acute and chronic effects of mechanical loading (by exercise) on bone. The goal of this study was to determine whether depleting levels of the antioxidant Vitamin E would impact osteocyte survival and bone adaptation during in vivo loading. We hypothesized that depletion of Vitamin E would lead to an increase in oxidative stress that would impair osteocyte survival.
Materials & Methods
Animals and diet.
All experiments followed NIH guidelines and were approved by the Institutional Animal Care and Use Committee at Augusta University. Male CD-1 mice were obtained from a commercial supplier (3 weeks old, Envigo, n = 50) and housed in standard rodent cages with Tek Fresh bedding (Teklad #7099) on a 12 hour light / 12 hour dark schedule. Mice were permitted water ad libitum, and were randomly assigned to be fed either a regular diet of standard rodent chow (RD: Teklad #2018; 110 IU/kg Vitamin E) or a Vitamin E-deficient diet (VEDD: Teklad #TD.88163; 0 IU/kg Vitamin E) ad libitum immediately upon arrival (n = 25 per group). Dietary composition and energy density were similar between the two diets with the exception of Vitamin E content (Supplemental Table 1). One VEDD mouse and one RD mouse died prior to conclusion of experiments.
Treadmill exercise.
Animals were subjected to 6 weeks of diet administration prior to the onset of exercise studies. To determine the effects of acute loading (one exercise bout) on osteocytes, a subset of mice from each dietary group (n=4 to 5 mice per group) were subjected to one strenuous exercise bout (−15° downhill, linearly increasing speed from 10 m/min to 40m/min over 30 minutes), as we previously reported [18] and sacrificed within 2 hours of loading. To determine the effects of chronic loading, the remaining mice were subjected to daily exercise on a flat treadmill (12 m/min for 30 min/day, 5 days/week) for 5 weeks, or served as sedentary (normal cage activity) controls (n=9 to 10 mice per group). This exercise regimen was selected as it was previously demonstrated to be osteogenic [19]. Vitamin E-deficient or control diets were administered throughout the exercise period. Sedentary control mice were exposed to the inactive treadmill environment on a daily basis. Calcein (10 mg/kg, subcutaneous injection) was administered at 5 and 1 days before sacrifice to label mineralizing surfaces for analysis of bone formation by histomorphometry. All mice received intraperitoneal injections of Evans Blue (50 mg/kg body mass) to act as an exogenous PMD tracer 18 hours prior to the final training bout and were sacrificed within 2 hours of loading. Mice were perfused with 10% formalin prior to tissue collection.
MicroCT.
At the conclusion of the study, cortical bone architecture was analyzed in mid-diaphysis of the left femur for the chronic exercise groups by ex vivo microCT (Skyscan 1174), as previously described [20]. The scanner was equipped with a 50-kV, 800-mA X-ray tube. Samples were maintained in a moist environment and scanned in air using a resolution of 16 microns, an integration time of 2000 ms, and a 0.5-degree rotation step. Reconstructions (grayscale threshold set from 60 to 220) were subjected to morphometric analyses using CTAn software (Skyscan); quantified properties included cortical bone area (Ct.B.Ar, mm2), polar moment of inertia (Ipolar, mm4), maximum moment of inertia (Imax, mm4), minimum moment of inertia (Imin, mm4), and cortical bone thickness (Ct.Th, mm).
Detection of PMD in cortical bone.
The right femur from each mouse was fixed in 10% neutral buffered formalin and cryosectioned longitudinally (undecalcified, 5 μm thickness) using Cryofilm IIC, as previously described [18]. Two RD exercise and one VEDD exercise samples were damaged during processing and were subsequently excluded from analysis. To detect Evans Blue staining, sections were imaged in PBS containing FM1-43 dye (used to label intracellular membranes and confirm the presence of an intracellular Evans Blue signal) on LSM780 multiphoton confocal microscope. Images were captured as a Z-stack (series of 20-30 images through section thickness) and processed into a maximum intensity projection. Five to ten images per bone were captured and analyzed in Bioquant Osteo software to quantify percentage of Evans Blue labelled osteocytes [18]. To validate Evans Blue staining, the left tibia from a subset of mice (n=4 to 7 mice per group) was decalcified, cryosectioned (7 μm) onto Histogrip (LifeTechnologies #008050) coated slides, and immunohistochemically stained to detect albumin as an endogenous PMD tracer, as we previously described [18]. Albumin was detected with a FITC-conjugated antibody (AIFAG3140, Accurate Chemical & Scientific Company, 1:100 dilution), and sections were mounted with DAPI (Vectashield H1500) and imaged with a confocal microscope (Zeiss).
Lacunar occupancy.
Decalcified tibial sections (n=8 to 10 mice per group) were stained with Goldner’s Trichrome to determine osteocyte lacunar vacancy as a measure of cell viability. Bone sections were examined at 400× magnification, and a total of 10 images per bone were collected at random locations throughout the cortical bone. The percentage of empty osteocyte lacunae was quantified with image analysis software (Bioquant OSTEO).
Reactive oxygen species.
To determine whether dietary Vitamin E deprivation affected oxidative stress in bone, histological sections from the left tibia (n=3 to 8 mice per group) were subjected to 4-HNE staining to detect lipid peroxidation as previously described [21]. Cryosections were incubated overnight with a rabbit anti-4HNE antibody (Alpha Diagnostic International) or a non-specific IgG control (Jackson ImmunoResearch #001-000-003) and developed with 3,3’-diaminobenzidine (DAB) peroxidase substrate (Sigma D3939). Sections were counterstained with Fast Green. Bone sections were imaged at 400× total magnification, and immunostaining was quantified as the ratio of 4-HNE-positive osteocytes to the total number of occupied osteocyte lacunae with image analysis software (Bioquant OSTEO).
Cortical bone formation.
The right tibial diaphysis from a subset of mice from the chronic exercise group (n=5 mice per group) was embedded in methyl methacrylate for dynamic histomorphometry as previously described [22,23]. Cross-sections were cut from the diaphysis with a low speed diamond saw (Buehler Isomet) and hand polished on a grinding wheel (Buehler Ecomet) to <100 micron thickness. Sections were mounted on glass slides (Permount) and imaged with a fluorescent microscope (Olympus IX-70) and digital camera (Qlcam). Calcein labels were imaged with a FITC cube, and periosteal and endocortical mineral apposition rates (MAR, μm/day), mineralizing surfaces (MS/BS, %), and bone formation rates (BFR/BS, μm3/μm2/day) were quantified with image analysis software (Bioquant OSTEO, Nashville TN) as previously described [22] For each envelope (periosteal or endocortical), mineralizing surface was defined as double labelled surface + ½ single labelled surface.
Cell culture
MLO-Y4 cells, courtesy of Dr. Lynda Bonewald, were maintained in growth medium (α-MEM Invitrogen) + 5% fetal bovine serum (FBS, Atlanta Biologicals) + 5% bovine calf serum (HyClone) + 1% Penicillin/Streptomycin). Cells were seeded onto type 1 collagen-coated glass coverslips and cultured for 24 h in either normal culture medium or culture medium supplemented with Vitamin E (220 μM alpha-tocopherol, Sigma #T3251), or Trolox (220 μM, Sigma #238813; a water soluble form of Vitamin E). Cells were treated with vehicle or H2O2 (1 mM) for 10 minutes and then subjected to mechanical wounding with glass beads in the presence of lysine-fixable fluorescein-conjugated dextran (10 kDa, 5 mg/ml + 10 mg/ml BSA), as we previously described [18]. Five minutes after wounding, cells were stained with propidium iodide (0.3 mg/ml) to detect dead cells (i.e., unrepaired PMD). Images were collected at randomized positions on the coverslip, where at least 10 cells were visible per field of view, using a multi-photon confocal microscope (Zeiss). The ratio of dead to repaired cells was calculated with image analysis software (Bioquant OSTEO). Three independent trials of each experimental condition were completed.
Statistics.
For each property, groups were compared by t-tests (acute exercise: 2 Diet) or 2-factor ANOVA with interaction (chronic exercise: 2 Diet × 2 Exercise) followed by Tukey’s HSD post hoc test when significant interaction effects were detected using JMP 13.0.0 (SAS Institute., Cary, NC). A log transformation was used when necessary to stabilize the variance of the residuals; this approach was used for Ps. MS/BS, Ps. MAR, Ps. BFR, and measurements of lacunar occupancy. For in vitro studies, groups were compared with 1-factor ANOVA and Tukey’s HSD post hoc test. Statistical significance was set at p<0.05 for all comparisons, and trends were considered when p<0.1. For quantification of histological/imaging studies, data were collected in a blinded fashion, and the same operator collected all data within a given experiment to limit inter-observer variability. Data are presented as mean ± standard error (SE), unless otherwise indicated. Sample sizes are indicated in each figure and/or caption.
Results
VEDD-fed mice had increased cortical bone osteocyte PMD formation in response to acute loading.
VEDD-fed mice demonstrated a significant increase in the number of osteocytes experiencing PMD after a single strenuous downhill training bout, suggesting either impaired PMD repair or increased membrane fragility (Fig. 1). All VEDD-fed mice completed the acute exercise bout, and did not demonstrate impaired running ability as compared to RD-fed mice, unlike longer-term (~11 months) Vitamin E deprivation studies in rats [3].
Figure 1: Effects of acute exercise on osteocyte PMD formation.
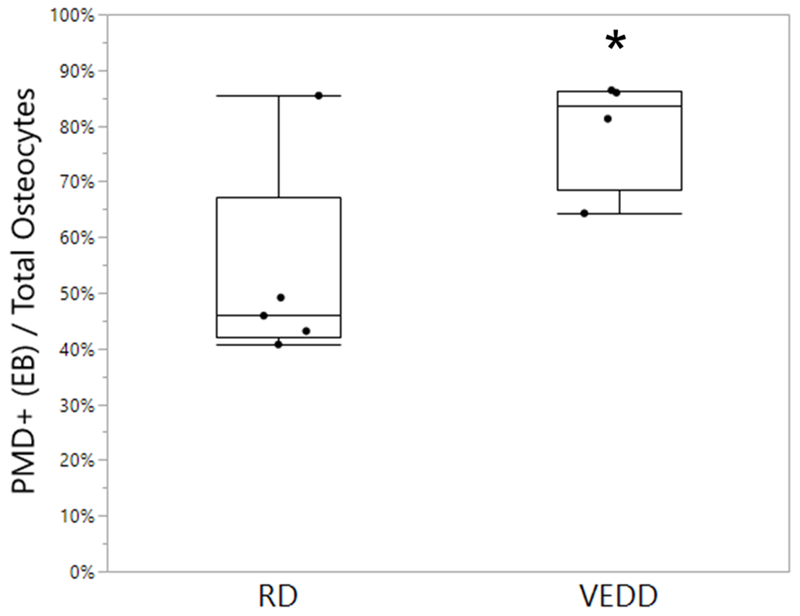
Mice fed a Vitamin E-deficient diet (VEDD) demonstrated an increase in the percentage of wounded osteocytes, as indicated by uptake of Evans Blue (EB) dye, after a single bout of downhill treadmill exercise. *p<0.05 vs. regular diet (RD). Box plots show median, quartiles, and outlier fences for each group.
VEDD-fed mice demonstrated enhanced periosteal bone formation in response to daily treadmill exercise, but RD-fed mice did not.
Since one bout of loading enhanced osteocyte membrane wounding in VEDD-fed mice, we next investigated the chronic effects of mechanical loading on bone in this model. Similar to the acute studies described above, no deficiency in treadmill running ability was noted in VEDD-fed as compared to RD-fed mice. Interestingly, there was a significant interaction effect observed between diet and exercise for cortical bone periosteal mineralizing surface, periosteal mineral apposition rate, and periosteal bone formation rate (Fig. 2), indicating that periosteal bone formation was enhanced by exercise in the VEDD-fed mice but not the RD-fed mice. Neither diet nor exercise affected endocortical mineralizing surface, endocortical mineral apposition rate, or endocortical bone formation rate (p>0.22), and there were no interactions observed for any of these properties (data not shown). From microCT studies, daily exercise increased cortical bone geometrical properties including cortical bone area (p=0.016), polar moment of inertia (p=0.047), and minimum moment of inertia (p=0.043), and tended to increase the maximum moment of inertia (p=0.056), but diet did not affect any of these properties (p>0.59) (Fig. 3). Neither diet nor exercise affected cortical bone thickness (p>0.33).
Figure 2: Effects of chronic exercise and Vitamin E deficiency on cortical bone formation.
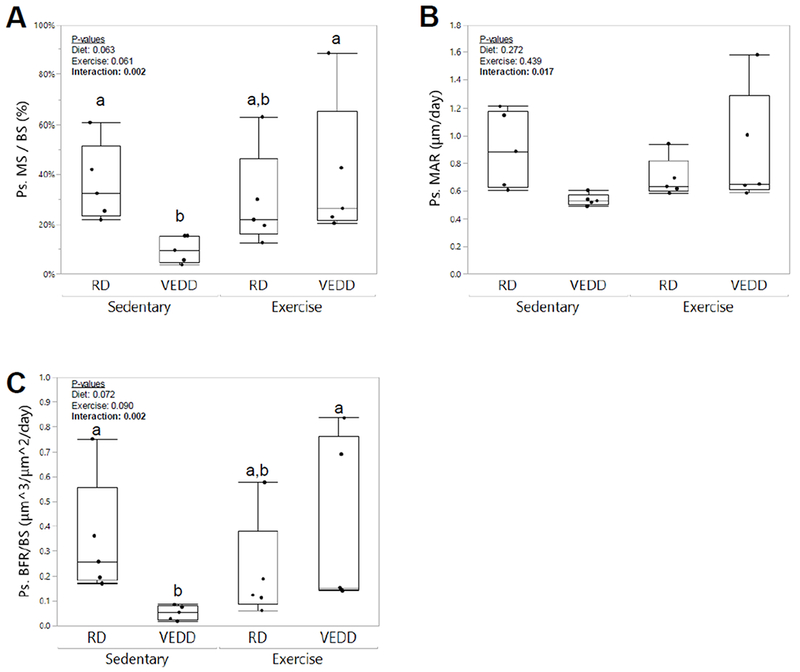
Exercise preferentially increased periosteal mineralizing surface (A), periosteal mineral apposition rate (B), and periosteal bone formation rate (C) in exercised VEDD-fed mice as compared to sedentary VEDD-fed mice. Groups with different superscript letters are significantly (p<0.05) different from one another by Tukey’s HSD post-hoc testing. P-values for factors in the 2-factor ANOVA with interaction analysis are shown. Box plots show median, quartiles, and outlier fences for each group.
Figure 3: Effects of chronic exercise and Vitamin E deficiency on bone geometry measured by microCT.
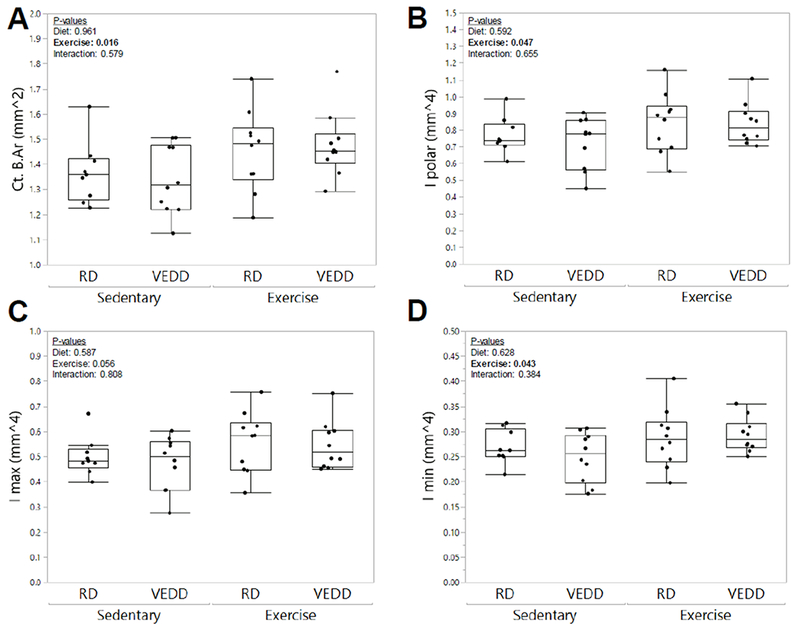
Exercise increased cortical bone area (A) in both RD- and VEDD-fed mice, but dietary administration had no synergistic effect with exercise. Polar moment of inertia (B), maximum moment of inertia (C), and minimum moment of inertia (D) demonstrated similar patterns. P-values for factors in the 2-factor ANOVA with interaction analysis are shown. Box plots show median, quartiles, and outlier fences for each group.
VEDD-fed mice had reduced lacunar occupancy.
Unexpectedly, after 5 weeks of training, VEDD-fed mice failed to show an exercise-induced increase in PMD-labeled osteocytes unlike their RD-fed counterparts. Identical trends were observed whether the PMD indicator was exogenous Evans Blue dye or endogenous albumin (Fig. 4). Since VEDD-fed mice showed enhanced wounding in response to a single bout of loading (Fig. 1), and Vitamin E deprivation impaired membrane repair and led to cell death in skeletal muscle [3], we hypothesized that VEDD-fed mice might be experiencing PMD repair failure and a subsequent reduction in osteocyte viability. Osteocyte lacunar vacancy was quantified as a metric of osteocyte cell death (Fig 5A). Both diet and exercise significantly (p<0.016) increased the percentage of vacant lacunae; a trend for a significant interaction effect was suggested (p=0.092) where exercise tended to increase lacunar vacancy in RD-fed mice but not in VEDD-fed mice. (Fig 5B).
Figure 4: Effects of chronic exercise and Vitamin E deficiency on cortical bone osteocyte PMD formation.
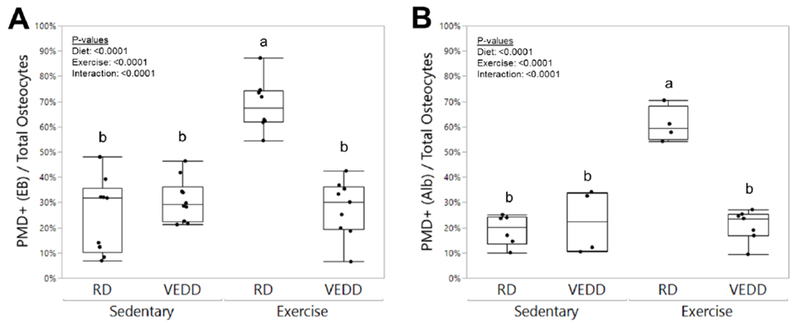
Measurements of intracellular Evans Blue (A) and intracellular staining for endogenous albumin (B) showed that while RD-fed mice demonstrated the expected increase in PMD-affected cells with exercise, this trend was not observed in VEDD-fed mice. Groups with different superscript letters are significantly (p<0.05) different from one another by Tukey’s HSD post-hoc testing. P-values for factors in the 2-factor ANOVA with interaction analysis are shown. Box plots show median, quartiles, and outlier fences for each group.
Figure 5: Effects of acute exercise and Vitamin E deficiency on osteocyte lacunar occupancy.
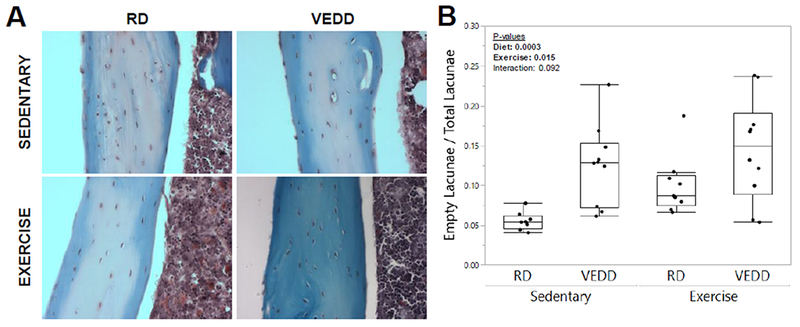
A) Representative sections demonstrating osteocyte lacunar occupancy (and vacancy) from each group. Original magnification: 400×. B) Both diet and exercise significantly increased the percentage of vacant osteocyte lacunae, suggesting an increase in osteocyte death. P-values for factors in the 2-factor ANOVA with interaction analysis are shown. Box plots show median, quartiles, and outlier fences for each group.
VEDD-fed mice demonstrate increased oxidative stress in osteocytes.
Tibial sections from RD- and VEDD-fed sedentary mice were stained for 4-HNE, a marker of lipid peroxidation. Both diet (p=0.047) and exercise (p=0.032) affected osteocytic levels of oxidative stress, as seen by 4-HNE staining; VEDD-fed mice had greater levels of 4-HNE staining, whereas interestingly 4-HNE staining was suppressed in the exercised groups (Fig 6). An interaction effect was not observed (p=0.493).
Figure 6: Oxidative stress is increased in VEDD-fed mice.
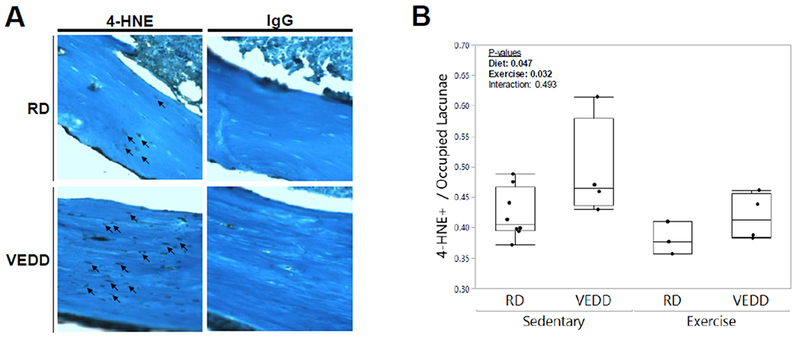
A) Longitudinal tibial sections of RD- and VEDD-fed mice were stained to detect 4-HNE, a marker of lipid peroxidation and oxidative stress; these representative samples are from the sedentary groups for each respective diet. Little to no staining was observed with a non-specific IgG control. Counterstain: Fast Green, 400× original magnification. B) Both diet and exercise significantly affected 4-HNE staining, with staining higher in VEDD-fed mice than RD-fed mice and surprisingly lower in exercised mice as compared to sedentary mice. P-values for factors in the 2-factor ANOVA with interaction analysis are shown. Box plots show median, quartiles, and outlier fences for each group.
Oxidative stress impairs post-wounding osteocyte survival but can be counteracted by antioxidant treatment.
Following observation of increased oxidative stress in VEDD-fed mice in vivo, we sought to mechanistically test the effects of reactive oxygen species and Vitamin E on osteocyte membrane repair and survival in vitro. We previously showed that impairing PMD repair processes in osteocytes leads to post-wounding cell death [18], and it has been demonstrated that oxidative stress (e.g., from H2O2) impairs PMD repair in other cells like myocytes [17,3]. To test this in osteocytes, we mechanically wounded osteocytes after introducing oxidative stress (via H2O2), with or without pre-treatment by Vitamin E, and stained cells with propidium iodide 5 min after wounding to detect non-repaired (necrotic) cells. In this timeframe, oxidative stress from H2O2 did not cause cell death in the absence of wounding (data not shown). With wounding, however, H2O2 treatment significantly increased cell death after injury, suggestive of impaired PMD repair. While Vitamin E antioxidant treatments did not substantially impact post-wounding cell death on their own, they significantly attenuated post-wounding cell death attributed to H2O2 treatment (Fig. 7).
Figure 7: Oxidative stress impairs post-wounding survival in osteocytes.
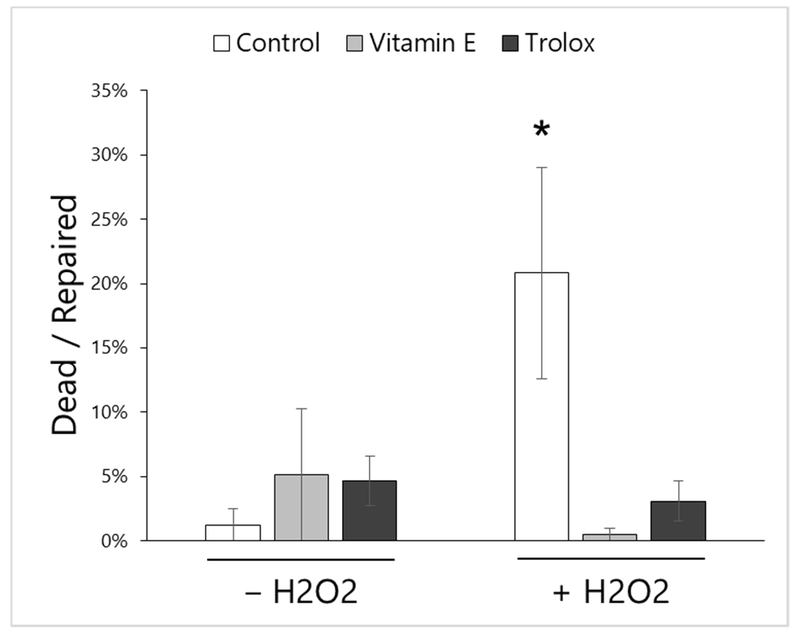
Osteocytes wounded in the presence of hydrogen peroxide (H2O2, 1 mM) demonstrated increased uptake of propidium iodide 5 minutes after wounding, indicative of membrane repair failure and cell death. However, pre-treatment with Vitamin E (220 μM) or Trolox (220 μM, a water-soluble form of Vitamin E) for 24 hours prior to wounding counteracted the negative impact of H2O2 on post-wounding cell survival. *p<0.05 vs. Control –H2O2 by Tukey’s HSD post-hoc testing. Mean±SE for each group is shown, and data are representative of 3 independent experiments.
From these data, we propose that plasma membrane repair in osteocytes is deleteriously affected by oxidative stress, suggesting an important role for antioxidants like Vitamin E in osteocyte survival.
Discussion
While the role of Vitamin E in skeletal health remains controversial, our data support the idea that Vitamin E has a beneficial function in bone. Compared to controls, Vitamin E-deprived mice showed increased osteocytic oxidative stress, increased lacunar vacancy, and decreased periosteal bone formation under conditions of normal cage activity. These results are consistent with previous studies supporting a beneficial role for Vitamin E in the skeleton. For example, alpha-tocopherol supplementation ameliorated some of the deleterious effects of ovariectomy in mice: medium and low doses of alpha-tocopherol increased bone mineralizing surface, while medium and high doses of alpha-tocopherol helped to offset the increased number of osteoclasts and erosion normally caused by ovariectomy [24]. Similarly, Vitamin E supplementation had beneficial roles in fracture healing [25], and in reducing the severity of disuse-induced bone loss [26]. A recent report also indicated that Vitamin E supplementation reduced osteocyte death caused by steroid and endotoxin treatment [27]. The increased presence of osteocytic oxidative stress in the tibias of the Vitamin E-deprived mice studied here supports an important antioxidant role for Vitamin E in bone, and our in vitro data suggest that Vitamin E may be important for counteracting skeletal oxidative stress to promote osteocyte survival. Important antioxidant effects of Vitamin E have also been shown in the human musculoskeletal system: in elderly subjects, activity levels of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx) were positively correlated with hip bone mineral density (BMD) measurements, and supplementation with high-dose antioxidants for 12 months reduced lipoperoxides and prevented BMD loss at the hip [13]. With regards to exercise, supplementation with Vitamin E reduced muscle damage in men exercising under hypoxic conditions known to cause enhanced oxidative stress [28]. Vitamin E is thought to reduce oxidative damage in humans via prevention of lipid peroxidation, which is the same mechanism suggested in rodent models [28,17]. Taken together, these data support Vitamin E’s beneficial role in bone, particularly under stressful conditions.
While reducing oxidative stress may be beneficial from a cell survival standpoint, some level of oxidative stress may be advantageous for tissue adaptation. For example, high doses of antioxidant supplementation constrained the favorable skeletal benefits of 12 weeks of resistance exercise in healthy elderly men [8], a result that mimicked the deleterious effects of high Vitamin E in rodent models [4]. In line with this concept, in the current study, exercise-induced changes in cortical bone formation were surprisingly only significant for VEDD-fed mice: periosteal mineralizing surface, MAR, and bone formation rate were increased with exercise in VEDD-fed mice as compared to sedentary VEDD-fed mice, but were not different between exercised RD-fed mice as compared to sedentary RD-fed mice at the conclusion of the study. Exercise increased cortical bone geometry measurements (e.g., cortical bone area) similarly in VEDD- and RD-fed mice, possibly because the short duration of loading was not sufficient for the differences in bone formation rate to manifest as differential structural changes in the bone. The enhanced response of periosteal bone formation to exercise in VEDD-fed mice may parallel the biology of skeletal muscle, where impaired PMD repair (e.g., dysferlinopathy-related muscular dystrophy) initially triggers a hypertrophic response that later evolves into tissue degeneration [29]. Consistent with this idea, in our previous study, we observed that osteocytes with membrane repair failure still undergo an initial burst of calcium signaling that can activate mechanotransduction in neighboring (non-wounded) cells, whereas enhancing osteocyte membrane repair rate with Vitamin E blunted the wounding-induced up-regulation of c-fos [18] and calcium signaling (data not shown). Therefore, it is possible that the VEDD-fed mice may have been sensitized to mechanical loading in this manner. However, since osteocyte death (increased lacunar vacancy) was also increased in VEDD-fed mice, it would be interesting to see if skeletal responses to longer-term Vitamin E deprivation and mechanical loading would be impaired by a potentially progressive depletion of the osteocyte population. Studies conducted in rats have shown that long-term Vitamin E-deprivation leads to a muscle phenotype consistent with diseases caused by myocyte repair failure, and that Vitamin E supplementation promoted membrane repair in these animals [3]. Together, our data support the idea that osteocytes experience PMD with mechanical loading in vivo and support the idea that osteocyte membrane repair rate is an important aspect of bone’s mechano sensitivity and adaptation to loading.
We were surprised to observe that VEDD-fed mice showed enhanced osteocyte wounding in response to a single, strenuous bout of exercise, but did not show an exercise-induced increase in osteocyte wounding at the conclusion of the chronic loading experiments. In contrast, RD-fed mice showed a robust increase in osteocyte PMD-labelling with exercise that was consistent with our previous findings in several experiments [18]. At present, we do not have a definitive explanation for this finding. Measurements for evidence of osteocyte membrane tears relied on cellular uptake of endogenous albumin, whether bound to the exogenous tracer Evans Blue or detected via immunostaining. Although mechanical loading has been shown to increase permeability of the lacuno-canalicular system to molecules the size of albumin (~70 kDa, [30]), we are confident that the signal measured was intracellular, as wounded cells were identified by co-staining with FM1-43 that labeled intracellular membranes. As both sedentary and exercised VEDD-fed mice had greater lacunar vacancy and greater 4-HNE staining indicative of oxidative stress, and even normal cage activity causes a baseline level of wounding (Fig 4), one possibility is that the osteocytes most vulnerable to wounding and/or death after wounding did not survive until the conclusion of the experiment, and that surviving osteocytes were selected for enhanced repair ability over the course of the study. Future studies will better address this issue by examining a more thorough time course for Vitamin E deprivation and loading, additional measurements of oxidative stress such as Amplex Red staining [31,32], and additional measurements of osteocyte viability such as lactate dehydrogenase (LDH) staining [33].
Our study has several limitations. First, we did not address the role of bone resorption in these experiments; however, it is important to note that cortical bone geometrical properties (e.g., cortical bone thickness) were not affected by dietary group, suggesting a lack of endocortical expansion that would have been driven by increased osteoclastic activity. Vitamin E’s effects on bone resorption are also reported to be independent from its antioxidant activity [4] which was the primary focus of the current study. A second limitation is that experiments were only conducted in male mice, so sex-specific effects cannot be determined from these studies. We chose to study male mice because they have stronger cortical bone responses to treadmill exercise than female mice [34], and because males may be more deleteriously affected by Vitamin E deficiency than females. For example, male αTTP-deficient mice show changes in bone mass as compared to wildtype controls, whereas female αTTP-deficient do not [6], and muscle damage from treadmill exercise is more apparent in male Vitamin E-deprived rats than in female Vitamin E-deprived rats [35].
Despite these limitations, our data imply that Vitamin E is important for mechanotransduction and adaptation of the musculoskeletal system, especially when considered in the context of Vitamin E’s effects on PMD repair and cell survival [18,3]. A previously unrealized role for Vitamin E in osteocytes may help explain some of the conflicting results regarding Vitamin E’s effects on skeletal biology in previous studies. For example, in elderly humans, hip BMD was increased by supplementation with Vitamins E and C in the absence of a prescribed exercise regimen [13], but supplementation with these same antioxidants blunted an exercise-induced increase in hip BMD and exercise-induced decreases in circulating sclerostin [8]. Osteocyte viability was not examined in these human studies, but the failure of antioxidant-treated individuals to suppress sclerostin with exercise is consistent with our earlier finding that Vitamin E treatment blunted the mechanotransduction response of wounded osteocytes [18,8]. The results of the current study, where Vitamin E-deprived mice demonstrated an enhanced bone formation response to short-term exercise but increased lacunar vacancy, suggest that there may be a window of optimal Vitamin E levels in skeletal health, and that the parameters of that window may differ depending on the physical activity of the subject. Future experiments will address the long-term effects of Vitamin E deficiency on bone adaptation and will examine the roles of osteoblast and osteoclast activity in the osteocytic control of PMD-driven bone adaptation.
Supplementary Material
Acknowledgements
Funding was received from the National Science Foundation (CMMI 1727949), the National Institute on Aging (P01 AG036675), and the Augusta University Medical Scholars Program. The authors wish to thank the Augusta University Cell Imaging Core Laboratory for assistance with imaging procedures and the Augusta University Electron Microscopy and Histology Core Laboratory for assistance with histology
Footnotes
Disclosure: All authors have no conflicts of interest.
References
- 1.Peh HY, Tan WS, Liao W, Wong WS (2016) Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol Ther 162:152–169. doi: 10.1016/j.pharmthera.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Satyamitra M, Ney P, Graves J 3rd, Mullaney C, Srinivasan V (2012) Mechanism of radioprotection by delta-tocotrienol: pharmacokinetics, pharmacodynamics and modulation of signalling pathways. Br J Radiol 85 (1019):e1093–1103. doi: 10.1259/bjr/63355844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labazi M, McNeil AK, Kurtz T, Lee TC, Pegg RB, Angeli JP, Conrad M, McNeil PL (2015) The antioxidant requirement for plasma membrane repair in skeletal muscle. Free Radic Biol Med 84:246–253. doi: 10.1016/j.freeradbiomed.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita K, Iwasaki M, Ochi H, Fukuda T, Ma C, Miyamoto T, Takitani K, Negishi-Koga T, Sunamura S, Kodama T, Takayanagi H, Tamai H, Kato S, Arai H, Shinomiya K, Itoh H, Okawa A, Takeda S (2012) Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat Med 18 (4):589–594. doi: 10.1038/nm.2659 [DOI] [PubMed] [Google Scholar]
- 5.Tennant KG, Leonard SW, Wong CP, Iwaniec UT, Turner RT, Traber MG (2017) High-Dietary Alpha-Tocopherol or Mixed Tocotrienols Have No Effect on Bone Mass, Density, or Turnover in Male Rats During Skeletal Maturation. J Med Food 20 (7):700–708. doi: 10.1089/jmf.2016.0147 [DOI] [PubMed] [Google Scholar]
- 6.Iwaniec UT, Turner RT, Smith BJ, Stoecker BJ, Rust A, Zhang B, Vasu VT, Gohil K, Cross CE, Traber MG (2013) Evaluation of long-term vitamin E insufficiency or excess on bone mass, density, and microarchitecture in rodents. Free Radic Biol Med 65:1209–1214. doi: 10.1016/j.freeradbiomed.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaelsson K, Wolk A, Byberg L, Arnlov J, Melhus H (2014) Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. The American journal of clinical nutrition 99 (1):107–114. doi: 10.3945/ajcn.113.064691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stunes AK, Syversen U, Berntsen S, Paulsen G, Stea TH, Hetlelid KJ, Lohne-Seiler H, Mosti MP, Bjornsen T, Raastad T, Haugeberg G (2017) High doses of vitamin C plus E reduce strength training-induced improvements in areal bone mineral density in elderly men. Eur J Appl Physiol 117 (6):1073–1084. doi: 10.1007/s00421-017-3588-y [DOI] [PubMed] [Google Scholar]
- 9.Chuin A, Labonte M, Tessier D, Khalil A, Bobeuf F, Doyon CY, Rieth N, Dionne IJ (2009) Effect of antioxidants combined to resistance training on BMD in elderly women: a pilot study. Osteoporos Int 20 (7):1253–1258. doi: 10.1007/s00198-008-0798-5 [DOI] [PubMed] [Google Scholar]
- 10.Yang TC, Duthie GG, Aucott LS, Macdonald HM (2016) Vitamin E homologues alpha- and gamma-tocopherol are not associated with bone turnover markers or bone mineral density in peri-menopausal and post-menopausal women. Osteoporos Int 27 (7):2281–2290. doi: 10.1007/s00198-015-3470-x [DOI] [PubMed] [Google Scholar]
- 11.Ochi H, Takeda S (2015) The Two Sides of Vitamin E Supplementation. Gerontology 61 (4):319–326. doi: 10.1159/000366419 [DOI] [PubMed] [Google Scholar]
- 12.Ostman B, Michaelsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, Basu S (2009) Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radic Biol Med 47 (5):668–673. doi: 10.1016/j.freeradbiomed.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Ramos M, Vargas LA, Fortoul Van der Goes TI, Cervantes-Sandoval A, Mendoza-Nunez VM (2010) Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J Nutr Health Aging 14 (6):467–472 [DOI] [PubMed] [Google Scholar]
- 14.Liu JF, Chang WY, Chan KH, Tsai WY, Lin CL, Hsu MC (2005) Blood lipid peroxides and muscle damage increased following intensive resistance training of female weightlifters. Ann N Y Acad Sci 1042:255–261. doi: 10.1196/annals.1338.029 [DOI] [PubMed] [Google Scholar]
- 15.Gozen I, Dommersnes P (2014) Pore dynamics in lipid membranes. The European Physical Journal Special Topics 223 (9):1813–1829. doi: 10.1140/epjst/e2014-02228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper ST, McNeil PL (2015) Membrane Repair: Mechanisms and Pathophysiology. Physiol Rev 95 (4):1205–1240. doi: 10.1152/physrev.00037.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard AC, McNeil AK, McNeil PL (2011) Promotion of plasma membrane repair by vitamin E. Nat Commun 2:597. doi: 10.1038/ncomms1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu K, Sellman DP, Bahraini A, Hagan ML, Elsherbini A, Vanpelt KT, Marshall PL, Hamrick MW, McNeil A, McNeil PL, McGee-Lawrence ME (2017) Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J Orthop Res doi: 10.1002/jor.23665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamrick MW, Skedros JG, Pennington C, McNeil PL (2006) Increased osteogenic response to exercise in metaphyseal versus diaphyseal cortical bone. Journal of musculoskeletal & neuronal interactions 6 (3):258–263 [PubMed] [Google Scholar]
- 20.Refaey ME, McGee-Lawrence ME, Fulzele S, Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG, Shi XM, Xu J, Hill WD, Johnson MH, Hunter M, Pierce JL, Yu K, Hamrick MW, Isales CM (2017) Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 32 (11):2182–2193. doi: 10.1002/jbmr.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liou GY, Storz P (2015) Detecting reactive oxygen species by immunohistochemistry. Methods in molecular biology 1292:97–104. doi: 10.1007/978-1-4939-2522-3_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGee ME, Maki AJ, Johnson SE, Nelson OL, Robbins CT, Donahue SW (2008) Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis). Bone 42 (2):396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGee-Lawrence ME, Wenger KH, Misra S, Davis CL, Pollock NK, Elsalanty M, Ding K, Isales CM, Hamrick MW, Wosiski-Kuhn M, Arounleut P, Mattson MP, Cutler RG, Yu JC, Stranahan AM (2017) Whole-Body Vibration Mimics the Metabolic Effects of Exercise in Male Leptin Receptor-Deficient Mice. Endocrinology 158 (5):1160–1171. doi: 10.1210/en.2016-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feresin RG, Johnson SA, Elam ML, Kim JS, Khalil DA, Lucas EA, Smith BJ, Payton ME, Akhter MP, Arjmandi BH (2013) Effects of vitamin e on bone biomechanical and histomorphometric parameters in ovariectomized rats. J Osteoporos 2013:825985. doi: 10.1155/2013/825985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuid AN, Mohamad S, Muhammad N, Fadzilah FM, Mokhtar SA, Mohamed N, Soelaiman IN (2011) Effects of alpha-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res 29 (11):1732–1738. doi: 10.1002/jor.21452 [DOI] [PubMed] [Google Scholar]
- 26.Smith BJ, Lucas EA, Turner RT, Evans GL, Lerner MR, Brackett DJ, Stoecker BJ, Arjmandi BH (2005) Vitamin E provides protection for bone in mature hindlimb unloaded male rats. Calcified tissue international 76 (4):272–279 [DOI] [PubMed] [Google Scholar]
- 27.Jia YB, Jiang DM, Ren YZ, Liang ZH, Zhao ZQ, Wang YX (2017) Inhibitory effects of vitamin E on osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells at early stage of steroid-induced femoral head necrosis. Mol Med Rep 15 (4):1585–1592. doi: 10.3892/mmr.2017.6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos SA, Silva ET, Caris AV, Lira FS, Tufik S, Dos Santos RV (2016) Vitamin E supplementation inhibits muscle damage and inflammation after moderate exercise in hypoxia. J Hum Nutr Diet 29 (4):516–522. doi: 10.1111/jhn.12361 [DOI] [PubMed] [Google Scholar]
- 29.Rocha CT, Hoffman EP (2010) Limb-girdle and congenital muscular dystrophies: current diagnostics, management, and emerging technologies. Curr Neurol Neurosci Rep 10 (4):267–276. doi: 10.1007/s11910-010-0119-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tami AE, Schaffler MB, Knothe Tate ML (2003) Probing the tissue to subcellular level structure underlying bone’s molecular sieving function. Biorheology 40 (6):577–590 [PubMed] [Google Scholar]
- 31.Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, Hill WD, Liu Y, Shi X, Fulzele S, Hamrick MW (2017) MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue engineering Part A 23 (21-22):1231–1240. doi: 10.1089/ten.TEA.2016.0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Votyakova TV, Reynolds IJ (2004) Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys 431 (1):138–144. doi: 10.1016/j.abb.2004.07.025 [DOI] [PubMed] [Google Scholar]
- 33.Jahn K, Stoddart MJ (2011) Viability assessment of osteocytes using histological lactate dehydrogenase activity staining on human cancellous bone sections. Methods in molecular biology 740:141–148. doi: 10.1007/978-1-61779-108-6_15 [DOI] [PubMed] [Google Scholar]
- 34.Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, Kohn DH (2007) Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40 (4):1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amelink GJ, van der Wal WA, Wokke JH, van Asbeck BS, Bar PR (1991) Exercise-induced muscle damage in the rat: the effect of vitamin E deficiency. Pflugers Arch 419 (3-4):304–309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


