Abstract
Background
Echocardiographic follow-up after pediatric heart transplantation is important due to the lifelong risk of rejection and resultant ventricular dysfunction. While adult studies have shown that echocardiographic measures of right ventricular (RV) function are changed after transplantation, similar results have not been reported in the pediatric population.
Methods
We performed a single-center retrospective study of echocardiograms performed in pediatric heart transplant recipients. All echocardiograms were selected remote from transplantation, rejection, or graft vasculopathy. These criteria identified 127 patients. RV systolic function was measured with tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC), and peak systolic tricuspid annular tissue velocity (S’). Results were compared to 380 healthy age-matched echocardiographic controls.
Results
TAPSE values in pediatric heart transplant recipients were significantly lower than controls at all ages (p<0.0001) with a mean Z-score of −3.38. FAC and S’ did not vary by age in control patients above 6 months old. FAC values in transplant patients were significantly decreased compared to controls (p<0.0001), but 83% of transplant patients had FACs within the control-derived normal range. S’ values were also significantly lower for transplants than controls (p<0.0001).
Conclusion
Heart transplant patients have significantly decreased quantitative metrics of RV function relative to healthy controls; longitudinal shortening (TAPSE and S’) is particularly affected. FAC is relatively preserved and may be a better metric in this population. These results establish nomograms of RV function in pediatric heart transplant patients and in normal pediatric controls, which may allow for quantification of changes in this vulnerable population.
Keywords: Two-dimensional echocardiography, Heart transplantation, Right ventricular systolic function, Pediatric Patients, Reference Values
Introduction
Accurate assessment of cardiac function by echocardiography is essential in a variety of clinical contexts. Specifically, pediatric heart transplantation requires lifetime follow-up due to the risk of rejection as well as coronary graft vasculopathy, and echocardiography is an important component of serial assessment1,2. While multiple methods exist using two-dimensional echocardiography to assess left ventricular volume and systolic function through ejection fraction (EF), similar methods cannot be applied to the right ventricle (RV). Due to this limitation, RV function is often assessed qualitatively. However, this method is subject to high inter- and intra-observer variability and is unable to detect subtle differences in function. Thus, it is important to better understand quantitative echocardiographic metrics in the pediatric transplant population.
Common quantitative metrics for assessment of RV function include tricuspid annular plane systolic excursion (TAPSE, a measure of longitudinal RV contraction), fractional area change (FAC, a metric which incorporates both longitudinal motion of the annulus and motion of the RV free wall), and the peak systolic annular velocity of the lateral tricuspid annulus (S’)3. These methods have geometric limitations, in particular difficulties in visualization of the endocardial border of the RV free wall4. While RV EF by cardiac MRI is more fully able to delineate the complex geometry of the RV, this methodology is currently impractical for clinical follow-up that occurs several times annually. As echocardiography is the mainstay of longitudinal follow-up, proper interpretation of quantitative metrics requires reference to normative values in the population of interest. While there are existing studies assessing healthy control values for TAPSE5 and S’6, normal values for FAC in children have not been established4. A value of ≥40% has been suggested, but not validated, in children3. Furthermore, the expected values of these quantitative metrics in the pediatric heart transplant population have never to our knowledge been fully assessed in the literature.
In clinical practice, we have observed lower TAPSE values in children after heart transplant relative to published normal values, even when clinically well. We thus set out to test this hypothesis using a retrospective cohort of transplant patients. As pericardiotomy and transplant alter the cardiac geometry (thus modifying the longitudinal motion of the RV), we also evaluated FAC and S’ in the same patients in order to determine if these metrics may be better preserved in the transplant population. As normative pediatric values are not available for all measures of RV function, we also retrospectively analyzed echocardiograms performed on a normal pediatric population. The creation of standards for seemingly well transplant patients will allow more accurate assessment of RV function and the detection of subtle decreases in RV function.
Methods
We performed a single center retrospective study to evaluate standard metrics of RV function in pediatric heart transplant patients and healthy controls and at the Children’s Hospital of Philadelphia. The study was approved by our Institutional Review Board; due to the retrospective nature of the study, consent was waived.
Study Population
For the transplant group, patients were eligible for inclusion (1) if they underwent heart transplantation at our institution and (2) if they had at least one echocardiogram performed between 1/1/2006 and 11/7/2016. As the goal was to study echocardiographic findings in stable post-transplant patients, echocardiograms were excluded if they occurred within six months of transplantation, within one year after any episode of rejection, after the diagnosis of coronary graft vasculopathy, or after retransplantation. Rejection was defined as biopsy-proven cellular grade greater than or equal to 2R, biopsy-proven antibody-mediated rejection, or any episode that required augmentation of immunosuppression (e.g., patients who were too unstable to undergo cardiac catheterization and endomyocardial biopsy, patients with so-called “biopsy negative” rejection, as well as those with symptomatic grade 1R acute cellular rejection)7. The one year period of exclusion after rejection is conservative, but as there are many instances in pediatric cardiology where cardiac function can remain depressed for a sustained period after an insult (e.g., myocarditis), we wanted to ensure that all included echocardiograms represented as close to a clinically well state as possible. Echocardiograms were also excluded if the quality of apical views was inadequate to calculate any of the three studied metrics. For each measure of RV function (TAPSE, FAC, and S’), the first echocardiogram (i.e., closest after transplant) that satisfied the above criteria was selected for inclusion. Using these criteria, we identified 127 transplant patients.
Healthy control patients were eligible for inclusion if they had an echocardiogram (performed during the above date range) with a diagnosis code indicative of no structural cardiac disease (e.g., “murmur”, “observation for condition not observed”, or “syncope”). Echocardiograms were excluded if they had any structural cardiac disease more significant than a patent foramen ovale or any valvar regurgitation above trivial. Controls were also excluded if there was any clinical history that would possibly affect cardiac function (e.g., myocarditis, cardiomyopathy, exposure to chemotherapy, or mitochondrial disease). In order to maximize the number of control echocardiograms with tissue Doppler imaging, controls were selected from the latter portion of the study window. The 380 echocardiograms in the control group were performed between 9/30/16 and 11/7/16.
Echocardiographic Measures
Imaging was performed on either a Philips iE33 or Sonos 5500 machine (Phillips Medical Systems, Andover, MA) with a probe selected based on patient size and image quality. The images were obtained during routine clinical care and retrospectively reviewed. Our clinical protocol includes apical four-chamber views, from which all calculations were made. The use of tissue Doppler imaging was introduced during the study window and was thus not universally available. All images were digitally stored using Syngo Dynamics (Siemens Healthcare, Ann Arbor, MI), which was used for all off-line calculations. All study measurements were made by the same investigator (BRW). A second reader (MJO) also performed measurements in a limited number of patients for the purpose of characterizing inter-reader reliability. The readers were blinded to any measurements of RV function made during the original clinical read of the echocardiogram.
For TAPSE, if an M-mode tracing from the apical position through the lateral tricuspid annulus was available, then this image was used to calculate TAPSE. Otherwise, TAPSE was calculated by measuring the displacement of the lateral tricuspid annulus in a 2D apical four-chamber view8. FAC was calculated by tracing the endocardium in systole and diastole from the apical view: FAC = 100% * (end diastolic area - end systolic area) / end diastolic area. S’ was calculated as the peak systolic velocity of the lateral tricuspid annulus determined by tissue Doppler imaging from the apical position. For all study metrics, multiple measurements were taken per echocardiogram (usually over three heart beats, or two if only shorter clips were available) and then averaged (for the purposes of inter-reader reliability, the second reader used three-beat averaging for TAPSE and S’ and single-beat calculations for FAC).
Statistical Analysis
Continuous variables are presented as means and standard deviations, or as medians with interquartile ranges. Categorical variables are presented as counts and percentages. Parameters were tested for normality using the Shapiro-Wilk test and visual inspection for skewness. To compare metrics between populations, Student’s t-test was used. Statistical significance was defined as p≤0.05. Reliability and repeatability of the measurements were assessed by obtaining intraclass correlation coefficients (ICCs) from linear mixed-effect models for TAPSE, FAC, and S’ as well as Bland-Altman analysis. To obtain reliability for FAC and S’, these calculations were based on 20 patients with two sets of measurements done at separate times at least one week apart. As TAPSE was calculated by two methods, the reliability across the two methods was also checked. For 20 patients each, TAPSE was calculated by M-mode twice or by 2D twice. In 20 patients, TAPSE was calculated by both M-mode and 2D. All ICC calculations were performed separately for the transplant and control populations.
To analyze changes over time, patients were divided into the same age groups as previously used to study TAPSE in Koestenberger et al.5: 0–30 days, 1–3 months, 3–6 months, 7–12 months, and then by year to age 18 years. As all transplant echocardiograms were performed at least 6 months after transplant, there are no transplant patients in the age groups under 6 months of age. As transplant patients are often small-for-age at the time of transplant, we additionally divided subjects by body surface area (BSA) as follows: BSA <0.2, then grouped by intervals of 0.3, and finally BSA ≥2.0.
TAPSE
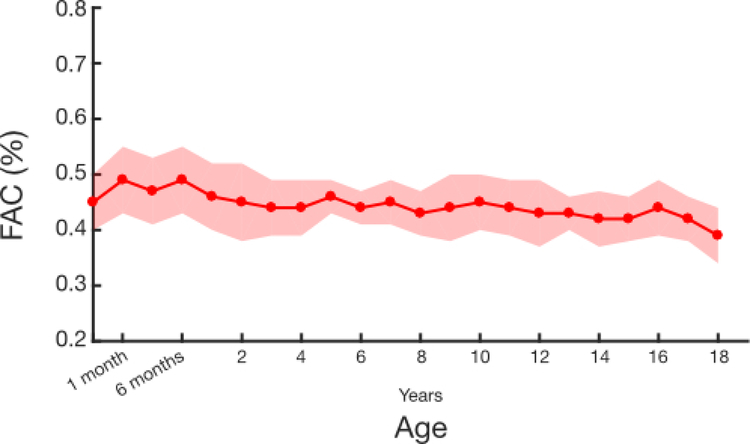
As TAPSE has been shown to vary with age and BSA5, descriptive statistics were computed by age or BSA to create an age- or BSA-specific mean and standard deviation for both transplant patients and controls. Published tabulated means and standard deviations of TAPSE by age5 were used as a second control data set for TAPSE analysis. Student’s t-test was used to compare mean TAPSE values between the control patients and the published control patients, within each age group. Z-scores were calculated for the transplant group using the control age-and BSA-specific means and standard deviations.
As the number of transplant subjects in this study was low at certain ages, means and Z-scores could not be accurately determined for each small increment of age or BSA. However, as will be seen in the Results (namely Figure 1 and Figure 2), it is apparent that the expected increase in TAPSE with increasing age or BSA is substantially attenuated in transplant patients. Thus, we divided the transplant patients into two groups by both age (<10 years old and ≥10 years old) and BSA (<1.0 m2 and ≥1.0 m2) to create nomograms for these subpopulations.
Figure 1.
Comparison of tricuspid annular plane systolic excursion (TAPSE) values by age in our controls (red), previously published controls (orange), and transplant patients (blue). The dark circles are the mean for each age with the shaded regions representing the one standard deviation above and below the mean.
Figure 2.
Comparison of tricuspid annular plane systolic excursion (TAPSE) values by body surface area (BSA) in transplant patients (blue) and controls (red). The dark circles are the mean for each BSA with the shaded regions representing the one standard deviation above and below the mean.
FAC
We first analyzed whether FAC changed with age (the predictor) in control subjects using linear regression. As FAC did not significantly vary with age (for ages greater than 6 months, see Results), all values for transplants and controls were computed across all ages greater than 6 months, as opposed to within age groups. Student’s t-test was used to compare FAC in transplant patients to controls.
When reporting systolic function with a percentile method (e.g., FAC or EF), patients are often classified based on ranges, such as “normal”, “mild ly diminished”, etc. rather than using Z-scores. To create such ranges using FAC from control subjects, we defined the lower range of normal as two standard deviations below the mean. Gradations of diminished function were further increments of standard deviations below the mean (e.g., “mildly diminished” indicates a value between two and three standard deviations below the mean).
S’
For S’, as with FAC, we first analyzed whether this metric changed with age in control subjects using linear regression. Similarly, S’ did not vary significantly with age (for ages greater than 6 months, see Results). Values for transplants and controls were thus computed across all ages greater than 6 months. Student’s t-test was used to compare S’ in transplant patients to controls. While normal values for S’ have been previously published6, those data were presented graphically and not numerically, so statistical comparison with our data is not possible.
Results
The age distribution of transplant patients (N=127) was bimodal, with the most common ages at transplant being less than one year and during the teenage years. The average time from the transplant to the included echocardiogram was 1.2 years (IQR 0.6 – 2.8 years, Table 1). A total of 47 patients (37%) experienced rejection prior to the included echocardiogram (cohort median: 0 episodes of rejection, IQR 0 – 1). All echocardiograms had normal LV function both quantitatively and qualitatively on their original clinical read (with clinical readers quantitatively determining LV function by at least one of: shortening fraction, EF by Simpson’s method, or EF by 3D volumes). RV pressures in the transplant cohort were slightly elevated with a systolic RV pressure measured at the most proximate cardiac catheterization of 27 mmHg +/− 7 mmHg and measured by tricuspid regurgitation jet when possible (N=91, 72%) of 25 mmHg +/− 6 mmHg above the right atrial pressure.
Table 1.
Demographic information of study participants. Dichotomous variables are presented as N (%), and continuous variables are presented as a median (25th-75th percentile range).
| Variable | Control Patients (N=380) | Transplant Patients (N=127) |
|---|---|---|
| Female | 164 (43%) | 60 (47%) |
| Age at transplant (years) | N/A | 5.8 (0.6, 13.5) |
| Age at echocardiogram (years) | 10.4 (3.0, 14.5) | 9.3 (3.1, 15) |
| Time from transplant to echocardiogram (years) | N/A | 1.2 (0.6, 2.8) |
| Episodes of rejection before echocardiogram | N/A | 0 (0, 1) |
TAPSE was able to be measured in all transplant patients (N=127) and controls (N=380). Images sufficient to calculate FAC were available in 121 (95%) transplant patients and in 327 (68%) controls. Tissue Doppler imaging to allow determination of S’ was available in 117 (92%) transplant patients and in 160 (42%) controls. There was good intra- and inter-reader repeatability for all metrics (Table 2).
Table 2.
Intraclass correlation coefficients (ICCs) and limits of agreement demonstrating repeatability of measurements of right ventricular (RV) function. In all groups, the number of subjects tested was 20. (TAPSE: tricuspid annular plane systolic excursion, FAC: fractional area change, S’: peak tricuspid annular systolic tissue velocity.)
| Intra-reader Reliability: Transplants | ICC (95% Confidence Interval) | Limits of Agreement | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| TAPSE by M-mode | 0.97 (0.94–1.00) | −0.09 – 0.14 | 0.025 (−0.003 – 0.052) |
| TAPSE by 2D | 0.79 (0.63–0.96) | −0.18 – 0.34 | 0.078 (−0.018 – 0.138) |
| TAPSE by M-mode and 2D | 0.95 (0.90–0.99) | −0.74 – 0.75 | 0.003 (−0.171 – 0.177) |
| FAC | 0.85 (0.72–0.97) | −0.08 – 0.07 | −0.005 (−0.022 – 0.012) |
| S’ | 0.99 (0.97–1.00) | −0.48 – 0.59 | 0.057 (−0.068 – 0.181) |
| Intra-reader Reliability: Controls | |||
| TAPSE by M-mode | 0.95 (0.91–0.99) | −0.11 – 0.35 | 0.123 (0.069 – 0.176) |
| TAPSE by 2D | 0.93 (0.86–0.99) | −0.37 – 0.38 | 0.004 (−0.083 – 0.091) |
| TAPSE by M-mode and 2D | 0.92 (0.85–0.99) | −1.17 – 0.97 | −0.100 (−0.350 – 0.150) |
| FAC | 0.77 (0.58–0.95) | −0.06 – 0.12 | 0.033 (0.012 – 0.055) |
| S’ | 0.93 (0.88–0.99) | −0.95 – 1.28 | 0.165 (−0.095 – 0.424) |
| Inter-reader Reliability: Transplants | |||
| TAPSE by M-mode | 0.88 (0.79–0.98) | −0.27 – 0.35 | −0.031 (−0.086 – 0.024) |
| TAPSE by 2D | 0.88 (0.74–0.98) | −0.31 – 0.20 | −0.056 (−0.115 – 0.004) |
| FAC | 0.76 (0.57–0.95) | −0.06 – 0.05 | −0.002 (−0.014 – 0.011) |
| S’ | 0.89 (0.80–0.98) | −1.68 – 0.81 | −0.438 (−0.730 - −0.146) |
| Inter-reader Reliability: Controls | |||
| TAPSE by M-mode | 0.97 (0.94–1.00) | −0.30 – 0.13 | −0.086 (−0.135 - −0.036) |
| TAPSE by 2D | 0.84 (0.72–0.97) | −0.29 – 0.58 | 0.145 (0.043 – 0.247) |
| FAC | 0.56 (0.26–0.87) | −0.08 – 0.09 | 0.006 (−0.014 – 0.026) |
| S’ | 0.92 (0.85–0.99) | −1.19 – 1.18 | −0.006 (−0.283 – 0.271) |
TAPSE
TAPSE values in the control subjects were overall similar to previously published values (Figure 1). However, at younger ages, there was a statistically significant difference in the distributions, with our measured values slightly higher than those previously published (Supplemental Table 1). Transplant TAPSE values were lower than those from either control data set (Figure 1); p < 0.0001 for all age groups. Using the control subject data to determine Z-scores for the transplant patients resulted in low Z-scores at all age groups with an overall mean Z score of - 3.38 (range: −9.36 to −0.41). Results are qualitatively similar when compared to published controls (Table 3), however, the Z-scores are often lower (likely reflecting the lower standard deviations and larger sample sizes in the published control data).
Table 3.
Tricuspid annular plane systolic excursion (TAPSE) values by age in transplant patients with corresponding Z-scores based on the two control data sets.
| Transplant patients | Z-Scores relative to controls | ||||
|---|---|---|---|---|---|
| Age ranges | N | Mean (cm) | Standard deviation (cm) | Current study controls | Published controls5 |
| 1–12 months | 11 | 0.92 | 0.16 | −2.17 | −3.25 |
| 1 year | 14 | 1.06 | 0.28 | −2.49 | −3.27 |
| 2 years | 5 | 0.91 | 0.27 | −3.59 | −4.93 |
| 3 years | 10 | 0.91 | 0.23 | −5.06 | −6.38 |
| 4 years | 5 | 1.15 | 0.18 | −3.37 | −5.15 |
| 5 years | 3 | 0.78 | 0.12 | −4.42 | −7.79 |
| 6 years | 5 | 1.05 | 0.21 | −7.23 | −6.07 |
| 7 years | 4 | 1.09 | 0.27 | −2.79 | −5.67 |
| 8 years | 6 | 1.12 | 0.19 | −3.73 | −5.67 |
| 9 years | 1 | 1.03 | - | −4.26 | −7.00 |
| 10 years | 6 | 1.27 | 0.26 | −3.17 | −6.00 |
| 11 years | 4 | 1.31 | 0.24 | −2.52 | −5.64 |
| 12 years | 1 | 0.81 | - | −3.60 | −8.87 |
| 13 years | 7 | 1.33 | 0.16 | −2.53 | −4.83 |
| 14 years | 13 | 1.15 | 0.39 | −3.60 | −5.55 |
| 15 years | 7 | 1.25 | 0.36 | −2.70 | −5.40 |
| 16 years | 7 | 1.18 | 0.37 | −4.15 | −5.76 |
| 17 years | 8 | 1.38 | 0.34 | −2.37 | −5.10 |
| 18 years | 4 | 1.38 | 0.27 | −3.30 | −5.19 |
| 19 years | 6 | 1.42 | 0.27 | N/A | N/A |
Similar to the data by age, the TAPSE values for normal patients increase with increasing BSA, and values for transplant patients were well below the normal values (p < 0.0001 for all BSA ranges, Figure 2 and Table 4). Transplant-specific nomograms were created for TAPSE by both age and BSA by dividing patients at age 10 years or at a BSA of 1.0 m2. Transplant patients 7 months to 9 years of age had an expected TAPSE of 1.00 +/− 0.23, which was similar to the expected TAPSE in patients with a BSA under 1.0 m2 of 1.01 +/− 0.25. Older patients (10–18 years) had an expected TAPSE of 1.25 +/− 0.32, and large patients (BSA ≥1.0 m2) had an expected TAPSE of 1.26 +/− 0.32.
Table 4.
Tricuspid annular plane systolic excursion (TAPSE) values by body surface area (BSA) in controls and transplant patients with corresponding Z-scores based on the control data set.
| Controls | Transplant patients | |||||||
|---|---|---|---|---|---|---|---|---|
| BSA | N | Mean (cm) | Standard Deviation (cm) | N | Mean (cm) | Standard Deviation (cm) | p-value | Transplant Z- score based on control data |
| 0.2–0.5 | 24 | 1.59 | 0.18 | 23 | 1.02 | 0.23 | < 0.0001 | −3.17 |
| 0.5–0.8 | 52 | 1.94 | 0.27 | 30 | 0.95 | 0.25 | < 0.0001 | −3.67 |
| 0.8–1.1 | 53 | 2.11 | 0.28 | 14 | 1.13 | 0.24 | < 0.0001 | −3.50 |
| 1.1–1.4 | 52 | 2.14 | 0.34 | 10 | 1.1 | 0.27 | < 0.0001 | −3.06 |
| 1.4–1.7 | 93 | 2.31 | 0.35 | 22 | 1.3 | 0.28 | < 0.0001 | −2.89 |
| 1.7–2.0 | 47 | 2.47 | 0.35 | 17 | 1.25 | 0.37 | < 0.0001 | −3.49 |
| 2.0+ | 13 | 2.58 | 0.30 | 5 | 1.4 | 0.3 | < 0.0001 | −3.93 |
FAC
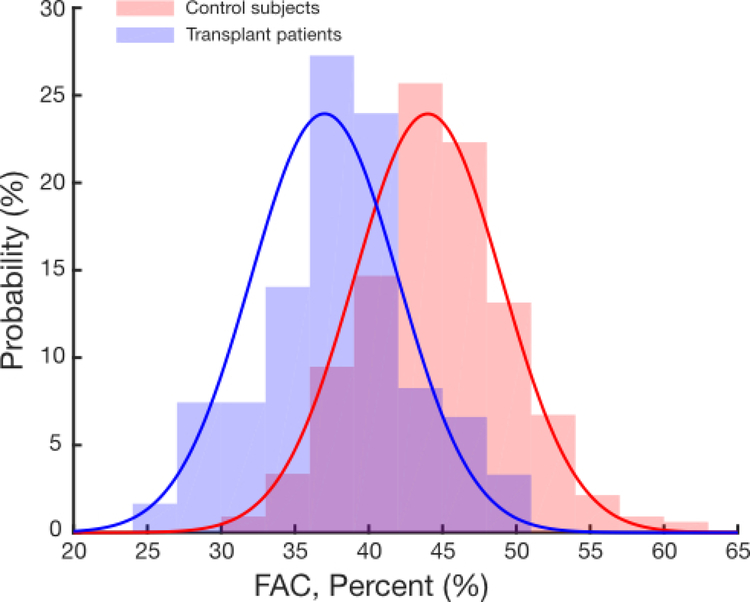
As no normal values of FAC have previously been published in children, we analyzed FAC vs age in the control group to determine whether an age-specific nomogram was necessary. For patients over 6 months of age, FAC did not vary significantly with changes in age by linear regression analysis (Supplemental Figure 1). Thus, the mean FAC values for transplant patients across all age groups were analyzed together and found to be significantly lower than FAC values in controls over 6 months of age (37% ± 5% vs 44% ± 5%, p<0.0001, Figure 3). The majority of transplant FAC values were within one standard deviation of control values. Ranges for FAC (e.g., “normal”, “mildly diminished”) were determined based the control population mean and standard deviation (Table 5). When classifying transplant patients using the ranges derived from controls, the majority of transplant FAC values fall in the normal range, albeit at the lower end of normal (Table 6).
Figure 3.
Comparison of fractional area change (FAC) values in transplant patients (blue) and controls (red), all >6 months of age. The bars denote the histogram of measured values of FAC with the solid lines are the calculated probability density function using the mean and standard deviation of the data.
Table 5.
Fractional area change (FAC) ranges determined from control subjects.
| Normal | ≥ 34% |
| Mildly Diminished | 29 – 33% |
| Moderately Diminished | 24 – 28% |
| Severely Diminished | < 24% |
Table 6.
Transplant patients classified by the fractional area change (FAC) range from normal controls, N (%).
| Normal | 99 (81.8%) |
| Mildly Diminished | 14 (11.6%) |
| Moderately Diminished | 8 (6.6%) |
| Severely Diminished | 0 (0%) |
S’
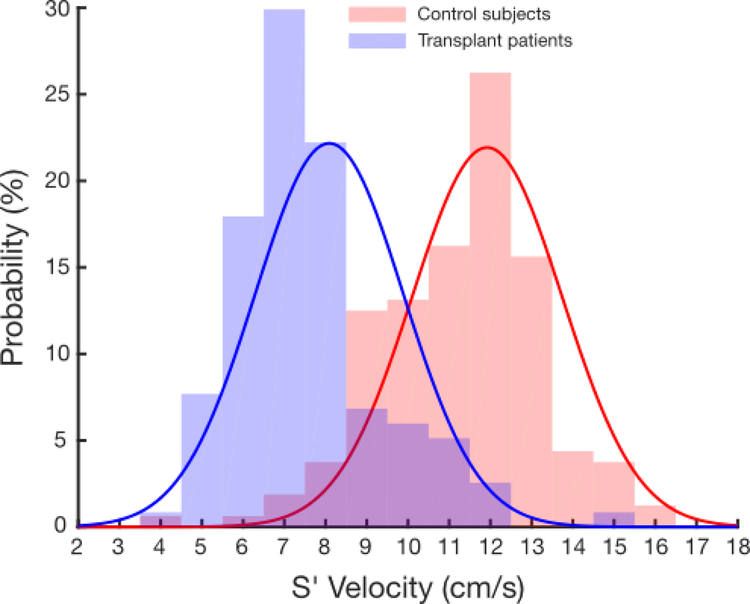
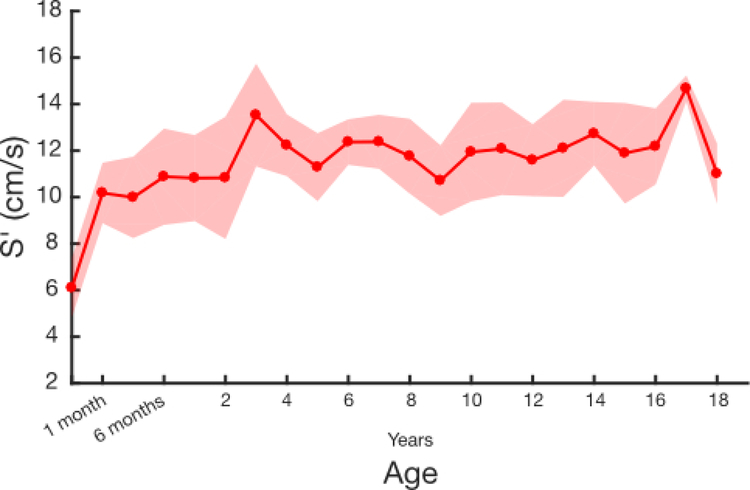
For analysis of S’, we examined control subjects by age. S’ values were lower for patients under 6 months of age, but linear regression showed that above this threshold, S’ did not vary significantly by age (Supplemental Figure 2). Thus, we restricted further analysis to patients older than 6 months of age. As with FAC, transplant patients had significantly lower mean S’ values compared with healthy controls (8.09 ±1.80 vs 11.92 ± 1.82, p < 0.0001, Figure 4).
Figure 4.
Comparison of peak tricuspid annular systolic tissue velocity (S’) values in controls (red) and transplant patients (blue), all >6 months of age. The bars denote the histogram of measured values of S’ with the solid lines are the calculated probability density function using the mean and standard deviation of the data.
Discussion
We performed a retrospective analysis in a relatively large sample size of clinically well transplant patients and controls to determine normal values for common metrics of right ventricular function. To our knowledge, this is the first comprehensive quantitative investigation of RV function in the pediatric transplant population, and it adds to our knowledge of normal pediatric control values, which have not been previously evaluated for all of these metrics.
TAPSE values in our control population were in general agreement with those published previously by Koestenberger et al.5 except at young ages, where our values were slightly higher. While this difference is statistically significant, the small changes in TAPSE are likely not clinically significant.
We have demonstrated that TAPSE values in pediatric patients after heart transplantation are well below the normal range for children. As these results hold for both age and BSA, the low TAPSE-for-age in transplant patients is not due to being small-for-age compared to healthy controls. While the age and BSA of the transplant patient were used by necessity, TAPSE in the transplanted heart is likely affected by the donor’s age and BSA as well; however, these data are not available in the medical record. Since TAPSE varies minimally with either age or BSA in transplanted hearts, the effect of any mismatch is mitigated.
As all of these echocardiograms were taken at times when these patients were clinically well, low TAPSE likely reflects changes in the mechanisms of right heart contractility in this population rather than right ventricular dysfunction. The pericardiotomy inherent to any cardiac surgery, such as transplant, alters the geometry of the heart in the chest and the external forces affecting right ventricular contraction. Studies performed in various postsurgical populations including atrial septal defect closure9,10, mitral valve repair11, and aortic valve repair12,13 have all shown decreased TAPSE values. While an early hypothesis attributed this finding to right heart injury during cardiopulmonary bypass and cardioplegia9,10, more recent studies have shown preserved postsurgical RV EF as measured by cardiac MRI11–14. It is likely that after pericardiotomy, the relationship between TAPSE and RV EF15 changes, as has been seen after the Fontan operation16 and tetralogy of Fallot repair8, but the exact mechanism for decreased TAPSE after cardiac surgery remains to be elucidated.
While normal values of FAC in adults have been reported as ≥32–35%17,18, normal values have not previously been reported in children. Our determined normal value of ≥ 34% is in keeping with the adult literature and not as strict as the previously asserted pediatric value3. We have shown that while FAC in transplant patients is on average lower than controls, the majority of patients have FAC values in the normal range. This finding may indicate that in the transplanted heart, the RV free wall provides a greater contribution to RV ejection to compensate for processes that decrease longitudinal contraction. Therefore, the use of FAC may be a more reliable measure in this population than TAPSE.
S’ values after cardiac transplantation have been assessed in prior smaller pediatric studies19–22. In general, these studies show that S’ is severely depressed immediately after transplant and recovers over the following 6 months, although not to normal values. Interestingly, these papers differ in their assumptions with some assuming a linear change in S’ with age22 and some assuming that S’ is invariant with age19–21. With a larger sample size in our study, we were able to perform linear regression analysis to see if S’ varied with age, and it did not. The prior studies of S’ demonstrate a wide range of expected values, from 6 cm/s to 10 cm/s. Our values in pediatric transplant patients, with the largest sample size to our knowledge to date, are in the middle of these prior results. Given that S’ and TAPSE are both measures of lateral tricuspid annular motion, it is reasonable to expect these values to change in tandem. However, as we found that S’ is independent of age and BSA, the clinical use of S’ may be simpler than TAPSE.
Over all three metrics, these results parallel those of prior studies of cardiac function after adult cardiac transplantation23–24, which also show decreased quantitative metrics of RV function. To judge post-transplant echocardiograms by normal pediatric values would present an overly-negative picture of function. If these echocardiograms are not examined closely and quantitatively, one could miss subtle changes in function that may be indicative of rejection or graft dysfunction. Thus, the use of transplant-specific nomograms is important as changes over time can be assessed relative to an appropriate population baseline.
One limitation of our study is that the population of patients was not evenly distributed over all ages with the majority of transplant patients occurring in infancy and the later teenage years. The bimodal distribution would not affect FAC and S’ where all patients were combined, but does affect the ability to adequately analyze TAPSE values for individual ages during the school age range. This limitation motivated our TAPSE nomograms which divide transplant patients into two large groups by age and BSA.
Additionally, most metrics of right ventricular function (including ejection fraction from cardiac MRI) are affected by preload and afterload. We did not control for the amount of tricuspid regurgitation, pulmonary stenosis, or pulmonary vascular resistance. While such a multivariate approach would be helpful in overall assessment of these patients, it would require a much larger sample size than presently available. RV strain is a promising parameter that may be less geometry-dependent and more indicative of myocyte contractility than the traditional metrics discussed here. However, retrospective analysis of echocardiograms that were not optimized for the image quality required for strain analysis was not possible. As the use of strain becomes more prevalent, an evaluation of its use in this population will become more feasible.
We attempted to include only healthy controls and clinically well transplant patients. However, some patients may have been experiencing unrecognized rejection at the time of the included echocardiogram. One area of future study will be in an expanded, longitudinal transplant cohort to explore whether prior or current rejection is associated with lower RV function metrics by echocardiography and how metrics evolve over time in individual patients as they get further from the time of transplant.
In conclusion, we have shown that seemingly well pediatric transplant patients have lower than normal quantitative metrics of RV function. Since quantitative measurement of RV function is important for follow-up in this population, the use of appropriate transplant-specific nomograms (presented in this work) should allow better assessment of RV function and perhaps allow earlier detection of subtle changes in RV function that could be associated with transplant-specific pathology such as rejection or graft vasculopathy.
Supplementary Material
Highlights.
Measures of RV function are decreased after pediatric heart transplantation.
TAPSE after pediatric transplantation is decreased at all ages and BSAs.
Post-transplant FAC is lower than in controls, but relatively preserved.
FAC and S’ do not vary by age in control patients above 6 months old.
Acknowledgments
Funding
This research was supported in part by the Cardiac Center Research Core at the Children’s Hospital of Philadelphia. BRW is funded by NHLBI grant T32HL007915 and the Children’s Hospital of Philadelphia Research Institute. LMR is funded by NHLBI Grant K01HL125521 and Pulmonary Hypertension Association Supplement to K01HL125521.
Abbreviations
- BSA
Body surface area
- EF
Ejection fraction
- FAC
Fractional area change
- RV
Right ventricle
- S’
Peak tricuspid annular systolic tissue velocity
- TAPSE
Tricuspid annular plane systolic excursion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thorn EM, de Filippi CR. Echocardiography in the cardiac transplant recipient. Heart Fail Clin 2007; 3:51–67. [DOI] [PubMed] [Google Scholar]
- 2.Estep JD, Shah DJ, Nagueh SF, Mahmarian JJ, Torre-Amione G, Zoghbi WA. The role of multimodality cardiac imaging in the transplanted heart. JACC Cardiovasc Imaging 2009; 2:1126–40. [DOI] [PubMed] [Google Scholar]
- 3.Mertens LL, Friedberg MK. Systolic ventricular function. In: Lai WW, Mertens LL, Cohen MS, Geva T, editors. Echocardiography in pediatric and congenital heart disease: From fetus to adult 2 ed: John Wiley & Sons; 2016: 96–131. [Google Scholar]
- 4.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the pediatric measurements writing group of the American society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr 2010; 23:465–95. [DOI] [PubMed] [Google Scholar]
- 5.Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, et al. Right ventricular function in infants, children and adolescents: Reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr 2009; 22: 715–9. [DOI] [PubMed] [Google Scholar]
- 6.Roberson DA, Cui W, Chen Z, Madronero LF, Cuneo BF. Annular and septal Doppler tissue imaging in children: Normal z-score tables and effects of age, heart rate, and body surface area. J Am Soc Echocardiogr 2007; 20:1276–84. [DOI] [PubMed] [Google Scholar]
- 7.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The international society of heart and lung transplant guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29:914–56. [DOI] [PubMed] [Google Scholar]
- 8.Mercer-Rosa L, Parnell A, Forfia PR, Yang W, Goldmuntz E, Kawut SM. Tricuspid annular plane systolic excursion in the assessment of right ventricular function in children and adolescents after repair of tetralogy of Fallot. J Am Soc Echocardiogr 2013; 26:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhillon R, Josen M, Henein M, Redington A. Transcatheter closure of atrial septal defect preserves right ventricular function. Heart 2002; 87:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanseus KC, Bjorkhem GE, Brodin LA, Pesonen E. Analysis of atrioventricular plane movements by Doppler tissue imaging and m-mode in children with atrial septal defects before and after surgical and device closure. Pediatr Cardiol 2002; 23:152–9. [DOI] [PubMed] [Google Scholar]
- 11.Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr 2009; 10:630–4. [DOI] [PubMed] [Google Scholar]
- 12.Okada DR, Rahmouni HW, Herrmann HC, Bavaria JE, Forfia PR, Han Y. Assessment of right ventricular function by transthoracic echocardiography following aortic valve replacement. Echocardiography 2013; 31:552–7. [DOI] [PubMed] [Google Scholar]
- 13.Keyl C, Schneider J, Beyersforf F, Ruile P, Siepe M, Pioch K, et al. Assessment of right ventricular function after aortic valve replacement: A pilot study comparing surgical and transcatheter procedures using 3D echocardiography. Eur J Cardiothorac Surg 2016; 49:966–71. [DOI] [PubMed] [Google Scholar]
- 14.D’Andrea A, Riegler L, Nunziata L, Scarafile R, Gravino R, Salerno G, et al. Right heart morphology and function in heart transplantation recipients. J Cardiovasc Med 2013; 14:648–58. [DOI] [PubMed] [Google Scholar]
- 15.Helbing WA, Bosch HG, Maliepaard C, Rebergen SA, van der Geest RJ, Hansen B, et al. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol 1995; 76:589–94. [DOI] [PubMed] [Google Scholar]
- 16.Avitabile CM, Whitehead K, Fogel MA, Mercer-Rosa L. Tricuspid annular plane systolic excursion does not correlate with right ventricular ejection fraction in patients with hypoplastic left heart syndrome after Fontan palliation. Pediatr Cardiol 2014; 35:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117:1436–48. [DOI] [PubMed] [Google Scholar]
- 18.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandresekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American society of echocardiography. J Am Soc Echocardiogr 2010; 23:685–713. [DOI] [PubMed] [Google Scholar]
- 19.Fyfe DA, Mahle WR, Kanter KR, Wu G, Vincent RN, Ketchum DL. Reduction of tricuspid annular Doppler tissue velocities in pediatric heart transplant patients. J Heart Lung Transplant 2003; 22:553–559. [DOI] [PubMed] [Google Scholar]
- 20.Mahle WT, Cardis BM, Ketchum D, Vincent RN, Kanter KR, Fyfe DA. Reduction in initial ventricular systolic and ventricular after heart transplantation in children: improvement over time identified by tissue Doppler imaging. J Heart Lung Transplant 2006; 25:1290–6. [DOI] [PubMed] [Google Scholar]
- 21.Lunze FI, Colan SD, Gauvreau K, Chen MH, Perez-Atayde AR, Blume ED, et al. Cardiac allograft function during the first year after transplantation in rejection-free children and young adults. Circ Cardiovasc Imaging 2015; 5:756–64. [DOI] [PubMed] [Google Scholar]
- 22.Pauliks LB, Pietra BA, Kirby S, Logan L, DeGroff CG, Boucek MM, et al. Altered ventricular mechanics in cardiac allografts: a tissue Doppler study in 30 children without prior rejection events. J Heart Lung Transplant 2005; 24:1804–13. [DOI] [PubMed] [Google Scholar]
- 23.Ingvarsson A, Werther Evaldsson A, Waktare J, Nilsson J, Smith GJ, Stagmo M, et al. Normal reference ranges for transthoracic echocardiography following heart transplantation. J Am Soc Echocardiogr 2018; 31:349–60. [DOI] [PubMed] [Google Scholar]
- 24.Clemmensen TS, Eiskjaer H, Logstrup BB, Andersen MJ, Mellemkjaer S, Poulsen SH. Echocardiographic assessment of right heart function in heart transplant recipients and the relation to exercise hemodynamics. Transpl Int 2016; 29:909–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.