Abstract
Purpose of Review
There are three liver-specific causality assessment tools currently available to guide clinical diagnosis of Drug Induced Liver Injury (DILI): Roussel-Uclaf-Causality-Assessment-Method (RUCAM), Digestive-Disease-Week Japan 2004 scale (DDW-J), and Clinical Diagnostic Scale (CDS). The purpose of this review is to assess these tools and discuss how to improve the causality assessment process as a whole.
Recent findings:
Existing DILI-specific causality assessment tools are surprisingly similar and exhibit only minor differences. We reviewed the literature on currently used causality assessment tools, identified areas for future improvement, and herein propose approaches for refinement. Opportunities to improve current models, as well as the assessment process in general include in particular provision of more precise clinical detail and to perhaps add new components to scoring systems. For example, the incorporation of drug-specific clinical signature patterns, accounting for a drug’s inherent hepatotoxicity potential, and/or incorporation of other drug properties to scoring systems may allow enhancement. Further, more systemic exclusion of competing diagnoses is needed. Finally, causality assessment processes will likely benefit from a data-driven and computer-assisted approach.
Summary
Current tools used for DILI adjudication are imperfect. Avenues to improve these tools are described.
Keywords: Causality, Drug induced liver injury, scoring system, liver disease, hepatotoxicity, drug toxicity, adverse liver events, differential diagnosis, diagnostic criteria
Introduction
Drug-induced liver injury (DILI) can be classified as either intrinsic DILI (inDILI) or idiosyncratic DILI (iDILI). The inDILI is sometimes referred as DILI caused by direct toxicity. The classic example of this includes hepatotoxicity caused by acetaminophen in which there is direct injury (to hepatocytes) as a result of the drugs action. There is a strong correlation between dose and hepatotoxicity, and the risk of hepatotoxicity is reasonably predictable at a high dose. This differs from iDILI, which is not necessarily dose-dependent. In the past it was believed that iDILI was thought to be largely independent of drug dose, but evidence suggests that the risk for iDILI may increase with the total daily dose [1, 2]. However, other factors such as fasting, alcohol consumption, genetic polymorphisms and race/ethnicity modify susceptibility and outcomes [3, 4, 5].
Hepatotoxicity has also historically been a major problem in drug development - and was a leading reason for drug withdrawal from a market in the US as well as worldwide. Drug withdrawals have been less of a problem in more recent years [6, 7], perhaps as models of hepatotoxicity during drug development have improved [8, 9]. Notwithstanding, DILI remains a significant challenge in post-marketing pharmacovigilance. In February 2018, the European Medicines Agency (EMA) recommended the removal of flupirtine from the market due to ongoing concerns over severe DILI.[10]
Perhaps most importantly, DILI is a common problem in clinical practice and leads to substantial morbidity and mortality in the US and worldwide [11, 12, 13, 14, 15]. The risk of chronic liver injury and chronic liver disease is also substantial. Chronic DILI may be defined as persistent elevation of liver tests at 3, 6 or 12 months [16, 17]. The frequency of chronicity reported in the literature has been highly variable, but has been reported to be as high as nearly 20% [16, 17]. Additionally, it was found that approximately 75% of patients with elevated liver tests at 6 months continue to have elevated liver test 6 months later[16]. Interestingly, older patients appeared to have a greater risk of chronic DILI. Additionally, for those with elevation in liver tests at 12 months, it was found that they had a reduced impaired quality of life [16].
In clinical medicine, DILI remains a diagnosis of exclusion. Liver test abnormalities may result from many different liver diseases, and even non-hepatic disorders [18, 19, 20]. For diseases such as viral hepatitis, a single diagnostic test may confirm or exclude the diagnosis with high sensitivity and specificity. In contrast, DILI is a diagnosis based on suspicion of an adverse drug reaction and the exclusion of competing causes of liver injury. Because of the difficulties in diagnosing DILI, a variety of causality assessment methods (CAMs) have been developed, many of which use point-scoring systems. The “Roussel Uclaf Causality Assessment Method” (RUCAM), also known as “Council for International Organizations of Medical Sciences” scale (CIOMS), was the first liver-specific causality tool[21], which is most often referred to as RUCAM. The “clinical diagnostic scale” (CDS) is another commonly used tool [22]. Finally, a variation of the RUCAM was developed by a Japanese hepatology working group, called “Digestive Disease Week Japan 2004 scale (DDW-J scale)[23]. While these scoring systems have considerable value, they fall short in inter-rater reliability and intra-rater reproducibility. [24] Currently, a structured expert-opinion process (SEOP), as described and utilized by the NIH-sponsored DILIN, appears to outperform RUCAM [25]. Unfortunately, a major drawback of this method is that it is impractical in clinical practice because several experts are required, and thus, it is not generally feasible and thus not widely applicable.
Here, we review the current state of DILI causality assessment by comparing existing causality assessment tools, and provide insight into how current scoring systems can be improved. While we believe that an ideal DILI causality assessment tool should be drug specific (when possible), until such an approach becomes feasible, it is likely that the best approach currently is to optimize existing causality assessment instruments.
Currently established causality assessment tools
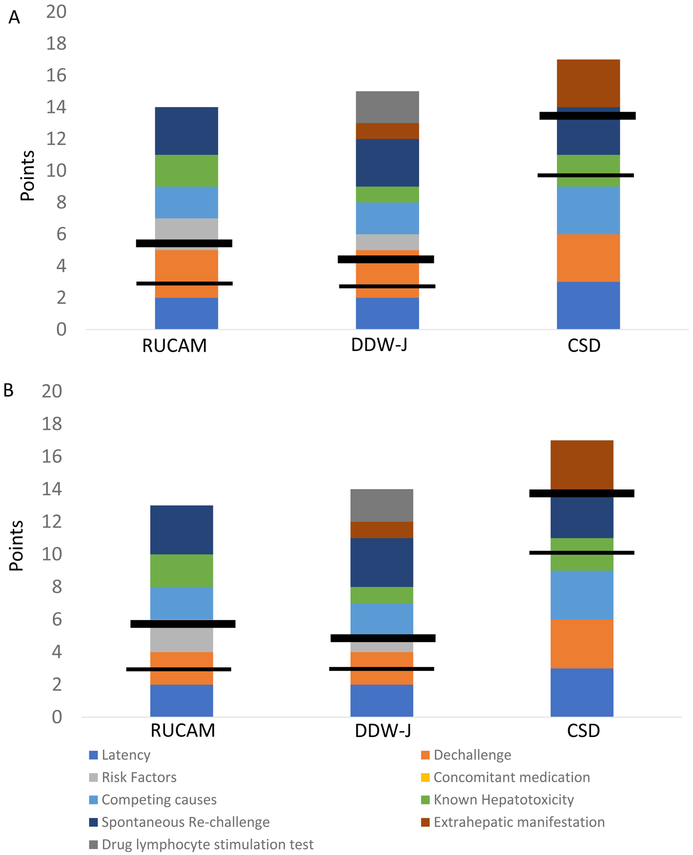
Here, we review 3 causality assessment tools specific for DILI, including the RUCAM (or CIOMS), Clinical Scale for the Diagnosis (CDS), and the DDW-J score (this has been published only in Japanese) (Table 1). All of these instruments utilize similar variables, and further have developed relatively similar scoring systems. Minor differences in the three scoring systems , CDS uses 6 categories, RUCAM uses 7 categories and DDW-J uses 8 categories. Each of the three systems consider drug latency to be important, and use a similar definition - days from drug start to DILI onset (when the patient presents while taking a medication), or days from drug stop to DILI onset (when a drug had already been stopped, as frequently occurs with defined courses of antibiotic use)[26]. Of note, although all three instruments have similar structures, and maximal points allocated for each category and total points achievable overall are only slightly different, the overall causality grading varies significantly (Table 1a, Figure 1); the CDS has a higher threshold to achieve scores consistent with DILI than RUCAM and DDW-J (Table 1a, Figure 1), though the differences in descriptions of the likelihood (or categories of DILI) are likely a matter of semantics.
Table 1a.
Comparison of different DILI causality scoring systems
| RUCAM/CIOMS | DDW-J | CDS | ||||
|---|---|---|---|---|---|---|
| Variables | Score | % of total score | Score | % of total score | Score | % of total score |
| Chronological criteria : 1a From drug start to onset* |
+1 to +2 |
+7.1 to +14,3% | +1 to +2 | +5.9 – +11.8% |
+1 to +3*,** |
+5.9 –+17.6 |
| 1b From drug stop to onset* | Incompatible *** to +1 | 0 to +7.1% | 0 to +1 | 0 – +5.9% |
−3 to +3*,** | −17.6 – +17.6 |
| 2 Dechallenge | −2 to +3 | −14.2 to +21.4% | −2 to +3$ | −11.8 to +17.6% | 0 to +3 | 0 – +17.6 |
| 4 Competing causes | −3 to +2 | −21.3 to +14.3% | −3 to +2 | −17.6 to 11.8% | −3 to +3 | −17.6 – +17.6 |
| 5 Rechallenge | −2 to +3 | −14.3% to +21.4% | −2 to +3 | −11.8 – +17.6% | +3 | 0 – +17.6 |
| 6 Known Hepatotoxicity | 0 to +2 | 0 to +14.3% | 0 to +2 | 0 – +5.9% | −3 to +2 | −17.6 – +11.8 |
| 3 Risk Factors | 0 to +2 | 0 to +14.3% | 0 to +2 | 0 – +12.5% | - | - |
| 7 Concomitant medication | −3 to 0 | −21.4 to 0% | - | - | - | - |
| Extrahepatic manifestations | - | - | 0 to +1 | 0 – +5.9% | 0 to +3 | 0 – +17.6 |
| Drug lymphocyte stimulation test |
- |
- |
0 to +2 |
0 – +11.8% |
- |
- |
| Total score | −10 to 14 | −5 to 17 | −6 to 17** | |||
| Assessment in each score | ||||||
| Definite | >8 | >8 | >17 | |||
| Probable | 6–8 | 5–8 | 14–17 | |||
| Possible | 3–5 | 3–4$ | 10–13 | |||
| Unlikely | 1–2 | 1–2$ | 6–9 | |||
| Excluded | ≤ 0 | ≤ 0 | ≤ 6 | |||
only either of these two categories 1a or 1b is to be counted;
the paper states a maximal of 20 points, but as you can only either be on a drug or have stopped it you would only the points of 1a or 1b would be counted resulting in a maximum total of 17 points;
in a “updated RUCAM” the incompatible scoring for onset after stop of drug has been eliminated.
Figure 1. Comparison of point distributions for causality instruments.
The figure depicts the maximal points given in each category for RUCAM, DDW-J and CDS, respectively. The horizontal lines indicate the points required to qualify for possible (thinner line) and probable (thicker line) DILI, respectively. In (A) is shown a hepatocellular injury pattern and in (B), mixed/cholestatic injury pattern. Of note, the category for concomitant medications is unique to RUCAM and gives negative points depending on the strength of concern that an alternative drug may have causes the liver injury. This category is ignored should there be identification of a competing cause such as acute viral hepatitis. Additionally, note CDS maximum was adjusted to 17, as 20 points in unattainable, since it is not possible to be “on” a drug and “off” a drug at the same time.
Abbreviations: CDS: Clinical diagnostic scale as proposed by Maria & Victorino, DDW-J: a modified version of RUCAM developed during Japanese Digestive Disease Week, HC: hepatocellular, MC: mixed/cholestatic, RUCAM.
Major differences in these 3 instruments are as follows:
The DDW-J scale is largely a modification of RUCAM with the following major differences: “DILI onset beyond 15 or 30 days after drug stop”, does not get any points, but are not considered incompatible with DILI like in RUCAM, no consideration for co-medication, simplification of hepatotoxicity into yes or no, elimination of risk factor “age”, addition of a drug-lymphocyte stimulation test (DLST), and consideration of eosinophilia [23,27]. To the best of the authors knowledge, the full DDW-J scale, 2002 version or 2004 version, has not been published in English, while it has been utilized in several English publications by Japanese investigators [28.29].
Though the CDS is similar to RUCAM in terms of clinical variables that are assessed, it differs from RUCAM in point values and timing of drug administration to onset of clinical features (see Table 1). In contrast to RUCAM, CDS allocates points for extrahepatic manifestation such as rash, fever, eosinophilia, arthralgia, and cytopenia [22].
An “updated” RUCAM, published in 2015 has 2 modifications to the original RUCAM [30]. This is that “DILI onset beyond 15 or 30 days after drug stop” does not get any point but is not considered incompatible with DILI anymore (similar to DDW-J). The other aspect is that for group I exclusion, HEV and HCV RNA testing would now be requested. This likely is self-explanatory and would have been likely considered by anyone completing RUCAM scores in 2019, based on knowledge that HEV and HCV can be mistaken for DILI if not checked for.[31,32]
Each of the tools also has unique features. For example, consideration of concomitant medication-use as a variable important in DILI assessment is unique to RUCAM, while drug lymphocyte stimulation is unique to DDW-J. Drug lymphocyte stimulation is technically demanding, difficult to reproduce and requires specialized laboratory expertise. Extrahepatic manifestations are not considered in RUCAM, and more weight is given to extrahepatic manifestations in CDS than in DDW-J scoring. Risk factors for DILI are not considered in the CDS, and have a higher weight in RUCAM than in DDW-J.
There is one scoring issue that might have been overlooked in the CDS scale. The reported maximum value for CDS is said to be 20 in the original report, which is only achievable if 3 points are allocated for the injury onset happening while on drug within 4 to 56 days from drug start plus 3 points for having the onset within 7 days of stopping a drug. However, logically one can only have either of these circumstances, not both. Thus, a score of >17, which is required for definite DILI in the original scoring system, should be unattainable, unless you would count both drug start to onset and drug stop to onset. Clear explanation on how to apply the criteria using days from drug initiation vs. the ones using days from drug discontinuation is lacking in the original RUCAM, which might have caused misleading and was somewhat clearer in the “updated RUCAM” [23]. In DDW-J 2004, they pointed out the problem in the report and provided clear guideline to score the latency by either using days from the drug initiation (DILI occurring while on suspected medication) or using days from the drug discontinuation (DILI occurring after drug discontinuation).
Limitations and considerations for improvement
Of note, all current scoring systems were initially developed largely based on expert opinions with only limited datasets to test their respective approaches. Thus, the approaches were not data-driven. For example, the heterogeneity of DILI manifestations and drug-specific aspects were not considered, perhaps due to the lack of aggregated data, at that time, of DILI phenotypes. Despite the shortcomings of the various instruments, they have been assessed on a variety of different levels, and have been demonstrated to correlate reasonably well with expert opinion and also with each other [21,22,33,34].
Ideally, there should be a confirmatory laboratory test to confirm suspected DILI. One such test reported is the lymphocyte transformation test (LTT), where proliferation of T-cells in response to drug exposure is assessed by measurement of incorporate 3H-thymidine or Interferon-gamma (IFN-γ production. However, a recent study reported limited reliability in test reproducibility when using a modified LTT with readouts for interleukin-2 (IL-2), IL-5, IL-13, granzyme B, and IFN-γ.35
A new and innovative approach towards a confirmatory (laboratory based) assay was recently described [36]; this assay uses monocyte-derived hepatocyte-like cells (MH) derived from patients with suspected DILI to assess drug-specific LDH release from MH as a diagnostic marker for DILI. This approach could be used to identify new biomarkers. For example, integrin beta 3 (ITGB3) was identified as being up-regulated in MH cells derived from patients with diclofenac DILI. The frequency of ITGB3 positive cells was found to be reduced in the peripheral blood of patients with diclofenac DILI compared to controls, while ITGB3 staining was found enriched in liver biopsy samples from patients with diclofenac DILI compared to others [37].
An additional important concept is that current causality approaches, including RUCAM, as currently designed, are general instruments, and not specific for any drug. This becomes an issue because drugs differ in their phenotype. For example, latencies were found to differ dramatically among different drugs (Table 2) [38,39,40,41,42,43,44]. As such, in principle, it appears that it is possible to develop a drug specific causality tool using clinical data [45]. Additionally, given advances in the understanding of the genetics, pathophysiology and molecular basis of disease, it is likely that enhanced drug specific causality tools can be refined and further developed.
Table 2:
Studies demonstrating drug specific variation in latency*.
| Hepatocellular | Cholestatic/ mixed |
P-value** | Reference | |
|---|---|---|---|---|
|
Statins |
n=14 153 (0 to 1866) |
n=8 153 (0 to 3600) |
p=0.913 | Russo et al.[38] |
|
Azithromycin |
n=10 20 (38 to 65) |
n=8 17 (2 to 38) |
p=0.887 | Martinez et al. [39] |
|
Cefazolin |
n=3 23 (21 to 23) |
n=16 20 (6 to 29) |
p=0.487 |
Alqahtani SA, et al.[40] |
|
Quinolones |
n=4 13 (5 to 27) |
n=8 2 (1 to 11) |
p=0.215 |
Orman ES, et al.[41] |
| Cyproterone acetate (CPA) | n=20 150 (105 to 215) |
n=2 150 & 151 |
p=0.866 |
Bessone F, et al. [42] |
|
Ceftriaxone |
n=2 11 & 21 |
n=13 8 (4 to 14) |
p=0.229. |
Nakaharai K, et al. [43] |
Latency is measured in days from drug start to abnormal liver tests
p-value for hepatocellular injury versus cholestatic injury (defined as R value: ALT times upper limit of normal (ULN) / alkaline phosphatase times upper limit of normal (ULN).
However, until drug specific tools become available, an initial simple improvement might be to develop a RUCAM process that is computerized. The advantage of this approach is that the computer calculates points based on raw data (such as date of drug start and date of drug stop, date of DILI onset), without human error. The latter component is important because it has been shown that different experts calculate the data differently, and thus have relatively poor interobserver agreement. [24] Several studies have reported automatization of RUCAM within electronic health records [46,47]. Not surprisingly, likely because of differences in data calculation, assessment via a computer algorithm correlated poorly with expert assessment [34]. The feasibility of using an automated computerized RUCAM for all implicated and concomitant drugs within the DILIN network has been assessed (data not shown). The advantage of this approach is that utilizing a computer to assign points, which in turn are used to assign causal likelihood, by definition, there is consistent data calculation. This, however, should always be followed up with clinical judgement.
Furthermore, there appear to be several areas which could be modified to improve existing causality tools. This would likely be an iterative process, using a computer would be most effective. Below are highlighted areas that could be address.
Risk factors. Risk factors included in the original RUCAM included age ≥55 years old and alcohol use or pregnancy in mixed/cholestatic injury patterns. Alcohol use was evaluated during the refinement of the DDW-J 2002 into the DDW-J 2004, and not found to be a risk factor and therefore not included in DDW-J 2004. Therefore, these risk factors are likely not associated with DILI in general, and probably should be abandoned in a general model.
Variation of liver tests after initial improvement following cessation of suspected drug is considered to reduce the likelihood of DILI according to RUCAM. This may be a relatively common occurrence, and is likely due to a variety of factors (laboratory variation, biological variability in the disease course, etc…). Currently, there is a lack of clarity as to how to handle this event in relation to what level of variation should be compatible with DILI, less likely to occur with DILI and incompatible with DILI. This should probably be iteratively assessed using data.
Exclusion of alternative causes. The required exclusion of competing causes should probably be separated by injury pattern. For example, exclusion of viral hepatitis is probably not required in cholestatic cases, unless patient is heavily immunosuppressed and at risk for HBV or HCV associated fibrosing cholangitits.
Latency may deserve greater discerning ability. If a liver injury occurs, while taking a drug, RUCAM adjudicates 1 or 2 points. It appears worthwhile testing if increasing the difference between short, medium and very long latencies can improve causality: i.e. assigning for example 0.5 instead 1 point for liver injury events occurring beyond 1 year and 0 or negative points for events beyond 3 years from last drug start or dose increase
Incorporation of drug specific properties reflecting a drug’s inherent hepatotoxicity potential. It is clear that the inherent risk of hepatotoxicity varies by drug. The specification of the risk is difficult, however, because different drugs are prescribed with far different frequencies. For example, amoxicillin/clavulunate is commonly used, and in addition has been reported to be one of the most common causes of DILI [48]. In contract, isoniazid is much less frequently prescribed, but is also a very common cause of DILI [12]. Thus, it follows that INH likely has a greater potential for hepatotoxicity than amoxicillin/clavulunate. Currently, grading of the relative risk of hepatotoxicity for a specific drug is simply based on (a) the label of a drug or (b) reported cases in the literature. A step forward was a report which graded hepatotoxicity based on the number of reported cases [49]. However, that approach does not take into consideration the frequency with which the drug was prescribed. Thus, an alternative approach would be to use drugs’ physiochemical properties such as daily dose, lipophilicity and hepatic metabolism,[2, 50] which may better reflect the risk for DILI associated with a given drug.
Consideration of extrahepatic manifestations. Both the CDS and DDW-J use extrahepatic manifestations to support the concern for DILI. It would be worth exploring whether careful and data driven inclusion of the presence (and if present the details) of extrahepatic manifestations may augment causality assessment.
In aggregate, utilization of these different approaches would likely provide greater accuracy for any causality instrument, including RUCAM. Whether such an approach could ultimately provide for greater correlation with expert opinion (currently the gold standard) is unknown.
Conclusion
Several causality instruments are currently available, and many are in widespread use. Of the available causality assessment methods, RUCAM, which was developed 25 years ago, appears to be the most commonly used DILI causality instrument. However, both the causality processes and instruments can be improved. For example, a model approach would be for causality instruments to be developed using data from known examples of bonafide DILI. Further, it would likely be useful for processes to be computerized as well as to be iterative in nature. In addition, risk factors such as genetic polymorphisms might be added, which may or may not be drug specific. A high-quality causality assessment tool would ideally have a drug specific component, since DILI phenotypes are likely to differ among different drugs. An ideal situation would likely include a computerized process which provides guidance, that is in turn used by the clinician to make a final judgement.
Key points.
The three currently available causality tools for drug induced liver injury (RUCAM, DDW-J, CDS) use very similar point structure for point allocation.
One causality tool, CDS, is much more stringent when adjudicating cases as “probable” requiring 14 of 17 possible points, while RUCAM and DDW-J require only 6 points of 14 or 5 points of 17 possible points, respectively.
Given a complex but strict logic behind those instruments, they could be computerized to eliminate human error, and test iterative changes if they result in improved performance.
Acknolegdments:
Financial Support and Sponsorship
There was no direct financial support for this manuscript, but all authors receive support in the subject area of “drug induced liver injury” either by the NIH or the VA.
Footnotes
Conflict of Interest
There was no direct conflict of interest. But Drs. Tillmann and Rockey served as advised to Trevena for a project, and Dr. Tillmann’s wife is full time employee of AbbVie and holds stocks in Abbott, AbbVie and Gilead.
The remaining authors have no conflict of interest.
References
- 1.Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC; Drug-Induced Liver Injury Network (DILIN) Study Investigators. Identification and Characterization of Cefazolin-Induced Liver Injury. Clin Gastroenterol Hepatol. 2015. July;13(7):1328–1336.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahnama-Moghadam S, Tillmann HL. DILI associated with skin reactions. Current Hepatology Reports 2018;17:225–234. [Google Scholar]
- 3.Hunt CM, Yuen NA, Stirnadel-Farrant HA, Suzuki A. Age-related differences in reporting of drug-associated liver injury: data-mining of WHO Safety Report Database. Regul Toxicol Pharmacol. 2014. November;70(2):519–26. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki A, Barnhart H, Gu J, Bonkovsky HL, Tillmann HL, Fontana RJ, Kleiner DE; Drug-induced Liver Injury Network (DILIN). Associations of gender and a proxy of female menopausal status with histological features of drug-induced liver injury. Liver Int. 2017. November;37(11):1723–1730. PMID: 28161910.*This study demonstrated for the first time, that in addition to gender differences, there is a difference in DILI phenotype depending on menopausal status in women.
- 5.Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, Stolz A, Navarro V, Hoofnagle JH. Idiosyncratic Drug Induced Liver Injury in African-Americans Is Associated with greater morbidity and mortality Ccompared to Caucasians. Am J Gastroenterol. 2017. September;112(9):1382–1388. PMID: 28762375.*Racial differences in severity in DILI outcome were reported, with more severe outcomes found in African Americans.
- 6.Fung M, Thornton A, Mybeck K, Wu HJ, Hornbuckle K, Muniz E. Evaluation of the Characteristics of Safety Withdrawal of Prescription Drugs from Worldwide Pharmaceutical Markets-1960 to 1999. Therapeutic Innovation & Regulatory Science 2001;35:293–317. [Google Scholar]
- 7.Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007. November;6(11):904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church RJ, Watkins PB. In silico modeling to optimize interpretation of liver safety biomarkers in clinical trials. Exp Biol Med (Maywood). 2018;243:300–307.***This study leveraged an in silico cell biologic approach to attempt to predict the risk of hepatotoxicity.
- 9.Regev A Drug-induced liver injury and drug development: industry perspective. Semin Liver Dis. 2014;34:227–39. [DOI] [PubMed] [Google Scholar]
- 10. [03-09-2018]; https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/EN/RV_STP/a-f/flupirtin-10-2017.html. accessed.
- 11.Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gómez M, Jimenez-Perez M, Bruguera M, Prieto M, Bessone F, Hernandez N, Arrese M, Andrade RJ; Spanish DILI Registry; SLatinDILI Network; Safer and Faster Evidence-based Translation Consortium.Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014. July;147(1):109–118.e5. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J; United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015. June;148(7):1340–52.e7. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, Serrano J, Lee WM, Chalasani N, Stolz A, Davern T, Talwakar JA; DILI. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014. July;147(1):96–108.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013. November;17(4):575–86, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi PH, Rockey DC, Fontana RJ, Tillmann HL, Kaplowitz N, Barnhart H, Gu J, Chalasani NP, Reddy KR, Sherker AH, Hoofnagle JH, for the Drug Induced Liver Injury Network (DILIN) Investigators. Death and Liver Transplantation within Two Years of Onset of Drug-Induced Liver Injury. Hepatology 2017;66:1275–1285. *This study described that there is increased liver-related mortality 6 months to 2 years following a DILI event. Interestingly, the risk of liver-related mortality was greatest in the initial 6 months following the DILI event.
- 16.Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, Lee WM, Stolz A, Phillips T, Serrano J, Watkins PB; DILIN Investigators. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega-Alonso A, Andrade RJ. Chronic liver injury induced by drugs and toxins. J Dig Dis. 2018;19:514–521.*This paper summarizes data on chronicity of DILI, as published until 2017.
- 18.Narjes H, Nehmiz G. Effect of hospitalisation on liver enzymes in healthy subjects. Eur J Clin Pharmacol 2000; 56: 329–333 [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig P, Miget N, Brohier S. Transaminase elevation on placebo during phase I trials: prevalence and significance. Br J Clin Pharmacol 1999; 48: 19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas IJ, Julia Langham J, Krishnan Bhaskaran K, Brauer R, Smeeth L Orlistat and the risk of acute liver injury: self controlled case series study in UK Clinical Practice ResearchDatalink. BMJ 2013;346:f1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danan G, Benichou C. Causality assessment of adverse reactions to drugs. I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323–1330. [DOI] [PubMed] [Google Scholar]
- 22.Maria VAJ, Victorino RMM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. HEPATOLOGY 1997;26: 664–669. [DOI] [PubMed] [Google Scholar]
- 23.Takikawa H, Takamori Y, Kumagi T et al. , “Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the International Consensus Meeting,” Hepatology Research 2003; 27: 192–195. [DOI] [PubMed] [Google Scholar]
- 24.Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB, Davern T, McHutchison JG; Drug-Induced Liver Injury Network (DILIN). Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008. October;48(4):1175–83. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M, Fontana RJ, Hayashi PH; US Drug-Induced Liver Injury Network. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010. June;51(6):2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC; Drug-Induced Liver Injury Network (DILIN) Study Investigators. Identification and Characterization of Cefazolin-Induced Liver Injury. Clin Gastroenterol Hepatol. 2015. July;13(7):1328–1336.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M, Shibuya A. Validity study of a new diagnostic scale for drug-induced liver injury in Japan-comparison with two previous scales. Hepatol Res. 2004. November;30(3):148–154. [DOI] [PubMed] [Google Scholar]
- 28.Hanatani T, Sai K, Tohkin M, Segawa K, Kimura M, Hori K, Kawakami J, Saito Y. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharmacoepidemiol Drug Saf. 2014. September;23(9):984–8. [DOI] [PubMed] [Google Scholar]
- 29.García-Cortés M, Stephens C, Lucena MI, Fernández-Castañer A, Andrade RJ. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol. 2011. September;55(3):683–691. [DOI] [PubMed] [Google Scholar]
- 30.Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015. December 24;17(1). pii: E14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalton HR, Fellows HJ, Stableforth W, Joseph M, Thurairajah PH, Warshow U, Hazeldine S, Remnarace R, Ijaz S, Hussaini SH, Bendall RP. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007. November 15;26(10):1429–35. Review. [DOI] [PubMed] [Google Scholar]
- 32.Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH, Tillmann HL, Gu J, Serrano J, Hoofnagle JH; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011. November;141(5):1665–72.e1–9. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001. January;33(1):123–30. [DOI] [PubMed] [Google Scholar]
- 34.Aithal GP, Rawlins MD, Day CP. Clinical diagnostic scale: a useful tool in the evaluation of suspected hepatotoxic adverse drug reactions. J Hepatol. 2000. December;33(6):949–52. [DOI] [PubMed] [Google Scholar]
- 35.Whritenour J, Ko M, Zong Q, Wang J, Tartaro K, Schneider P, Olson E, Van Volkenburg M, Serrano J, Hayashi P, Fontana R, Chalasani N, Bonkovsky HL. Development of a modified lymphocyte transformation test for diagnosing drug-induced liver injury associated with an adaptive immune response. J Immunotoxicol. 2017. December;14(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benesic A, Rotter I, Dragoi D, Weber S, Buchholtz ML, Gerbes AL. Development and Validation of a Test to Identify Drugs That Cause Idiosyncratic Drug-Induced Liver Injury. Clin Gastroenterol Hepatol. 2018. September;16(9):1488–1494.e5.*This study described a new laboratory method for validating causality in suspected DILI cases.
- 37.Dragoi D, Benesic A, Pichler G, Kulak NA, Bartsch HS, Gerbes AL. Proteomics Analysis of Monocyte-Derived Hepatocyte-Like Cells Identifies Integrin Beta 3 as a Specific Biomarker for Drug-Induced Liver Injury by Diclofenac. Front Pharmacol. 2018. July 4;9:699.*This study described the use of the MH cell assay to confirm DILI diagnosis. The study used simple flow cytometry of peripheral blood to identify integrin beta 3 as a putative biomarker for diclofenac DILI. The implication is that a biomarker might be utilized to support a DILI diagnosis.
- 38.Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, Chalasani N, Bonkovsky HL. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014; 60: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, et al. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015; 13:369–376.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC; Drug-Induced Liver Injury Network (DILIN) Study Investigators. Identification and Characterization of Cefazolin-Induced Liver Injury. Clin Gastroenterol Hepatol. 2015; 13: 1328–1336.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, et al. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011; 9: 517–523.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessone F, Lucena MI, Roma MG, Stephens C, Medina-Caliz I, Frider B, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver Int. 2016; 36: 302–310. [DOI] [PubMed] [Google Scholar]
- 43.Nakaharai K, Sakamoto Y, Yaita K, Yoshimura Y, Igarashi S, Tachikawa N. Drug-induced liver injury associated with high-dose ceftriaxone: a retrospective cohort study adjusted for the propensity score. Eur J Clin Pharmacol. 2016; 72: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 44.Bermúdez J, Brahm M, Poniachik J, Contreras J, Valera J, Smok G, Brahm J. Espectro clínico de la hepatotoxicidad por nitrofurantoína en una serie clínica de 12 pacientes.Gastroenterol Latinoam 2012; 23: 129–133 [Google Scholar]
- 45.Tillmann HL, Barnhart HX, Serrano J, Rockey DC. A Novel Computerized Drug Induced Liver Injury Causality Assessment Tool (DILI-CAT). Hepatology 2016; 64(Suppl.1): A320–A321.*This study presented, for the first time, a drug specific approach to assess causality by characterizing a drug’s typical DILI phenotype.
- 46.Cheetham TC, Lee J, Hunt CM, Niu F, Reisinger S, Murray R, Powell G, Papay J. An automated causality assessment algorithm to detect drug-induced liver injury in electronic medical record data. Pharmacoepidemiol Drug Saf. 2014;23:601–8. [DOI] [PubMed] [Google Scholar]
- 47.Hanatani T, Sai K, Tohkin M, Segawa K, Kimura M, Hori K, Kawakami J, Saito Y. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharmacoepidemiol Drug Saf. 2014;23:984–8. [DOI] [PubMed] [Google Scholar]
- 48.Rahnama-Moghadam S, Tillmann HL. DILI associated with skin reactions. Current Hepatology Reports 2018;17:225–234.*This review compared the dominant drugs for DILI and drug associated skin reactions, where Augmentin was found the leading causal agent in several DILI registries, while it was not the most common drug related to allergic skin manifestions.
- 49.Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology.2016;63:590–603.*Study in which the authors catalog DILI cases associated with different drugs, and present a scoring system for the relative likelihood or risk of hepatotoxicity based on the number of reported cases. Five categories were proposed, based on the number of cases reported, as follows: A, ≥50; category B, 12–49; category C, 4–11; category D, 1–3; category E, none.
- 50.McEuen K, Borlak J, Tong W, Chen M. Associations of drug lipophilicity and extent of metabolism with Drug-Induced Liver Injury. Int J Mol Sci. 2017. June 22;18(7). pii: E1335. doi: 10.3390/ijms18071335.*This study explored whether a determination of the hepatic metabolism of a drug might be able to be used to improve the prediction of hepatotoxicity caused by a drug.