Abstract
Many of the fungicides and antibiotics currently available against plant pathogens are of limited use due to the emergence of resistant strains. In this study, we examined the effects of diphenyleneiodonium chloride (DPIC), an inhibitor of the superoxide producing enzyme NADPH oxidase, against fungal and bacterial plant pathogens. We found that DPIC inhibits fungal spore germination and bacterial cell proliferation. In addition, we demonstrated the potent antibacterial activity of DPIC using rice heads infected with the bacterial pathogen Burkholderia glumae which causes bacterial panicle blight (BPB). We found that treatment with DPIC reduced BPB when applied during the initial flowering stage of the rice heads. These results suggest that DPIC could serve as a new and useful antimicrobial agent in agriculture.
Keywords: Antimicrobial activity, Diphenyleneiodonium chloride, Fusarium head blight, Fusarium graminearum, Bacterial panicle blight, Burkholderia glumae
1. Introduction
Plants are continuously exposed and attacked by a variety of pathogenic microorganisms, including bacteria, fungi, and viruses, in their environments, and these infections lead to severe crop losses worldwide [1–3]. The pathogenic fungi that cause the most severe losses are in the genera Fusarium, Botrytis, Magnaporthe, Colletotrichum, and Cylindrocarpon [4,5]. Bacteria in the genera Burkholderia, Pseudomonas, and Ralstonia are major plant pathogens that exist widely in the environment [6–8]. Recently, it has been shown that Fusarium graminearum, which causes Fusarium head blight (FHB) and Burkholderia glumae, which causes bacterial panicle blight (BPB), interact cooperatively in their development and dispersal on rice [9]. This finding suggests that comprehensive studies are required to control plant diseases in fields.
Many of the antimicrobial agents currently used in agriculture are highly toxic and non-biodegradable and often cause long-term environmental pollution [10–12]. Moreover, the continuous use of the antimicrobial agents frequently results in the occurrence of antimicrobial-resistant strains [10,13], for example, antimicrobial-resistant fungi have emerged worldwide even though azole-type fungicides have broad spectrum antifungal activity and an improved safety profile [14,15]. Thus, we need to develop new alternative compounds that are not toxic to environment and that minimize the occurrence of resistant strains.
Diphenyleneiodonium chloride (DPIC) not only affects NADPH oxidase but also interferes with other flavoenzymes, including nitric oxide synthase and xanthine oxidase [16–18]. DPIC inhibits superoxide production in intact mitochondria and cell redox metabolism and blocks ion channels nonselectively [19]. DPIC also shows potent and broad spectrum antimicrobial activity against human pathogenic bacteria in vitro [20]. In addition, DPIC prevents the proliferation of cancer cells and may be non-toxic to healthy tissues in animals [21]. In plants, DPIC inhibits the accumulation of hydroxycinnamic acid, which is caused by wounds, in carrots [22] and inhibits suberization and lignification in potatoes, thus DPIC can also improve the quality of agricultural products [23,24].
In this study, we found that DPIC has potent antifungal and antibacterial activities against phytopathogenic fungi and bacteria. We tested the efficacy of DPIC against B. glumae in vitro and in vivo, and we found that rice heads treated with DPIC had significantly reduced disease severity caused by B. glumae. DPIC showed an intense dissolution mode and powerful antimicrobial effects suggesting that it is a good candidate for the control of plant pathogens.
2. Materials and methods
2.1. Fungal and bacterial strains
The fungal and bacterial strains used in this study were obtained from the Centre for Fungal Genetic Resources (Seoul National University, Seoul, Korea) and the Korean Agricultural Culture Collection (National Agrobiodiversity Center, Jeonju, Korea). F. graminearum, Fusarium oxysporum, Fusarium fujikuroi, Fusarium solani, Colletotrichum gloeosporioides, Cylindrocarpon destructans, Botrytis cinerea, and Magnaporthe oryzae were inoculated on potato dextrose agar (PDA) at 25 °C. Spore production was induced in carboxymethyl cellulose (CMC) [25] or carrot agar (CA) [26], and spore germination was tested in minimal medium (MM). B. glumae, Bacillus megaterium, Escherichia coli, Pseudomonas putida, and Ralstonia solanacearum were cultured in lysogeny broth (LB) [27] at 30 °C with shaking at 200 rpm for 24 h. All of the strains were stored in 15% (v/v) glycerol at −70 °C.
2.2. Spore preparation and antifungal activity assay
To induce spore production, strains in the genus Fusarium were incubated in 50 ml of CMC medium as previously described [25], B. cinerea and M. oryzae were incubated in CA for 7 days at 25 °C under a blue light, and C. destructans was incubated in 50 ml of PDB + G (potato dextrose broth supplemented with ginseng powder) as previously described [28]. The cultures were filtered with two layers of miracloth (Calbiochem, La Jolla, CA), and the spores were harvested by centrifugation at 13,000 rpm. The harvested spores were washed twice with distilled water and resuspended in 1 ml of MM. To test effects of DPIC (Sigma-Aldrich, St. Louis, MO) on spore germination, spores of each fungal strain were incubated at a final concentration of 106 spores/ml in 20 ml of liquid MM containing 0 or 0.1 mM DPIC, and germination was observed at 4, 8, and 12 h. To determine whether the inhibition of germination was temporary, spores were treated with DPIC for 24 h, centrifuged at 13,000 rpm for 5 min, the supernatant was removed, and the pelleted spores were resuspended in distilled water; this procedure was repeated two more times. Then, the pelleted spores were resuspended in fresh liquid MM and the rate of spore germination was determined by counting the number of germinated and total spores every 12 h.
2.3. Antibacterial activity assay
Bacteria were cultured overnight at 30 °C in liquid LB for the preparation of cell suspensions. Each cell suspension was adjusted spectrophotometrically to approximately 104 CFU/ml, and 100 μl of each bacterial cell suspension was added to 20 ml of liquid MM containing 0.1 mM DPIC. The cultures were incubated at 30 °C with shaking at 200 rpm, and cell growth (OD600) was measured every 4 h for 20 h.
2.4. Rice seedling growth assay
Rice seeds were soaked in 1% (w/v) sodium hypochlorite for 5 min, rinsed in sterile water for 5 min, and then these sterilized rice seeds were germinated in distilled water at 28 °C for 2 days. The pre-germinated seeds were incubated in 10 ml of distilled water or DPIC solution (0.1 mM) for 1 h in an orbital shaker (200 rpm), were dried on a clean bench for 1 h, and then the seeds were transplanted on a seedbed with filter paper. Growth was determined by measuring shoot and root lengths after incubation at 28 °C with high relative humidity (close to 100%) for 7 days. The experiments were repeated three times with three replicates, and the Tukey test in the R software package version 3.1.2 was performed to evaluate significant differences (p < 0.05).
2.5. Efficacy assay of DPIC against FHB and BPB on rice heads
The effects of DPIC treatment on rice heads exposed to F. graminearum or B. glumae were tested using the “Dongjin” rice cultivar at initial- or mid-anthesis. Rice heads were dipped in 30 ml of distilled water or DPIC solution (0.1 mM) containing F. graminearum (107 spores/ml) or B. glumae (104 CFU/ml) for 1 min and then were sealed individually in plastic bags for 2 days. For the controls, rice heads were treated with distilled water or DPIC solution without F. graminearum or B. glumae. The infected plants were placed in a green house, and the rice grains that exhibiting blight symptoms were counted 3 weeks after inoculation. The disease index was calculated as the number of diseased grains per rice head.
3. Results
3.1. Effect of DPIC on fungal spore germination
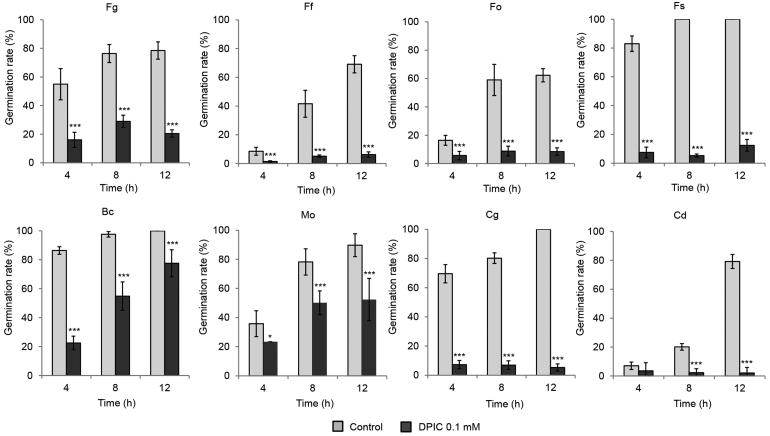
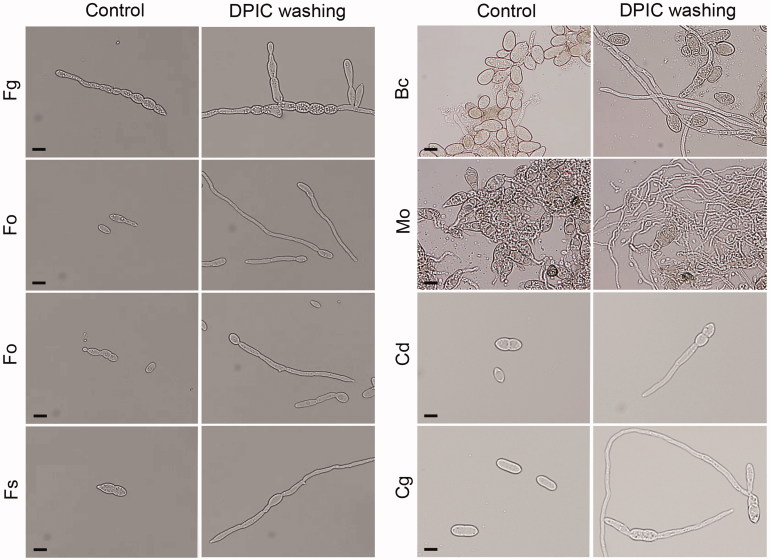
Spore germination was inhibited strongly by 0.1 mM DPIC for all eight of the fungal species tested in this study. Germination was determined by the length of the germ tube relative to the spore. The spore germination rate decreased to less than 50% after 12 h for C. destructans, C. gloeosporioides, and Fusarium spores treated with DPIC. The germination rate for B. cinerea and M. oryzae spores treated with DPIC was >50% after 12 h, but the germinated hyphae lengths were shorter with DPIC treatment than without DPIC treatment (Supplementary Figure S1; Figure 1). However, when the spores treated with DPIC were washed with water and transferred to fresh liquid MM, the germination rates were recovered (Supplementary Figure S2; Figure 2).
Figure 1.
Effect of DPIC on fungal spore germination. Spores were treated with sterile distilled water (Control) or 0.1 mM DPIC (DPIC 0.1 mM). Fungal spore germination rates were determined after 4, 8, and 12 h of incubation at 25 °C. Asterisks indicate significant differences between Control and DPIC 0.1 mM samples (***p < 0.001). Fg: F. graminearum; Ff: F. fujikuroi; Fo: F. oxysporum; Fs: F. solani; Bc: B. cinerea; Mo: M. oryzae; Cg: C. gloeosporioides; Cd: C. destructans.
Figure 2.
Effect of washing with distilled water on spore germination. Spores exposed to DPIC were washed with distilled water, resuspended in fresh liquid MM, incubated for 12 h, and then spore germination was observed using an optical microscope. Spores that were continuously exposed to DPIC without washing were used as the control (Control). Scale bars, 10 μm. Fg: F. graminearum; Ff: F. fujikuroi; Fo: F. oxysporum; Fs: F. solani; Bc: B. cinerea; Mo: M. oryzae; Cg: C. gloeosporioides; Cd: C. destructans.
3.2. Effect of DPIC on bacterial cell growth
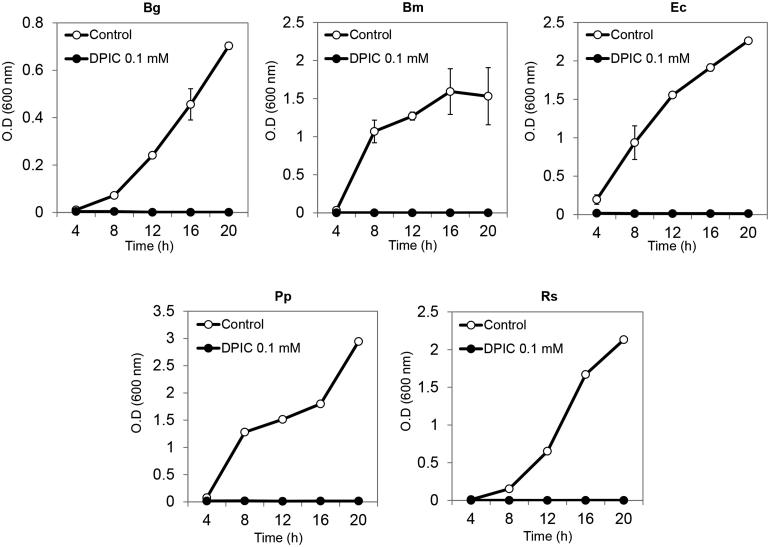
DPIC inhibited B. glumae, B. megaterium, E. coli, P. putida, and R. solanacearum cell growth. Bacteria at an OD600 of 0.1 were inoculated into 20 ml of fresh liquid LB with 0.1 mM DPIC and incubated for 24 h at 30 °C with shaking. Bacterial growth was completely arrested in 0.1 mM DPIC and OD values did not increase in 0.1 mM DPIC (Figure 3).
Figure 3.
Bacterial growth inhibition assays. Bacterial suspensions were inoculated into liquid MM with 0.1 mM DPIC (DPIC 0.1 mM) or without DPIC (Control) and incubated at 30 °C with shaking. OD600 measurements were taken every 4 h for 20 h and growth curves were generated. Bg: B. glumae; Bm: B. megaterium; Ec: E. coli; Pp: P. putida; Rs: R. solanacearum.
3.3. Effect of DPIC on rice seed germination and seedling growth
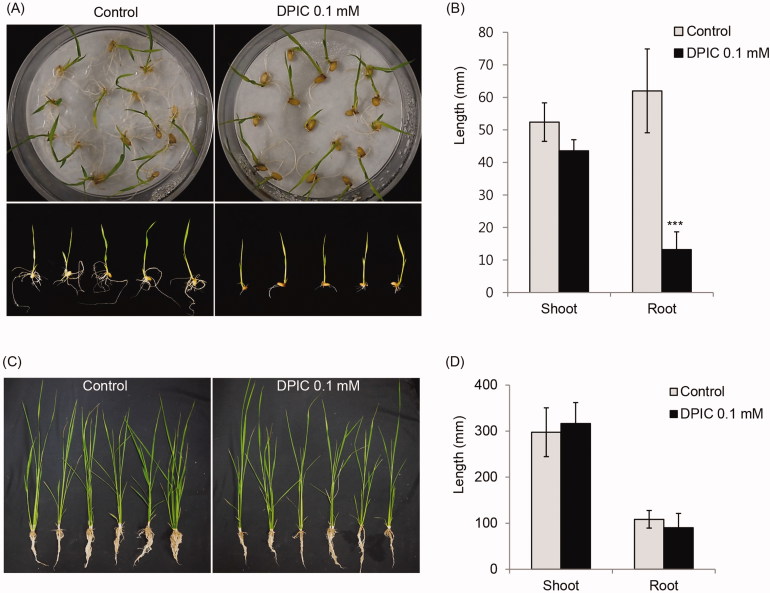
There was no difference in the germination rates of rice seeds that were soaked in 0.1 mM DPIC and rice seeds that were not treated with 0.1 mM DPIC. The germination rate of DPIC-treated and untreated rice seeds was 80–90%. Although the root length was significantly shorter in rice treated with 0.1 mM DPIC than in untreated rice, the shoot length did not differ significantly in the DPIC-treated and untreated rice. However, after 7 days, the final shoot and root lengths did not differ in DPIC-treated and untreated rice (Figure 4).
Figure 4.
Effects of DPIC on rice seedling growth. Rice seeds were soaked in 0.1 mM DPIC or distilled water for 1 h, incubated for 7 days at 30 °C, and then the shoot and root lengths were measured (A,C). The germinated seeds were transferred to egg pots, were grown for another 7 days, and then the shoot and root lengths were measured (B,D). The plants were grown under a 14 h light and a 10 h dark cycle at 30 °C with 70% humidity. The shoot and root lengths of ten rice seedlings per condition (with or without DPIC) were measured and averaged. Asterisks indicate significant differences between the Control and DPIC 0.1 mM samples (***p < 0.001).
3.4. Efficacy of DPIC against FHB and BPB on rice
The rice heads that were dipped in F. graminearum or B. glumae showed typical FHB or BPB symptoms after 3 weeks, but disease symptoms were not observed in the rice heads treated with either DPIC or water (Supplementary Figure S3 and Table 1). Rice heads that were treated with DPIC in the initial-anthesis stage showed reduced BPB severity compared to untreated rice heads (Supplementary Figure S3 and Table 1). However, DPIC treatment did not affect the disease severity of FHB caused by F. graminearum, and DPIC treatment did not reduce FHB or BPB severity when rice heads were treated with DPIC in the mid-anthesis stage (Supplementary Figure S3 and Table 1).
Table 1.
Efficacy of DPIC against F. graminearum and B. glumae.
| Percentage of grains with disease symptomsa |
||
|---|---|---|
| Initial-anthesis inoculation | Mid-anthesis inoculation | |
| DW | 6.52Ab | 0.91A’ |
| DPIC | 1.94A | 0.66A’ |
| DW + Fg | 26.10B | 13.28B’ |
| DW + Bg | 52.34C | 34.65C’ |
| DPIC + Fg | 17.08B | 14.56B’ |
| DPIC + Bg | 25.10B | 38.01C’ |
aRice heads were inoculated by the dip inoculation method. DW: Distilled water; DPIC: Diphenyleneiodonium chloride; Fg: F. graminearum; Bg: B. glumae.
bValues with different letters within a column differ significantly according to Tukey’s test (p < 0.05).
4. Discussion
In this study, we showed that DPIC has strong antifungal and antibacterial effects on various fungal and bacterial plant pathogens. In fungi, reactive oxygen species (ROS) produced by NADPH oxidases have been shown to associate with various physiological processes and cellular differentiations, including the development of sexual fruiting bodies, ascospore germination, and hyphal growth [29]. Furthermore, it has been shown that the NADPH oxidase family of enzymes is also present in bacteria [30]. Appropriate levels of ROS produced by NADPH oxidases are required for proper fungal and bacterial cell growth and proliferation [31,32]. DPIC inhibits NADPH oxidase activity, thus the DPIC-induced inhibition of spore germination and cell proliferation observed in this study may be related to a reduction in the cellular ROS produced.
In this study, we found that DPIC inhibits germination of all of the fungal spores tested and inhibits the growth of all of the bacteria tested (Figure 1). We focused on F. graminearum and B. glumae in this study because there has been an increase in FHB and BPB in rice fields in Korea due to climate change [33,34]. In addition, we showed previously that F. graminearum plays an important role in the disease dispersal and survival of B. glumae [9]. Therefore, alternative compounds that have broad-spectrum activity against both fungi and bacteria, such as DPIC, are needed to control plant diseases, such as F. graminearum and B. glumae.
Although DPIC displayed strong inhibitory effects on various plant pathogens in vitro, application of DPIC on rice heads was not enough to control the diseases caused by F. graminearum and B. glumae. DPIC slightly reduced the incidence of disease caused by B. glumae when it was applied during initial-anthesis but not during mid-anthesis (Supplementary Figure S3 and Table 1) suggesting that DPIC can protect rice plants from B. glumae when applied during initial flowering.
Recent studies have shown that Bacillus amyloliquefaciens JCK-12 extracts and chemical fungicides reduce the incidence of FHB and that the combination of biological control agents and chemical fungicides reduces the incidence of fungicide resistance and lowers the effective dose of chemical fungicides, thereby reducing environmental damage and improving the efficacy of antifungal agents [35]. Therefore, we hypothesize that treatment with DPIC in combination with existing chemical fungicides will be more effective in controlling pathogens.
The indiscriminate use of fungicides and antibiotics in agriculture has led to the rapid emergence of drug-resistant pathogens, which are difficult to control [36,37]. For example, the application of tebuconazole, guazatine, or iminoctadine significantly reduces F. graminearum mycelial growth but increases production of the F. graminearum mycotoxin 3-acetyldeoxynivalenol four-fold as has been previously described for fungicide-resistant F. graminearum [15]. Furthermore, the application of tebuconazole has resulted in an increase in the extent of grain colonized by Microdochium nivale, which causes pink snow mold in cool season turf grass species [15,38]. Therefore, we need to develop alternative compounds with different modes of action than the chemicals currently used.
Importantly, we showed that fungal spores exposed to DPIC germinated normally when washed with distilled water and transferred to fresh medium (Figure 2) indicating that although DPIC can arrest germination of fungal spores and proliferation of bacterial cells, it does not have long-term toxicity to microorganisms. This characteristic of DPIC can be useful for controlling pathogens that infect plants during specific and short growth stages, such as flowering. Therefore, we expect that proper use of DPIC during pathogen infection can prevent pathogens from invading and weaken the appearance of resistant mutations. DPIC has potential as a safe and broad-spectrum antifungal and antibacterial agent to combat the increased resistance of plant pathogens to the currently available antimicrobial agents and to remove toxic chemicals from agriculture.
Supplementary Material
Funding Statement
This work was supported by the Rural Development Administration (PJ010119).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Savary S, Teng PS, Willocquet L, et al. Quantification and modeling of crop losses: a review of purposes. Annu Rev Phytopathol. 2006;44:89–112. [DOI] [PubMed] [Google Scholar]
- 2.Strange RN, Scott PR. Plant disease: a threat to global food security. Annu Rev Phytopathol. 2005;43:83–116. [DOI] [PubMed] [Google Scholar]
- 3.Makovitzki A, Viterbo A, Brotman Y, et al. Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Appl Environ Microbiol. 2007;73:6629–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyraz N, Özcan M. Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int J Food Microbiol. 2006;107:238–242. [DOI] [PubMed] [Google Scholar]
- 5.Rahman M, Punja ZK. Factors influencing development of root rot on ginseng caused by Cylindrocarpon destructans. Phytopathology. 2005;95:1381–1390. [DOI] [PubMed] [Google Scholar]
- 6.Denny TP. Plant pathogenic Ralstonia species. In: Gnanamanickam SS, editor. Plant-associated bacteria. Dordrecht: Springer; 2007. p. 573–644. [Google Scholar]
- 7.Ham JH, Melanson RA, Rush MC. Burkholderia glumae: next major pathogen of rice? Mol Plant Pathol. 2011;12:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemanceau P. Beneficial effects of rhizobacteria on plants: example of fluorescent Pseudomonas spp. [plant growth promoting rhizobacteria, PGPR, microbial antagonism, siderophore, bacterial inoculation]. Agronomie. 1992;12:413–437. [Google Scholar]
- 9.Jung B, Park J, Kim N, et al. Cooperative interactions between seed-borne bacterial and air-borne fungal pathogens on rice. Nat Commun. 2018;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daoubi M, Hernández-Galán R, Benharref A, et al. Screening study of lead compounds for natural product-based fungicides: antifungal activity and biotransformation of 6α, 7α-dihydroxy-β-himachalene by Botrytis cinerea. J Agric Food Chem. 2005;53:6673–6677. [DOI] [PubMed] [Google Scholar]
- 11.Russell P. Fungicide resistance: occurrence and management. J Agric Sci. 1995;124:317–323. [Google Scholar]
- 12.Knight S, Anthony V, Brady A, et al. Rationale and perspectives on the development of fungicides. Annu Rev Phytopathol. 1997;35:349–372. [DOI] [PubMed] [Google Scholar]
- 13.Brent KJ, Hollomon DW Fungicide resistance in crop pathogens: how can it be managed? GIFAP Brussels; 1995. [Google Scholar]
- 14.Walsh T, Viviani M-A, Arathoon E, et al. New targets and delivery systems for antifungal therapy. Med Mycol. 2000;38:335–347. [PubMed] [Google Scholar]
- 15.Pirgozliev SR, Edwards SG, Hare MC, et al. Strategies for the control of Fusarium head blight in cereals. Eur J Plant Pathol. 2003;109:731–742. [Google Scholar]
- 16.Park SE, Song JD, Kim KM, et al. Diphenyleneiodonium induces ROS-independent p53 expression and apoptosis in human RPE cells. FEBS Lett. 2007;581:180–186. [DOI] [PubMed] [Google Scholar]
- 17.Dodd ‐ o. JM, Zheng G, Silverman HS, et al. Endothelium-independent relaxation of aortic rings by the nitric oxide synthase inhibitor diphenyleneiodonium. Br J Pharmacol. 1997;120:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders SA, Eisenthal R, Harrison R. NADH oxidase activity of human xanthine oxidoreductase-generation of superoxide anion. Eur J Biochem. 1997;245:541–548. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Hatano N, Muraki Y, et al. The NADPH oxidase inhibitor diphenyleneiodonium activates the human TRPA1 nociceptor. Am J Physiol Cell Physiol. 2014;307:C384–C394. [DOI] [PubMed] [Google Scholar]
- 20.Pandey M, Singh AK, Thakare R, et al. Diphenyleneiodonium chloride (DPIC) displays broad-spectrum bactericidal activity. Sci Rep. 2017;7:11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogasawara MA, Zhang H. Redox regulation and its emerging roles in stem cells and stem-like cancer cells. Antioxid Redox Signal. 2009;11:1107–1122. [DOI] [PubMed] [Google Scholar]
- 22.Jacobo-Velázquez DA, Martínez-Hernández GB, del C. Rodríguez S, et al. Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J Agric Food Chem. 2011;59:6583–6593. [DOI] [PubMed] [Google Scholar]
- 23.Bernards MA, Razem FA. The poly(phenolic) domain of potato suberin: a non-lignin cell wall bio-polymer. Phytochemistry. 2001;57:1115–1122. [DOI] [PubMed] [Google Scholar]
- 24.Razem FA, Bernards MA. Reactive oxygen species production in association with suberization: evidence for an NADPH-dependent oxidase. J Exp Bot. 2003;54:935–941. [DOI] [PubMed] [Google Scholar]
- 25.Cappellini R, Peterson J. Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia. 1965;57:962–966. [Google Scholar]
- 26.Leslie JF, Summerell BA. The Fusarium laboratory manual. Hoboken (NJ): John Wiley & Sons; 2008. [Google Scholar]
- 27.Maniatis T, Fritsch EF, Sambrook J Molecular cloning: a laboratory manual. Vol. 545. Cold spring harbor laboratory Cold Spring Harbor, NY; 1982. [Google Scholar]
- 28.Kang Y, Kim MR, Kim KH, et al. Chlamydospore induction from conidia of Cylindrocarpon destructans isolated from ginseng in Korea. Mycobiology. 2016;44:63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takemoto D, Tanaka A, Scott B. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet Biol. 2007;44:1065–1076. [DOI] [PubMed] [Google Scholar]
- 30.Hajjar C, Cherrier MV, Mirandela GD, et al. The NOX family of proteins is also present in bacteria. mBio. 2017;8:e01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cano-Domínguez N, Álvarez-Delfín K, Hansberg W, et al. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell. 2008;7:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashmiri Z, Mankar S. Free radicals and oxidative stress in bacteria. Int J Curr Microbiol App Sci. 2014;3:34–40. [Google Scholar]
- 33.Vaughan M, Backhouse D, Ponte ED. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: a review. World Mycotoxin J. 2016;9:685–700. [Google Scholar]
- 34.Schaad NW. Emerging plant pathogenic bacteria and global warming. In: Fatmi M, et al., editors. Pseudomonas syringae pathovars and related pathogens–identification, epidemiology and genomics. Dordrecht: Springer; 2008. p. 369–379. [Google Scholar]
- 35.Kim K, Lee Y, Ha A, et al. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front Plant Sci. 2017;8:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar DJ, Mukherjee I, Shakil NA, et al. Antibiotics in agriculture: use and impact. Ind J Ethnophytopharm. 2018;4:4–19. [Google Scholar]
- 37.Hollomon D. Does agricultural use of azole fungicides contribute to resistance in the human pathogen Aspergillus fumigatus? Pest Manag Sci. 2017;73:1987–1993. [DOI] [PubMed] [Google Scholar]
- 38.Becher R, Hettwer U, Karlovsky P, et al. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology. 2010;100:444–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.