Abstract
An evaluation of the relationship between predictors and immune response was conducted using data obtained from a clinical trial in 200 Czech healthy adults aged 24–65 years receiving a booster dose of a monovalent tetanus vaccine in 2017. The response was determined from ELISA antibody concentrations of paired sera obtained before and 4 weeks after the immunisation. While all subjects with initial antibody levels <1.2 IU/ml achieved a 100% seroconversion rate (at least a fourfold rise in antibodies), only 8% seroconversion was documented in subjects with initial levels >2.2 IU/ml. The immune response was not affected by sex, age, tetanus vaccine type, concomitant medication, related adverse events or post-vaccination period since there were no significant differences in geometric mean concentrations or seroconversion rates. The seroconversion rate of 56% in smokers was significantly lower than that of 73% achieved in non-smokers. Although the seroconversion rates did not differ between individuals with normal or higher body weight, the adjusted odds ratio (1.3; 95% Cl 1.08–1.60) revealed a positive correlation between seroconversion rate and body mass index (BMI). Although the vaccine-induced response was influenced by pre-vaccination antibody levels, smoking or BMI, the booster immunisation against tetanus produced a sufficient response regardless the predictors.
Key words: BMI, pre-vaccination levels, seroconversion rate, smoking, tetanus vaccination
Introduction
Tetanus vaccines are the most commonly used vaccines worldwide and generally considered successful in the immunoprophylaxis of tetanus, also due to the relatively high vaccination rate of children and regular booster immunisation in adulthood.
A monovalent vaccine is usually used only for booster or post-exposure vaccination after an injury if there is a risk of tetanus infection. The availability of international standards and reference samples has contributed to the development of standardised production and quality requirements for tetanus vaccines, which can thus be currently considered to have the same quality with no markedly different safety and efficacy.
Some countries require the booster dose to be repeated every 10 years, either with a monovalent or combined vaccine containing tetanus toxoid since a significant decrease in tetanus antibody concentrations is generally known to occur in some vaccinees about 10 years later. Czech law requires regular booster immunisation against tetanus every 10–15 years in adults. The vaccination date including the vaccine name is recorded in the respective patient's medical record kept by their practitioner.
As immunisation against tetanus has been carried out worldwide for more than 80 years, one would expect that most of its aspects are well explored. Despite that, knowledge about predictors impacting the immune response to booster vaccination against tetanus is still scant. We therefore decided to focus on potential predictors of the seroconversion rates based on data obtained from a clinical trial designed to evaluate immunogenicity by comparing two monovalent alum-adjuvanted tetanus vaccines.
Material and methods
Two hundred healthy adults aged between 24 and 65 years requiring routine booster immunisation against tetanus were enrolled in the trial designed as a single-blind, randomised, parallel-group one. All vaccinees were selected from four outpatient clinics participating in the study. Previous vaccination against tetanus carried out from 10 to 15 years before was confirmed by physician documentation and individual statement. All participants had been properly and completely vaccinated with five doses of a combined or monovalent vaccine against tetanus in childhood.
Subjects were randomly assigned (at a 1:1 ratio) to one of two vaccine groups stratified by sex and age. From March through May 2017, they received either Tetavax; batch number M74627V (Sanofi Pasteur SA, Lyon, France) or Vacteta 40 IU/0.5 ml; batch number 03716001 (Biodrug s.r.o., Bratislava, Slovakia) vaccines. Vaccines were administered intramuscularly in the deltoid region according to manufacturers’ recommendations to minimise occurrence of local adverse reactions. While the former vaccine was supplied in a pre-filled syringe, the latter one was available in an ampule whose content needed to be drawn into a syringe with a 25–30 mm needle prior to administration.
Single blinding was achieved by simply covering a subject's eyes with a mask prior to vaccine administration. The immune response to the booster dose of the tetanus vaccine was assessed by serological analysis of paired serum samples obtained from each subject before and 4 weeks after vaccination. The samples were collected, stored at ‒20 °C and measured with the ELISA method using the VaccZyme Anti-Tetanus Toxoid IgG Enzyme Immunoassay Kit with a measuring range from 0.01 to 7.0 IU/ml (The Binding Site Group Ltd, Birmingham, UK) in one central laboratory (Interimun s.r.o., Pardubice, Czech Republic) at the end of the study. To ensure accuracy, paired samples were determined in one run and all samples were blinded for the laboratory staff.
This clinical trial was conducted in compliance with the rules of Good Clinical Practice and approved by the Ethics Committee of the Czech Ministry of Health and State Institute for Drug Control (EudraCT 2016-004934-11). All the subjects were informed in detail about the experimental procedure and provided written informed consent.
Seroconversion rates for booster response were adopted from WHO requirements [1], defined as a multiple increase in antibody levels from pre-vaccination to post-vaccination. In our study, seroconversion rate was determined by either a fourfold (SCR4) or twofold (SCR2) rise in antibody including the attainment of at least four times the protective level if pre-vaccination levels were lower than 1.0 IU/ml. Furthermore, both seroconversion rates could be attained only in subjects with at least 0.4 IU/ml after the booster dose. The SCR2 was proposed in accordance with WHO requirements to demonstrate any variation of seroconversion results, especially in subjects with higher pre-vaccination levels of tetanus antibodies (i.e. >1.0 IU/ml) who need not achieve the fourfold rise. Antibody levels were assessed using geometric mean concentrations (GMCs) of tetanus antibodies.
As antibody levels before and after immunisation as well as a rise in antibodies may be non-normally distributed, the Mann–Whitney U test for intergroup comparison and the Wilcoxon test for intragroup comparisons were used where appropriate. The proportions including rates were statistically evaluated with Fisher's exact test.
Logistic regression was used to assess any potential association of predictors with seroconversion rates. A total of 10 variables were selected as potential predictors of seroconversion rate, i.e. dichotomous variables: sex, vaccine, cigarette smoking, with or without concomitant medication, occurrence of adverse events related to this vaccination and continuous ones: age and body mass index (BMI) of vaccinees, pre-vaccination levels of tetanus antibodies, time since the last immunisation against tetanus and length of the post-vaccination period. The sample size of 200 subjects was justified for logistic regression using 10 covariates [2]. The power of the test ranges between 75% and 85% for any of the predictors. McFadden's R2 of this approach was higher than 0.7 indicating a very good predictive ability of this model for the selected predictors. The sensitivity and specificity of the model achieved more than 90%. The association was evaluated with the odds ratio (OR) mutually adjusted for all selected predictors, including a 95% confidence interval (CI).
The study population was divided into two groups according to the median of each predictor represented by a continuous variable (i.e. age, BMI, period or antibody levels). The characteristics of the study population are reported in Table 1.
Table 1.
Characteristics of the study population according to predictors expressed with proportions or means including the 95% confidence interval
| Predictors | N | Proportion % (95% Cl) | Predictors | N | Mean (95% Cl) | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 100 | 50 (42.9–57.1) | Age (years) | ⩽43.8 | 99 | 32.6 (31.3–33.8) |
| Female | 100 | 50 (42.9–57.1) | >43.8 | 101 | 52.8 (51.7–53.9) | |
| Vaccine | ||||||
| Tetavax | 100 | 50 (42.9–57.1) | BMI (kg/m2) | ⩽25.8 | 99 | 22.9 (22.5–23.3) |
| Vacteta | 100 | 50 (42.9–57.1) | >25.8 | 101 | 30.0 (29.2–30.9) | |
| Smokers | ||||||
| Yes | 41 | 20.5 (15.1–26.8) | Pre-vaccination period (years) | ⩽12.6 | 98 | 11.2 (11.0–11.3) |
| No | 159 | 79.5 (73.2–84.9) | >12.6 | 102 | 13.9 (13.7–14.0) | |
| Concomitant medication | ||||||
| Yes | 73 | 36.5 (29.8–43.6) | Pre-vaccination GMC (IU/ml) | ⩽1.2 | 103 | 0.6 (0.5–0.6) |
| No | 127 | 63.5 (56.4–70.2) | >1.2 | 97 | 2.9 (2.6–3.3) | |
| Related adverse events | ||||||
| Yes | 56 | 28 (21.9–34.8) | Post-vaccination period (days) | ⩽28 | 107 | 27.8 (27.7–27.9) |
| No | 136 | 68 (61.1–74.4) | >28 | 93 | 31.2 (30.8–31.7) | |
N, number of subjects.
Continuous variables were divided according to median of the entire population.
All tests were two-tailed, and the level of significance was set at 0.05. Statistical analyses and logistic regression were performed with Prism 7 (GraphPad Software, Inc., La Jolla, California, USA) and STATA version 14.2 software (StatCorp, Lakeway Drive, Texas, USA), respectively.
Results
All subjects but one achieved antibody levels higher than 1.0 IU/ml after booster vaccination against tetanus except one (0.5 IU/ml). No significant differences in the immune response to booster vaccination were found between men and women, smokers and non-smokers or the tetanus vaccines used (Table 2). The GMCs of tetanus antibodies and geometric mean of rise in antibodies after vaccination were independent of adult age (range 24–65 years) and BMI.
Table 2.
Post-booster geometric mean concentrations (GMCs) of tetanus antibody and pre- to post-booster level ratio including the 95% confidence interval
| Predictors | Post-GMCs (95% CI) in UI/ml | P | Pre- to post-booster level ratio (95% Cl) | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 7.7 (7.1–8.4) | 0.162 | 6.9 (5.7–8.3) | 0.114 |
| Female | 7.9 (7.1–8.9) | 8.3 (6.7–10.1) | ||
| Vaccine | ||||
| Tetavax | 8.3 (7.6–9.1) | 0.186 | 7.3 (6.1–8.9) | 0.860 |
| Vacteta | 7.4 (6.6–8.2) | 7.8 (6.3–9.5) | ||
| Smoker | ||||
| Yes | 7.3 (6.2–8.6) | 0.213 | 6.7 (4.8–9.4) | 0.182 |
| No | 8.0 (7.4–8.7) | 7.8 (6.7–9.1) | ||
| Concomitant medication | ||||
| Yes | 7.4 (6.4–8.5) | 0.588 | 7.1 (5.9–8.7) | 0.588 |
| No | 8.1 (7.5–8.8) | 7.8 (6.4–9.4) | ||
| Related adverse events | ||||
| Yes | 8.6 (7.4–9.9) | 0.071 | 7.6 (5.9–9.6) | 0.993 |
| No | 7.6 (7.0–8.3) | 7.4 (6.2–8.9) | ||
| Age (median) | ||||
| ⩽43.8 years | 7.7 (6.9–8.5) | 0.641 | 8.6 (7.0–10.6) | 0.103 |
| >43.8 years | 8.0 (7.3–8.8) | 6.6 (5.5–7.9) | ||
| BMI (median) | ||||
| ⩽25.8 kg/m2 | 7.8 (7.0–8.7) | 0.856 | 7.5 (6.1–9.3) | 0.997 |
| >25.8 kg/m2 | 7.9 (7.2–8.6) | 7.6 (6.3–9.1) | ||
| Pre-vaccination period (median) | ||||
| ⩽12.6 years | 8.1 (7.4–8.9) | 0.615 | 7.1 (5.9–8.5) | 0.331 |
| >12.6 years | 7.6 (6.8–8.5) | 8.0 (6.5–9.8) | ||
| Pre-vaccination GMC (median) | ||||
| ⩽1.2 IU/ml | 7.1 (6.3–8.0) | 0.033 | 15.8 (13.9–18.1) | <0.0001 |
| >1.2 IU/ml | 8.7 (8.0–9.3) | 3.4 (3.1–3.9) | ||
| Post-vaccination period (median) | ||||
| ⩽28 days | 7.4 (6.7–8.3) | 0.221 | 7.3 (6.0–8.7) | 0.715 |
| >28 days | 8.3 (7.6–9.2) | 7.9 (6.4–9.7) | ||
P, statistical significance calculated with the Mann–Whitney U test.
Although the study was conducted in healthy adults, it was not possible to exclude subjects with concomitant diseases that did not constitute a contraindication to tetanus vaccination, such as hypertensive disease (15.5%), metabolic disorders (6.5%), impaired thyroid function (5.5%), chronic lower respiratory tract diseases (3%), dermatitis and eczema (2%), diseases affecting the oesophagus, stomach and duodenum (2%), and other (16.5%). A total of 73 subjects with concomitant treatment did not exhibit any signs of a lower immune response compared with those not receiving such treatment.
Also the pre-vaccination period, i.e. the period between the last and current immunisation against tetanus in this study (from 9.9 to 15.9 years) did not influence the post-vaccination GMCs. A second blood sample was taken between 24 and 35 days after vaccination and no impact of this interval on the immune response was identified because the GMCs of tetanus antibody as well as the antibody rise did not vary between subjects with a period of ⩽28 days and those with a period of >28 days (Table 2).
Adverse events related to vaccination reported in a total of 56 subjects did not influence either post-vaccination GMCs or antibody rise compared with those of subjects without adverse events.
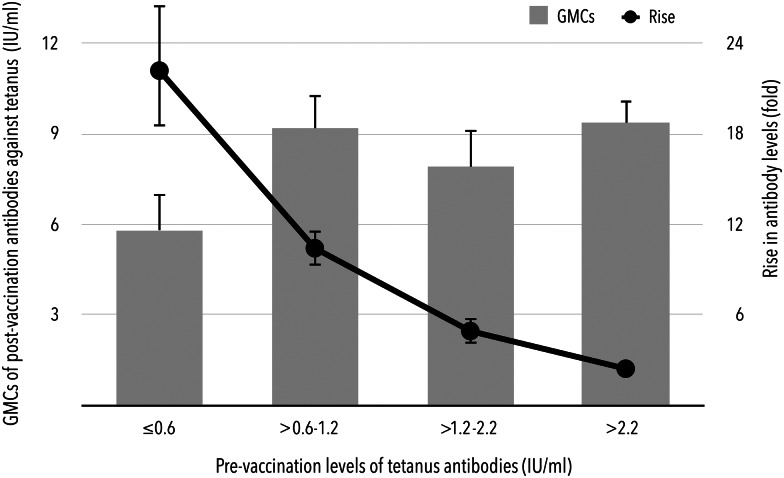
Prior to booster vaccination, there were 98.5% (95% CI 95.7–99.7%) and 57.5% (95% CI 50.3–64.4%) of subjects with levels of tetanus antibodies higher than 0.1 and 1.0 IU/ml, respectively. All subjects had pre-vaccination levels higher than 0.01 IU/ml. Although higher levels before booster immunisation contributed to higher GMCs after vaccination, a statistically significant inverse correlation was found between pre-vaccination GMCs expressed in a range of quartiles, i.e. 0.6 IU/ml (25% quartile), 1.2 IU/ml (median) and 2.2 IU/ml (75% quartile) and the rise in post-vaccination antibodies (Fig. 1). If the pre-vaccination levels were lower than the median of the entire study population, the antibody levels increased 15.8-fold (95% CI 13.9–18.1) after booster immunisation. Conversely, in subjects with pre-vaccination antibody levels exceeding 2.2 IU/ml (75% quartile of pre-vaccination levels), only a 2.4-fold (95% CI 2.1–2.7) increase in post-booster antibodies was observed.
Fig. 1.
Dependence of GMCs and rise in antibodies after booster immunisation on the pre-vaccination levels of tetanus antibodies.
The SCR4 defined by at least a fourfold rise in antibody levels after booster immunisation achieved 69.5% (95% CI 62.6–75.8%) in the entire study population. The SCR4 was linked to the pre-vaccination levels of tetanus antibodies, since only 37% of subjects with pre-vaccination levels >1.2 IU/ml showed a fourfold rise of antibodies (Table 3). This relationship was confirmed not only by crude OR, but also by OR mutually adjusted for all study predictors (P < 0.0001). In addition, the SCR4 achieved only 8% (95% CI 2.2–19.2%) in individuals with the highest pre-vaccination levels (i.e. >2.2 IU/ml).
Table 3.
Seroconversion rates, crude and mutually adjusted odds ratios (cOR, aOR) including the 95% confidence interval
| Predictors | Seroconversion, at least a fourfold increase in antibodies (SCR4) | Seroconversion, at least a twofold increase in antibodies (SCR2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Rate (%) | cOR | aOR | P | Rate (%) | cOR | aOR | P | |
| Sex | ||||||||
| Male | 70.0 (60.0–78.8) | 92.0 (84.8–96.5) | ||||||
| Female | 69.0 (59.0–77.9) | 0.95 (0.52–1.74) | 1.22 (0.26–5.78) | 0.800 | 90.0 (82.4–95.1) | 0.78 (0.30–2.07) | 0.53 (0.07–4.25) | 0.554 |
| Vaccine | ||||||||
| Tetavax | 68.0 (57.9–77.0) | 89.0 (81.2–94.4) | ||||||
| Vacteta | 71.0 (61.1–79.6) | 1.15 (0.63–2.10) | 0.89 (0.22–3.60) | 0.871 | 93.0 (86.1–97.1) | 1.64 (0.61–4.42) | 0.90 (0.14–5.85) | 0.916 |
| Smoker | ||||||||
| No | 73.0 (65.3–79.7) | 90.6 (84.9–94.6) | ||||||
| Yes | 56.1 (39.7–71.5) | 0.47 (0.23–0.96) | 0.11 (0.02–0.69) | 0.019 | 92.7 (80.1–98.5) | 1.32 (0.36–4.79) | 4.13 (0.40–42.12) | 0.232 |
| Concomitant medication | ||||||||
| No | 68.5 (59.7–76.5) | 89.8 (83.1–94.4) | ||||||
| Yes | 71.2 (59.4–81.2) | 1.14 (0.61–2.14) | 1.63 (0.33–8.14) | 0.549 | 93.2 (84.7–97.7) | 1.55 (0.53–4.54) | 2.64 (0.29–24.32) | 0.392 |
| Related adverse events | ||||||||
| No | 69.4 (61.2–76.8) | 88.9 (82.6–93.5) | ||||||
| Yes | 69.6 (55.9–81.2) | 1.01 (0.52–1.97) | 2.93 (0.42–20.40) | 0.278 | 96.4 (87.7–99.6) | 3.38 (0.75–15.19) | 17.3 (0.71–421.57) | 0.080 |
| Age (years) | ||||||||
| ⩽43.8 | 73.7 (63.9–82.1) | 92.9 (86.0–97.1) | ||||||
| >43.8 | 65.3 (55.2–74.5) | 0.67 (0.37–1.23) | 0.99 (0.91–1.08) | 0.897 | 89.1 (81.3–94.4) | 0.62 (0.23–1.68) | 0.95 (0.85–1.06) | 0.347 |
| BMI (kg/m2) | ||||||||
| ⩽25.8 | 66.7 (56.5–75.8) | 91.9 (84.7–96.4) | ||||||
| >25.8 | 72.3 (62.5–80.7) | 1.30 (0.71–2.38) | 1.31 (1.08–1.60) | 0.007 | 90.1 (82.5–95.1) | 0.80 (0.30–2.12) | 0.98 (0.79–1.20) | 0.815 |
| Pre-vaccination period (years) | ||||||||
| ⩽12.6 | 71.4 (61.4–80.1) | 92.9 (85.8–97.1) | ||||||
| >12.6 | 67.6 (57.7–76.6) | 0.84 (0.46–1.53) | 0.54 (0.32–0.91) | 0.021 | 89.2 (81.5–94.5) | 0.64 (0.24–1.71) | 0.61 (0.31–1.19) | 0.146 |
| Pre-vaccination GMC (IU/ml) | ||||||||
| ⩽1.2 | 100.0 (96.5–100) | 100.0 (96.5–100) | ||||||
| >1.2 | 37.1 (27.5–47.5) | 0.00 (0.00–0.02) | 0.01 (0.00–0.06) | <0.001 | 81.4 (72.3–88.6) | 0.00 (0.00–0.16) | 0.21 (0.11–0.40) | <0.001 |
| Post-vaccination period (days) | ||||||||
| ⩽28 | 69.2 (59.5–77.7) | 89.7 (82.3–94.8) | ||||||
| >28 | 69.9 (59.5–79.0) | 1.04 (0.57–1.89) | 1.40 (0.95–2.08) | 0.088 | 92.5 (85.1–96.9) | 1.41 (0.52–3.79) | 1.42 (0.87–2.29) | 0.157 |
P, statistical significance determined by logistic regression.
Both crude (0.47; 95% Cl 0.23–0.96) and adjusted ORs (aORs) (0.11; 95% Cl 0.02–0.69) showed a reduced SCR4 in smokers, i.e. only 56% of smokers (95% CI 40–72%) had a fourfold increase in antibodies compared with 73% of non-smokers (95% CI 65–80%).
Furthermore, the SCR4 was affected by BMI and the pre-vaccination period. Although no statistically significant difference was observed between seroconversion rates in subjects with BMI lower and higher than the median (25.8 kg/m2), the aOR revealed a significant positive correlation of SCR4 with BMI, i.e. aOR = 1.31 (95% CI 1.08–1.60). Conversely, the period between previous and current vaccinations had a significant inverse effect on the SCR4 as demonstrated with the aOR of 0.54 (95% CI 0.32–0.91) for increasing length of time. No other monitored predictors such as sex, concomitant treatment, age or related adverse events were found to correlate with the seroconversion rate. The rate was independent of the vaccine used because the seroconversion rate difference between Vacteta and Tetavax vaccines was not higher than 10%, i.e. the lower limit of the 95% CI was −9.8%.
The observed link between pre-vaccination status and seroconversion rate was further tested with the modified seroconversion rate (i.e. SCR2) to determine whether or not it can change the results of the associations found (Table 3). While the ORs mutually adjusted for all predictors again confirmed the strong inverse correlation of pre-vaccination antibody concentrations with seroconversion rate, i.e. aOR = 0.21 (95% CI 0.11–0.40), no other predictors exhibited any signs of an association with the SCR2. As could be expected, this rate (91.0%; 95% CI 86.1–94.6%) exhibited a higher value than the SCR4.
Adverse events were reported by a total of 64 subjects, i.e. 32% (95% Cl 25.6–38.9%). Neither serious nor severe adverse events were documented during the entire study. Most of the related adverse events, i.e. 91.1% (95% CI 82.7–98.0%) were mild. All related adverse events resolved spontaneously without any treatment and the outcome was full recovery. Their occurrence was not influenced by the selected predictors, including the pre-vaccination antibody concentrations.
The most common adverse event was pain at injection site in 10.5% of immunised subjects independently of the vaccine used. Common adverse events were consistent with those listed in the summary of product characteristics of both tetanus vaccines: redness, swelling, induration, elevated temperature, itching and spot at injection site, arm or shoulder pain, fatigue, low-grade fever, headache or pruritus (exacerbation of eczema). The safety profile of both tetanus vaccines showed good tolerability with no difference.
Discussion
The immune response, i.e. both seroconversion rates and post-vaccination GMCs as well as antibody rise after booster immunisation against tetanus, was independent of the monovalent tetanus vaccine used, i.e. no difference was noted in individuals immunised with either Vacteta or Tetavax vaccine.
The sex and age (range 24–65 years) of healthy adults did not have any impact on the seroconversion rates or response induced by both vaccines. As expected, concomitant treatment did not influence the immune response because only subjects with no medication stimulating or suppressing the immune system (including other types of active or passive immunisation) within the last 30 days before booster vaccination were enrolled.
The occurrence of adverse events presumably related to this vaccination did not change the post-vaccination response although a slightly higher seroconversion rate determined by at least a twofold antibody increase was found in subjects experiencing adverse events (96.4%) compared with those without them (88.9%) – data not shown in the Results section.
In this trial, the post-vaccination period was determined by the period of time when the blood sample was taken after booster immunisation. The variance of the period was small (from 24 to 35 days), hence no expected significant difference in seroconversion rates or post-vaccination GMCs was confirmed between intervals shorter or longer than 28 days.
While almost all of the subjects had sufficient antibody concentrations (i.e. >0.1 IU/ml) before booster immunisation and, consistent with recently published data [3], more than half of them had concentrations even higher than 1.0 IU/ml before booster immunisation.
The interval since the last tetanus vaccination may have decreased the SCR4 since the aOR revealed declining seroconversion rates with increasing time. Interestingly, the SCR2 as well as the increase in antibodies or post-vaccination levels of antibodies did not verify this correlation. It is likely that the interval between both vaccinations, limited in this study to a range of 9.9–15.9 years, was not long enough to detect any relevant differences in the immune response. An acceptable response is achieved after booster doses of a tetanus vaccine even when intervals longer than 20 years have elapsed since the last dose, although the briskness and magnitude of the response and duration of protective antibody levels are somewhat dependent on the length of time since previous vaccination [4–6].
The booster dose-induced response was obviously dependent on the pre-vaccination tetanus antibody levels. The lowest levels seem to hold promise for a good immune response reflected in the pre- to post-booster levels ratio as well as the seroconversion rates. The robust rise in antibodies was extremely higher in subjects with pre-vaccination concentrations of ⩽1.2 IU/ml or even <0.6 IU/ml. These individuals achieved an almost 16-fold enhancement of antibody levels after booster immunisation compared with the 3.4-fold increase observed in subjects with pre-vaccination concentrations higher than 1.2 IU/ml.
Also, the SCR2 and SCR4 were significantly lower in those with antibody levels >1.2 IU/ml before immunisation, i.e. 81.4% and 37.1%, respectively. However, this result comes as no surprise as it could not be expected that subjects with a sufficient quantity of antibodies would respond to booster immunisation with more than a fourfold rise. Moreover, this situation could be similar to that seen in infants with maternal antibodies. These antibodies can block the induction of higher levels of post-vaccination antibodies against tetanus as documented in immunised infants with and without maternal antibodies prior to vaccination [7]. This finding could be explained either by the epitopes masking with pre-vaccination antibodies thus preventing antigen binding by B cells and limiting their priming or by inhibition of B-cell activation by Fc-γ receptor-mediated signalling [8, 9]. Naturally, one cannot rule out the possibility of elimination of vaccine antigens by binding to pre-existing tetanus antibodies.
Nevertheless, all subjects but one achieved post-vaccination levels higher than 1.0 IU/ml and are thus supposed to be protected for at least 10 years [10].
The influence of cigarette smoking on the post-vaccination response of the immune system has been generally accepted [11]. Although we did not find any significant difference in the GMCs of antibodies against tetanus between smokers and non-smokers, a significantly higher SCR4 was documented in non-smokers (73%) compared with smokers (56%). In addition, the same outcome was confirmed with mutually aOR. The effect of smoking on the immune response found in this trial is in line with published results [12]. We found that the chance of achieving a fourfold rise in antibodies was decreased in smokers by almost 90% compared with non-smokers. Nevertheless, cigarette smoking did not substantially suppress the immune response since the proportion of smokers and non-smokers with an at least twofold increase in post-vaccination antibody levels was similar. This may be why vaccination failure is not documented in smokers more often than in non-smokers.
The limited body of data concerning the effect of obesity on vaccine immunogenicity and efficacy suggests that obesity could be a factor increasing the likelihood of a poor vaccine-induced immune response. A correlation between obesity and a poor vaccine-induced immune response was first observed in 1985 when obese hospital employees received a hepatitis B vaccine [13].
One study demonstrated that children of 8–17 years of age with a BMI above the 85th percentile had significantly reduced tetanus-specific IgG levels when compared with healthy-weight children several years after infant or childhood vaccination [14]. Conversely, our results suggested that overweight or obese individuals had even a higher chance to achieve at least a fourfold rise in antibodies compared with those with normal weight (aOR of 1.3). This observation was in line with published results after vaccination against influenza [15, 16]. However, when considering post-booster GMCs or SCR2, no association – consistent with the results published after immunisation against hepatitis A [17], influenza [18], pertussis [19] or rabies [20] – was demonstrated.
The lack of data regarding the influence of obesity on vaccine-induced immunity limits our ability to predict if vaccination is sufficient to protect obese or overweight individuals. There is an urge to further improve our understanding of the burden that obesity places on vaccine immunogenicity and efficacy.
Booster immunisation against tetanus in adults carried out with either investigated monovalent tetanus vaccines produced a highly robust response. The post-vaccination concentrations of tetanus antibodies as well as the seroconversion rates were obviously influenced by the antibody levels before immunisation. It is evident that individuals with high pre-vaccination levels cannot induce more than a twofold increase in antibodies.
Based on our data, we can further predict a higher increase in tetanus antibodies is achieved more often in non-smokers, in individuals revaccinated at an interval shorter than 12.6 years or in overweight or obese ones. If these factors have any clinical impact on protection against tetanus is yet to be established.
Acknowledgements
The authors have seen and approved the content and have contributed significantly to the work. The authors would like to thank the following physicians participating in the study as investigators: Lenka Pennigerová, Helena Sedláková, Alena Ježdíková and Aneta Knittelová. They specially thank Jana Havlasová from the central laboratory of Interimun s.r.o. (Czech Republic) for her help and support of the study.
Conflict of interest
MP has no conflicts of interest to declare. VO has received lecture fees from Biodrug s.r.o. (Slovakia), the study sponsor.
Financial support
This work was supported by Biodrug s.r.o. Slovakia.
References
- 1.WHO Expert Committee on Biological Standardization (2014) Sixty-third report. Recommendations to assure the quality, safety and efficacy of tetanus vaccines (adsorbed). WHO Technical Report Series No. 980. Available at http://www.who.int/biologicals/vaccines/Tetanus_Recommendations_TRS_980_Annex_5.pdf (Accessed 10 December 2017).
- 2.Peduzzi P et al. (1996) A simulation study of the number of events per variable in logistic regression analysis. Journal of Clinical Epidemiology 49, 1373–1379. [DOI] [PubMed] [Google Scholar]
- 3.Borella-Venturini M et al. (2017) Tetanus vaccination, antibody persistence and decennial booster: a serosurvey of university students and at-risk workers. Epidemiology and Infection 145, 1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonsen O, Kjeldsen K and Heron I (1984) Immunity against tetanus and effect of revaccination 25–30 years after primary vaccination. The Lancet 2, 1240–1242. [DOI] [PubMed] [Google Scholar]
- 5.Simonsen O et al. (1986) The fall-off in serum concentration of tetanus antitoxin after primary and booster vaccination. Acta Pathologica Et Microbiologica Scandinavica 94, 77–82. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen O et al. (1987) Evaluation of vaccination requirements to secure continuous antitoxin immunity to tetanus. Vaccine 5, 115–122. [DOI] [PubMed] [Google Scholar]
- 7.Jones C et al. (2014) The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 32, 996–1002. [DOI] [PubMed] [Google Scholar]
- 8.Niewiesk S (2014) Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Frontiers in Immunology 5, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegrist CA (2003) Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21, 3406–3412. [DOI] [PubMed] [Google Scholar]
- 10.Amanna IJ, Carlson NE and Slifka MK (2007) Duration of humoral immunity to common viral and vaccine antigens. The New England Journal of Medicine 357, 1903–1915. [DOI] [PubMed] [Google Scholar]
- 11.Younas M et al. (2017) Immune activation, smoking, and vaccine response. AIDS (London, England) 31, 171–173. [DOI] [PubMed] [Google Scholar]
- 12.Jafarzadeh A et al. (2012) Lower immunity to tetanus in cigarette smoker subjects. Journal of Occupational Health and Epidemiology 1, 124–131. [Google Scholar]
- 13.Weber DJ et al. (1985) Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. The Journal of the American Medical Association 254, 3187–3189. [PubMed] [Google Scholar]
- 14.Eliakim A et al. (2006) Reduced tetanus antibody titers in overweight children. Autoimmunity 39, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan PA et al. (2012) Obesity is associated with impaired immune response to influenza vaccination in humans. International Journal of Obesity 36, 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot HK et al. (2012) Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine 30, 3937–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J et al. (2014) The immunogenicity of a single dose of hepatitis A virus vaccines (Havrix(R) and Epaxal(R)) in Korean young adults. Yonsei Medical Journal 55, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan ST et al. (2014) Impact of body mass index on immunogenicity of pandemic H1N1 vaccine in children and adults. The Journal of Infectious Diseases 210, 1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi M et al. (2015) The effect of body mass index on post-vaccination maternal and neonatal pertussis antibody levels. Journal of Reproductive Immunology 112, 34–37. [DOI] [PubMed] [Google Scholar]
- 20.Sirikun J et al. (2018) Immunogenic response in obese patients undergoing rabies post-exposure prophylaxis with combined equine rabies immunoglobulin and rabies vaccination. Vaccine 36, 285–291. [DOI] [PubMed] [Google Scholar]