Abstract
Aims
To present the novel application of combining continuously measured glucose with continuous accelerometer measured physical activity and sedentary behaviour data and discusses the principles used and challenges faced in combining and analysing these two sets of data in the context of diabetes management.
Methods
The background and rationale for exploring glucose, physical activity and sedentary behaviour in people with Type 2 diabetes is presented, the paper outlines the technologies used, the individual data extraction and finally the combined data analysis. A case study approach is used to illustrate the application of the combined data processing and analysis.
Results
The data analytic principles used could be transferred to different conditions where continuous data sets are being combined to help individuals or health professionals better manage and care for people with long term conditions.
Conclusions
Future work should focus on generating validated techniques to visualise combined data sets and explore ways to present data back to the individual in an effective way to support health care management and rehabilitation.
Keywords: Mobile technology, diabetes, continuous measurement, glucose, sedentary behaviour, physical activity
Introduction
Mobile technology is increasingly being developed and made available in both the commercial and research setting, allowing continuous measurement of behaviour and health outcomes. There is opportunity to improve the management of many chronic conditions if these data could be collected, managed and analysed in meaningful ways. Limited focus, however, has been given towards understanding these data and developing methodologies to combine relevant datasets in ways that can improve long-term condition management.
This paper presents the application of combining continuously measured glucose data and accelerometer-measured physical activity and sedentary behaviour data and discusses the challenges faced and possible solutions in combining and analysing these two sets of data in meaningful ways. We start by presenting the background and rationale for exploring glucose, physical activity and sedentary behaviour, then outline the technologies used for this data collection, the individual data extraction and finally the combined data analysis approaches used. A case study approach was used to illustrate the application of the developed methodology.
With mobile technology increasingly being used to support healthcare management and rehabilitation, the challenges and solutions discussed could easily be transferred to conditions where continuous datasets are being combined to help individuals or health professionals better manage and care for people with long-term conditions.
Background and rationale
Type 2 diabetes is a metabolic condition characterised by inadequate insulin sensitivity and/or impaired insulin secretion, and poor management can lead to serious and costly health complications.1 The number of people worldwide with Type 2 diabetes is projected to reach 628.6million by 2045.1 Lifestyle changes, such as incorporating a healthy balanced diet, increasing levels of physical activity2–4 and reducing prolonged sedentary behaviour5 can contribute successfully to the management of Type 2 diabetes.
Ekelund et al.6 report high levels of physical activity per day (∼60–75 min) reduces the risk of all-cause mortality in those sitting for more than 8 h per day. Suggesting that the negative impact of sitting for long periods of time can be nullified by high levels of moderate physical activity. As technology is progressing, people are increasingly finding themselves in settings where time being spent sedentary is the dominant behaviour. Matthews et al.7 reported that adults spend approximately 70% of their waking day in sedentary behaviours. A recent study conducted by Dempsey et al.8 found when prolonged sitting down is broken up with regular, short (3 minute) breaks of light intensity physical activity, glucose profiles in those with Type 2 diabetes are improved, and this improvement was shown to persist for at least a 24-h period.9
In summary, Type 2 diabetes is a chronic disease with increasing prevalence. Glucose management is important within Type 2 diabetes care to reduce risk of additional health complications and improve overall patient quality of life. Increasing physical activity and reducing prolonged sedentary behaviour both have favourable effects on glucose management and are recommended components of Type 2 diabetes care.10 Mobile technologies are now available that independently provide continuous measurement of glucose, physical activity and sedentary behaviour. Developing methodologies to combine these datasets presents the opportunity for in-depth exploration of the relationship between glucose, physical activity and sedentary behaviour and enables tailoring of physical activity and sedentary behaviour interventions for optimal glucose control and disease management in people with Type 2 diabetes.
Selected technology
activPAL
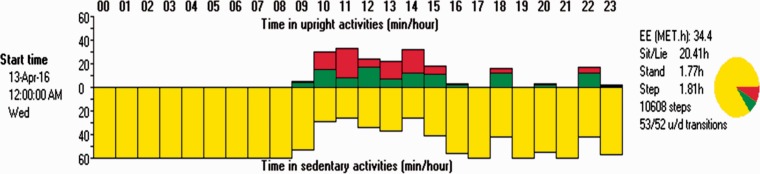
Numerous wearable technologies are available to monitor physical activity and sedentary behaviour. The overall focus of our research was to investigate how patterns of sedentary behaviour affect glucose outcomes in people with Type 2 diabetes. With focus towards sedentary behaviour, the activPAL™ (PAL Technologies Ltd, Glasgow, UK) was selected. In a study conducted by Kozey-Keadle et al.,11 the activPAL correlated with direct observation of sedentary time 94% of the time. The activPAL is a small electronic device (measuring 53 × 35 × 7 mm; weighing 15 g) worn on the front of the thigh, midway between the knee and the hip.12 The activPAL is the first validated instrument to be developed to quantify postural allocation, allowing sedentary behaviour to be accurately identified.12 The activPAL contains an accelerometer and an inclinometer, allowing the participant’s physical activity and sedentary behaviour patterns to be measured in a freeliving context for up to 14 days at a time. Step count, cadence and postural transitions and energy expenditure estimates are also provided.12 Figure 1 illustrates the hour-by-hour summary of activity over a 24-h period. Each line symbolises an hour and the different colour shows the proportion of the hour spent sitting/lying (yellow), standing (green) and stepping (red). The summary output also provides information regarding 24-h step count and the number of transitions from sitting to standing.
Figure 1.
Example of summary data output showing behavioural categorisation by hour in a 24-h period.
The output from the activPAL contains periods categorised as sitting/lying that are not considered sedentary behaviour, such as sleep and non-wear.13 Therefore, a 24-h wear protocol is used and a daily wear diary noting sleep time, wake time and any time where the device was removed and reattached is completed. This allows researchers to remove sleep prior to data analysis.
FreeStyle Libre
Flash Glucose Monitoring is one of the newest methods of glucose monitoring, providing multiple continuous glucose readings compared with conventional ad hoc capillary blood glucose data whilst being more affordable than continuous glucose monitors. The FreeStyle Libre is a flash glucose monitoring system that continuously measures a person’s glucose through their interstitial fluid.14 The FreeStyle Libre consists of a small sensor and a reader.
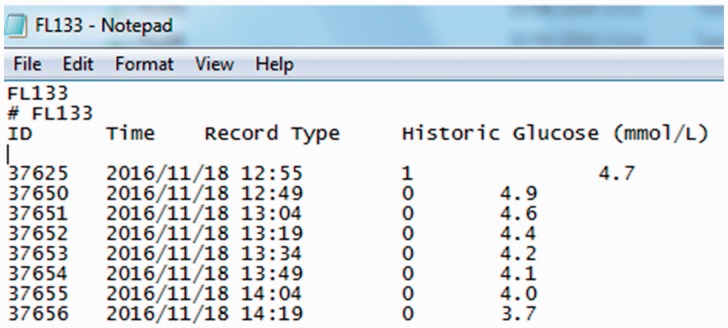
The sensor is applied to the arm where a thin flexible filament (5 mm) is inserted just below the skin. The sensor measures glucose every minute but summarises this over 15 minutes continuously for up to 14 days with date and time also recorded. The sensor has the ability to store up to 8 h of data; therefore, the reader must be scanned over the sensor in order to capture and store continuous data. The data can be uploaded from the reader to desktop software and can be presented as summary data in user-friendly graphs and tables or the raw data can be exported to a text file (Figure 2).
Figure 2.
Example of FreeStyle Libre raw data output.
The FreeStyle Libre is a relatively new device and is predominantly targeted at the consumer market. There are some factors that must be considered when interpreting the data from this device. The FreeStyle Libre is measuring glucose through the interstitial fluid and not through the blood, so there is a physiological lag between the measurements and this lag can be different for each individual, making it difficult to account for. To address this, participants could provide blood glucose measures at regular intervals throughout the day; however, it was decided that the participant burden would be too much. There have been some issues reported where the sensor fails to record at all, is not reading the glucose correctly or is producing unusually low readings. It should also be noted that CGM only measures glucose levels, and it does not provide any estimation of insulin sensitivity or beta-cell function which might also provide important information to fully understand glycaemic responses. It was decided when researching the available devices that the FreeStyle Libre was most suitable to this project, but the methods discussed could be used for any CGM dataset.
Individual device data extraction
Prior to combining datasets, it was important to first screen the datasets and check for any anomalies or outliers and remove any unsuitable data. The challenge then presented was to extract manageable and meaningful information from a large dataset without losing the context and detail held within the continuous objective dataset. Data extracted also need to be relevant to current healthcare practice and research evidence to allow comparison of findings with data presented in clinical practice guidelines and relevant research studies.
Activity data
Once the activPAL data were downloaded, the summary output files for each participant were checked to make sure the data were valid (for example, no large periods of missing data) and that there was a minimum of three days of data, once the first and last days of recording were removed. A day was counted if there were 10 h or more recorded wear time. Datasets where there were less than three days of data or the device had not recorded were removed from the dataset. This is in line with findings of Rich et al.15 who suggested that data collected on two or more days is sufficient for providing reliable results. The activPAL categorises all behaviour in a sitting or lying position together, meaning that sleep time is categorised as sedentary behaviour.12 In order to use sedentary behaviour as a meaningful variable, sleep time must be reliably identified and removed from the dataset. Removing sleep time enables exploration of sedentary behaviour and physical activity patterns over the waking day period and calculation of daily proportion of waking time spent sitting, standing and stepping. Recent studies have examined the use of automated algorithms for identifying and removing sleep/ non-wear time;15 however, in the case of Winkler et al.,13 the automated method was validated against the usual method of the monitor-corrected diary and as yet, is not common practice. For the purpose of this study, sleep was removed manually using the sleep diary completed by the participant. This is a high burden method, particularly with large datasets, and therefore an automated method is currently being developed by researchers.
Sleep removal provides an overview of the waking day, but gives no indication of the more specific daily pattern of behaviour, for example, periods of the day that were more active or sedentary than others rather than an average day. To look at sedentary behaviour in more depth, the proportion of time spent in each behaviour per hour was examined, allowing specific times of day to be isolated and compared. Research has identified that both total sedentary time and continuous uninterrupted periods of sedentary behaviour are detrimental to health.16 Therefore, further analysis was conducted to isolate behaviour based on events to explore continuous periods of sedentary behaviour. This involved breaking the data into sedentary and non-sedentary behaviours and examining sedentary bouts of varying durations, for example, sedentary bouts ≥ 30 min and ≥ 60 min in duration. Breaking up the data into these smaller, more focused intervals allowed us to pull meaningful segments of information from a larger dataset. MATLAB was used to enable us to automate this process and allow data extraction from a large sample (i.e. up to 14 days of individual data and a target sample size for the full study of ∼ 50 participants).
Glucose data
As with the activPAL data, the glucose data from the FreeStyle Libre were downloaded, and the summary output files were checked for accuracy and consistency. Once the first and last days of recording were removed, participants with less than three days of data were removed from the dataset. The data were also checked to make sure there were no issues with the sensor; as aforementioned, there have been some issues reported where the sensor fails to record at all or is not reading the glucose correctly or producing unusually low readings. In these cases, the data were also removed from the dataset. Sleep time was not removed from the glucose dataset, allowing for the data to be examined over a 24-h period in addition to hourly and shorter, more specific bout durations.
For people with Type 2 diabetes, improved blood glucose control substantially decreases the development and progression of diabetic complications and improves overall patient quality of life.17 HbA1c is the most commonly used indicator for glucose control and is a measure of average glucose over a three-month period.18 Another measure of glucose control is daily mean glucose, which is the average glucose level calculated using six glucose readings over a 24-h period. Research has documented a close relationship between HbA1c and daily mean glucose.19
More recent research has identified daily glucose variability as a possible contributor to developing diabetes complications. Glucose variability is the measurement of variation in glucose levels in a day and should not be confused with postprandial glucose excursions, which is the measurement of glucose after a meal. Increased variability was shown to be associated with markers for cardiovascular damage in those with Type 2 diabetes,20 and it has been suggested that variability in glucose levels could be more damaging to long-term health than consistently higher average glucose levels.21 Wearable technology with continuous measurement offers a unique ability to explore within and between individual variability. Currently, there is no consensus on the best measurement of glucose variability to use. Examples of parameters used are: mean average glucose, the average changes in glucose over time of measurement; mean of daily differences, the glucose variability between consecutive days; continuous overall net glycaemic action, measure of continuous glucose variability using continuous monitoring and requires 288 glucose readings in a 24-h period;22 mean amplitude of glycaemic excursions, the average differences between consecutive blood glucose values that are more than one standard deviation from the mean.
More widely used measures of variability and dispersion, such as standard deviation, coefficient of variation and range have all been used to measure glucose variability and are easily determined.22 Standard deviation is easily calculated and is widely supported as a suitable method of measuring variability in glucose profiles.23
It was decided that several measures of glucose variability would be included in the analysis to ensure the effect of sedentary behaviour and physical activity on glucose was fully examined. Although the data could have been analysed using all the above measures of variability, using too many methods would increase the chance of finding a false positive in the results. However, research identifies that both mean glucose and glucose variability can impact overall health of people with Type 2 diabetes. The following variables were therefore extracted and included in the preliminary analysis: daily mean glucose, standard deviation, range and coefficient of variance. Similar to the activPAL data, MATLAB was used to allow the process of data extraction to be automated.
During the study, participants will complete a food and medication diary alongside the wear diary. This information would allow the relationship between sedentary behaviour patterns surrounding meal times and postprandial glucose. For the purpose of this paper however, it was decided that the focus would remain on combining the activity and glucose datasets. The food diary data may be used in future analysis of the dataset.
Combining datasets
Once the data from each device was checked and extracted, the activity, glucose and demographic datasets were imported into MATLAB, where a final output file was produced for data analysis. We took a case study approach to present the individual and combined data analysis. Participant A was a 68-year-old retired male, with a body mass index of 29.2 kg/m2, who has been diagnosed with Type 2 diabetes for two years. Participant A spent, on average, 70% of their waking day sitting/lying, 18% standing still and just 12% of their day stepping, mean daily glucose was 7.53 mmol/l. Table 1 illustrates results from the analysis of data from participant A where mean glucose, standard deviation, range and coefficient of variance were examined in sedentary bouts of 30–60 min and sedentary bouts ≥60 min.
Table 1.
Results from analysis for participant A.
| n = 1 | Mean glucose | Standard deviation | Range | Coefficient of variation |
|---|---|---|---|---|
| 30–60-min bouts | 7.51 | 0.32 | 0.52 | 0.04 |
| ≥60 min bouts | 7.58 | 0.46 | 1.28 | 0.07 |
Subsequent analysis with the full study sample (N = 50) will explore the relationship between overall daily mean glucose and the daily proportion of time spent sitting/lying during wake time. Additionally, the relationship between specific sedentary bout durations and mean glucose and glucose SD, range and coefficient of variation will be examined.
From preliminary analysis, examining the overall glucose response and sedentary bout duration is providing us with more meaningful results than isolating specific sedentary events and the glucose response within those events. Isolating sedentary bouts with a non-sedentary period pre and post-bout was more difficult than anticipated due to the variable nature of behaviour in a freeliving setting.
Conclusions
The aim of this paper was to present the challenges associated with the novel application of combining continuously measured glucose and activity data for people with Type 2 diabetes, and to outline the rationale and principles followed in exploring the combined analysis. Authors suggest using validated devices and visually checking summary data prior to processing and analysis, to check for any errors or unsuitable data. Although not used in this study, the use of heat maps, as described by Edwardson et al.,24 could enhance the robustness of the visual checking of data. It is important to identify specific and meaningful outcome variables prior to processing and analysis of the data and where possible, the use of automated methods for processing and combining datasets would remove a significant burden from the researcher.
We have discussed the process taken during individual data extraction and presented an individual case study of combined data analysis. The principles used could be transferred to different situations or health conditions where continuous datasets are being combined to help individuals or health professionals better manage and care for people with long-term conditions.
Collecting and combining such rich data provides the opportunity for this analysis to be expanded to further explore the temporal patterns and relationships between physical activity, sedentary behaviour and glucose outcomes. A possible focus for this analysis could be significant daily events such as the timing and content of meals and the timing and dose of medication in addition to giving focus to different periods of the day.
Future work needs to give focus towards generating validated techniques to visualise combined datasets and exploring ways to present data back to the individual in an effective way to support healthcare management and rehabilitation. An automated algorithm for the removal of sleep and non-wear time from the activPAL data would be beneficial in larger datasets. Furthermore, the development of multisensory devices allowing measurement of physical activity, sedentary behaviour and glucose, in addition to other behaviours and health outcomes, will enable further exploration of the interaction of multiple behaviours and health outcomes.
Acknowledgements
None.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The PhD project was funded through contribution from PAL technologies and the Partnership Contribution Fund which is a joint fund created by Capita IT Enterprise Services and the University of Strathclyde to provide education and employment benefits to students and graduates.
Guarantor
KAM
Contributorship
KAM, AK, AH, SFMC and ML were involved in the conception and design of the study and study question. KAM participated in data collection and analysis, interpreted the results, drafted and revised the manuscript. AK and AH contributed to data collection and processing. KAM, AK, AH and ML contributed to the revision of the manuscript. The final manuscript was approved by all authors.
References
- 1.International Diabetes Federation. IDF diabetes atlas, 8th ed. Brussels, Belgium: International Diabetes Federation, www.diabetesatlas.org/resources/2017-atlas.html (2017, accessed 30 May 2018).
- 2.Avery L, Flynn D, Van Wersch A, et al. Changing physical activity behavior in Type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care 2012; 35: 2681–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011; 305: 1790–1799. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006; 3: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015; 162: 123–132. [DOI] [PubMed] [Google Scholar]
- 6.Ekelund U, Steen-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016; 388: 1302–1310. [DOI] [PubMed] [Google Scholar]
- 7.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 2008; 167: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dempsey PC, Larsen RN, Sethi P, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 2016; 39: 964–972. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey PC, Blankenship JM, Larsen RN, et al. Interrupting prolonged sitting in type 2 diabetes: nocturnal persistence of improved glycaemic control. Diabetologia 2017; 60: 499–507. [DOI] [PubMed] [Google Scholar]
- 10.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016; 39: 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozey-Keadle S, Libertine A, Lyden K, et al. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc 2011; 43: 1561–1567. [DOI] [PubMed] [Google Scholar]
- 12.PAL Technologies Limited. Products, www.paltechnologies.com/products (accessed 26 February 2017).
- 13.Winkler EAH, Bodicoat DH, Healy GN, et al. Identifying adults’ valid waking wear time by automated estimation in activPAL data collected with a 24h wear protocol. Physiol Measur 2016; 37: 1653–1668. [DOI] [PubMed] [Google Scholar]
- 14.Abbott. FreeStyle Libre, www.freestylelibre.co.uk/libre (accessed 26 February 2017).
- 15.Rich C, Geraci M, Griffiths L, et al. Quality control methods in accelerometer data processing: defining minimum wear time. PLoS One 2013; 8: e67206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chastin SFM, Egerton T, Leask C, et al. Meta-analysis of the relationship between breaks in sedentary behaviour and cardiometabolic health. Obesity 2015; 23: 1800–1810. [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 18.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. http://www.who.int/diabetes/publications/report-hba1c_2011.pdf (2011, accessed 4 June 2018).
- 19.Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol 2011; 5: 1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol 2008; 2: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegelaar SE, Holleman F, Hoekstra JB, et al. Glucose variability; does it matter? Endocrine Rev 2010; 31: 171–182. [DOI] [PubMed] [Google Scholar]
- 22.Service FJ. Glucose variability. Diabetes 2013; 62: 1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Therap 2009; 11: S55–S67. [DOI] [PubMed] [Google Scholar]
- 24.Edwardson CL, Winkler EAH, Bodicoat DH, et al. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci 2017; 6: 162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]