Abstract
Introduction
The objective of this article is to introduce the robotic platform KIINCE and its emphasis on the potential of kinetic objectives for studying and training human walking and standing. The device is motivated by the need to characterize and train lower limb muscle coordination to address balance deficits in impaired walking and standing.
Methods
The device measures the forces between the user and his or her environment, particularly the force of the ground on the feet (F) that reflects lower limb joint torque coordination. In an environment that allows for exploration of the user’s capabilities, various forms of real-time feedback guide neural training to produce F appropriate for remaining upright. Control of the foot plate motion is configurable and may be user driven or prescribed. Design choices are motivated from theory of motor control and learning as well as empirical observations of F during walking and standing.
Results
Preliminary studies of impaired individuals demonstrate the feasibility and potential utility of patient interaction with kinetic immersive interface for neuromuscular coordination enhancement.
Conclusion
Applications include study and rehabilitation of standing and walking after injury, amputation, and neurological insult, with an initial focus on stroke discussed here.
Keywords: Biofeedback, design requirements, evaluation, gait rehabilitation, neurorehabilitation, rehabilitation devices, robot-assisted rehabilitation, stroke rehabilitation
Introduction
Walking and standing are typically an integral part of the human experience. For those who are impaired due to disease or injury, independent standing and walking are often high-priority functional goals.1–3 The mechanism of impairment across individuals varies widely, but the high-level physical goals are always the same: support one’s self using only two legs without falling down or tipping over. In the case of walking, those goals must be met while also moving through space. While these overall objectives are stated simply, the details of their execution and the mechanisms humans use to achieve them are far more complex.
Diverse approaches4 have been used on various impairments in attempts to train walking and standing with mixed effectiveness. With neural insults such as stroke, traumatic brain injury (TBI) and spinal cord injury (SCI), classic motor learning and neurophysiological approaches,5 as well as task-specific repetitive training (e.g. treadmill training, robotic approaches),6–8 are plagued with insufficient evidence to establish their precise utility in recovery.9–12 More appropriate user challenge level, active user participation, and the latitude to train balance have been cited as much needed improvements in interventions.9,13 A complete survey of rehabilitation options and their typical outcomes, which are too often unsatisfactory,1,12,14 readily leads one to conclude that better understanding of impairment mechanisms and more effective therapies directed precisely at those mechanisms are needed. This article introduces a research tool and rehabilitation device that intends to provide such understanding and therapeutic potential based on its foundation in addressing the fundamental goals of the walking and standing mentioned above.
Walking and standing can be viewed as having kinematic and/or kinetic objectives, as these two perspectives are linked by Newton’s second law. The goal of “not falling down,” for instance, is a kinematic constraint on the vertical translation of the center of mass (CM) and, equivalently, a kinetic constraint on the magnitude of the ground-on-foot force (F). “Not tipping over” is a kinematic constraint on CM translation to stay near or within the base of support without the angle of the whole body deviating too far from vertical; from a kinetic perspective, this is a constraint on the location, magnitude, and direction of F relative to the CM. The precise control variables that the central nervous system uses are not well understood,15–17 so the best training target is not apparent.
The ready observability of kinematics makes movement training an intuitive approach, and thus, the basis of most rehabilitation approaches is to focus on retraining appropriate motion and posture. Modern rehabilitation strategies based on the concept of task-specific repetitive training even refer to their class of therapies as movement therapy.18 This approach is exemplified in devices such as the driven gait orthosis exoskeletons (Lokomat, AutoAmbulator)19–22 and movable footplate interfaces (Gait Trainer, HapticWalker)23,24 which target training of motion patterns and impose movement patterns on the user, particularly in the swing phase of walking. With these approaches, however, kinematics may look nonimpaired while atypical joint torque coordination persists.25,26 Similar issues occur with the use of bodyweight-supported (BWS) treadmill training, where the harness support system also provides substantial lateral and rotational stabilizing support.27 The theoretical rationale of these rehabilitation approaches is incomplete, given that humans depend on supraspinal control for tuning standing and walking beyond the basic rhythmic stepping patterns of spinal origin.28 The essential balance-related aspect of maintaining upright posture, that is the appropriate joint torque coordination that produces appropriate F, is not explicitly addressed. Devices that address the need for practice of task-specific active balance control, such as LOPES29,30 still focus on movement goals rather than appropriate limb endpoint force.
The relatively less observable kinetics—the internal and external forces and torques acting within and on the whole human body—that coordinate to drive those movements and postures are often overlooked or incompletely addressed despite their essential contribution to successful standing and walking. Various orthoses, prosthetics, and techniques such as functional electrical stimulation (FES) have been designed with the objectives of measuring and supplementing joint torques during standing and walking to produce more typical values.29,31–34 These techniques address the importance of adequate joint torques; however, they do not retrain precisely appropriate generation or coordination. This is evidenced by the common reliance on assistive devices for balance with these techniques. Kinetic immersive interface for neuromuscular coordination enhancement (KIINCE) measures and provides feedback on F, the metric that emerges from the coordination of multiple joint torques. It also measures any other external forces on the body if used for assistance or training via the instrumented harness and hand rail. When used in conjunction with a motion capture system, internal joint forces and torques can be estimated via inverse dynamics to provide joint-specific kinetics. These kinetic objectives are as essential as kinematics, and focusing on them as training objectives may better align with structure of the walking and standing process in humans.17,35,36 Thus, if addressed from a kinetic perspective, more effective therapy approaches may result.
KIINCE prototype design
Based on these concepts, the kinetic feedback-based robotic environment called KIINCE has been designed and built to provide a more kinetic goal-oriented approach to studying and training standing and walking (Figure 1). The device is a walking environment external to the user with instrumented and controllable features to customize metrics and intervention. The research objectives of KIINCE are to better understand the neuromechanical deficits that manifest as impaired standing and walking and develop the appropriate training paradigm to address them. The general training approach of KIINCE is to provide real-time feedback of parameters such as F, along with guidance toward more appropriate performance, such that the user can learn appropriate neuromuscular coordination. Device use focuses on exploring and training people’s capabilities to produce particular forces on their environment that will keep them balanced while standing and walking, allowing natural kinematics to emerge.
Figure 1.
Robotic force plates support the standing or walking human who can receive visual feedback on the display or kinematic feedback from plate anterior–posterior motion. Forces applied to the human via the force plates, handrail, or harness (see Figure 4) are measured and incorporated into feedback.
The main feature of the device is a custom-designed multiaxis force plate under each foot (Figure 1, mechanically analogous to design by McLeish and Arnold37). The motion of each plate is programmable along a linear path in the anterior–posterior direction of the user. The programmability allows for highly variable means of interaction between each force plate and the user. The plates may be programmed to recreate common study paradigms such as split- or tied-belt-speed treadmill walking or quiet standing, but they are far more versatile. Anterior–posterior standing perturbation, motion driven by some characteristic of the user’s force on the plate or any other real-time metric, or virtually any realistic velocity profile one can imagine are possibilities for operation. A PC running LabVIEW (National Instruments, Austin, TX) is currently used to control and record data. Many control strategies for rehabilitation robotics have been developed,18,38 and the detailed discussion of which are most effective when used to control the foot plates on KIINCE is both application specific and beyond the scope of this article. The purpose here is to emphasize KIINCE’s capacity and versatility in incorporating kinetic variables, particularly F, into studying and training balance strategies.
The feet interface with the plates via custom-designed foot harnesses allows heel and toe liftoff, as well as some lateral, vertical, and yaw motion relative to the plate (Figure 2). They restrict anterior–posterior translation of the foot relative to the plate to prevent the foot from stepping off the plate. Some lateral foot excursion is allowed while still preventing stepping over the centerline. This connection is present during both swing and stance phases. When mounting the foot harness, manual tensioning of a hook and loop fastener adjusts the stiffness of the harness in the vertical direction. Typical tension allows the foot to lift off the plate by about 3 cm, which is within the range of normal foot clearance. In the current algorithm to approximate walking, swing-phase foot motion is accommodated by the plate moving forward with a velocity profile that approximates overground velocity either from a scaled normative or adaptive learning algorithm. An example of F during overground walking compared to walking on KIINCE after use of an adaptive algorithm is presented in Figure 3. A brief video of a participant walking on the device is also available as online supplementary material to this manuscript. Further comparison to walking overground and on other devices, as well as iterations of the walking algorithm, needs to be carried out to validate a transparent walking mode on the device.
Figure 2.
A harness prevents the feet from translating anterior-posteriorly with respect to the force plate but allows the natural heel and toe rise of walking. Each foot is coupled to a plate with three nylon straps. One attaches to a foot harness near the heel and to the plate anterior to the toes. The other pair of straps straddles the first and is attached to a foot near the toes and to the plate posterior to the heel.
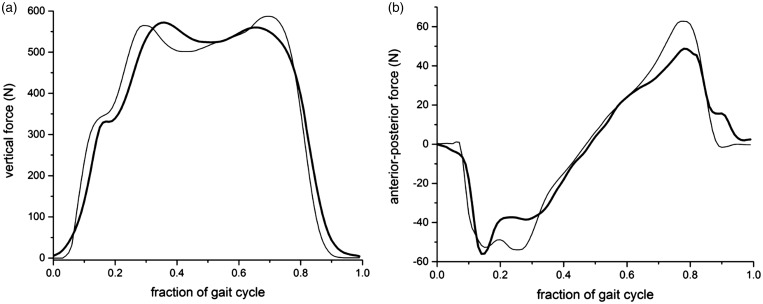
Figure 3.
The primary kinetic features of walking are shared between an initial walking algorithm on KIINCE and overground walking. The vertical (a) and anterior-posterior (b) components of F for overground walking (thin line) and walking on KIINCE (thick line) are shown as the mean of multiple cycles for a representative nonimpaired individual.
The use of programmable footplates is not novel.24 The Gait Trainer and HapticWalker, for example, have designed and argued for the benefits of a movable footplate interface24 over exoskeletons. The plates allow therapist access to the limbs and allow for more unrestricted degrees of freedom in the user’s lower limbs. KIINCE’s design adds to these benefits with its compact footplate and control design. It differs in that the plates move on a linear track, but the foot is not rigidly fixed to the plate and thus, the leg is not tightly restricted to a motion path. This allows subtle variability39 in foot placement and natural heel-to-toe center of pressure (CP) progression during walking. The versatile plate–foot coupling can physically accommodate users with various footwear including bulky ankle-foot orthoses. Irregular gait behaviors such as foot-drop can be accommodated, measured, and targeted for intervention with customized programming. Feedback of F can be used to promote typical heel-to-toe CP excursion, a characteristic of gait shown to have significant importance in walking.36 The plate trajectory also need not be preprogrammed to impose motion on the user as in other devices.23,24 In the interest of more actively involving the user in therapy, which has been considered essential for optimal recovery,40 the plates may be driven by the force of the user on the plate to minimize external assistance and reward appropriate F production by the user. The open footplate design enhances patient access and enables system compatibility with technologies such as motion capture, immersive visual flow environments, metabolic metrics, bodyweight support, electromyography, FES, perturbation mechanisms, prostheses, and robotic exoskeletons.
The custom force plate design provides for a relatively low sprung-mass sensing surface compared to many commercially available systems (plates and treadmills41). This results in a natural frequency that is sufficiently higher than the fundamental frequencies of walking and thus, improved temporal resolution in force sensing (43 Hz unloaded, 55 Hz with human). The design also allows for accurate CP measurement with significant off-axis loading, a common limitation on commercial six-axis sensors. The force-sensing accuracy of the plates was evaluated by applying dynamic forces of similar magnitude and direction variability to that observed during walking. The forces were applied with a commercial force/torque sensor (ATI Delta 660, ATI Industrial Automation, Apex, NC, USA) and the difference between the two measurements was analyzed. The RMS difference was 0.35 N (0.2% of applied force) for each of the horizontal axes and was 0.6 N (0.1% of applied force) for the vertical axis. The CP RMS difference across the surface of the plate was 0.1 mm (0.01% of signal range) for both axes. Theoretical resolution based on analog–digital conversion limitations and electrical noise contributions was significantly smaller than the experimentally derived uncertainties for both force and CP metrics. During human walking the stance phase force plate velocity varied from the commanded velocity by 1.5% (RMS).
Many people with impaired gait facilitate their walking and/or standing by means of an additional force on their hands, typically with a cane, walker, or in the case of a treadmill, a handrail. Effects of handrail hold have been acknowledged in many studies;42–44 however, the precise mechanical contribution of that handrail force is often overlooked. If one is to understand the deficit in motor control facilitating the need for such additional support, i.e. handrail contribution to balance, the force and torque the handrail exerts on the person must be quantified. Some commercial instrumented treadmill manufacturers (i.e. Bertec FIT) recognize the importance of fully characterizing external forces on the body and include this feature. Therefore, in addition to sensing force at the feet, KIINCE provides a front handrail instrumented with a commercial multiaxis force sensor (ATI Industrial Automation Delta F/T, Figure 1).
The device also features the option of an instrumented torso stabilization harness that can provide measured lateral force as well as pure torque on the torso (Figure 4). Four near-orthogonal horizontal tensile straps at each of the shoulder and hip levels attach to a harness worn by the user. The straps have adjustable stiffness and slack length to produce variable stabilization conditions. The angle and force magnitude in each strap is measured so that the user’s mechanical reliance on the harness for balance is quantifiable. Note that the harness does not produce a vertical force on the user due to the nearly horizontal orientation of the straps. The harness provides measured stabilizing force such the user can explore his/her ability to produce various forces on the plates (discussed in “Neuromuscular foundation of standing and walking rehabilitation as motivation for design” section) and the user’s motor control preference can be studied without him or her falling over. Further study of lateral and/or rotational stabilization on walking can also be performed.45
Figure 4.
A slack safety harness arrests falls while a stability harness can restrict torso pitch and roll via four straps at the shoulder and hip levels (posterior attachment points shown as asterisks). The straps are length and compliance adjustable and instrumented to measure force.
The final feature is a screen in front of the user that can be used to guide patient performance and deliver visual feedback on whichever variable(s), such as F direction and/or location, are of interest to the experimenter or clinician (Figure 1). Previous studies using visual feedback have been limited to providing information on the CP,46 but KIINCE can provide feedback that better reflects coordination, such as F direction. Visual and other modes of biofeedback based on kinetic objectives lie at the heart of KIINCE’s approach and are described further in “Feedback modes” section.
Safety: Various mechanical, software, and electronic measures are built into the device to ensure user safety. These mechanisms serve to protect patients from injurious force and excessive joint stress that could conceivably result from actuator malfunction. Manual emergency stop buttons are installed within reach of the user and the operator that will command software to stop the plates from moving if needed. The operator also has an emergency stop button to cut power to the motors driving plate motion. As the motors are back-drivable, a mechanical brake can be engaged when power to the motors is cut. The plate tracks have electronic limit switches that engage before mechanical stops at each end of the plate range of motion. These stops are directly connected to the motor control drives to cut motor power. Kinetic and kinematic limits on the motors that drive plate motion can be limited to provide a safety margin appropriate for a particular application. The foot harnesses are connected to the plates via hook/loop attachments such that the foot can break away from the plate in the event of excessive force. The foot harnesses also activate a magnetic switch that will stop plate motion if foot breakaway occurs. The user wears a safety harness to prevent falls by supporting bodyweight and prevent tipping over if support from the user’s feet is lost. Esthetic, safety, and logistical functional considerations were taken from a licensed physical therapist with gait rehabilitation experience in order make clinically and user-friendly design choices. Both impaired and nonimpaired users of the device have reported feeling comfortable and safe and expressed interest in further experience on the device. Further population-specific study is needed to quantify user perception of the device in order to ensure an optimal, healthy, rehabilitation environment.
Neuromuscular foundation of standing and walking rehabilitation as motivation for design
KIINCE is designed to enable new research as well as develop and encourage a shift to the novel training paradigm of guiding the user to produce a desired force with their foot/feet on the ground rather than produce a specific motion pattern. Kinetic study of walking has shown specific lower limb joint torque coordination patterns during walking25,47 that result in a particular F pattern.35,36 A variety of studies have characterized attributes of F during standing in unimpaired and impaired populations.48–51 KIINCE provides the appropriate feedback channels and mechanically equipped environment to make replication of those nonimpaired F characteristics (direction, magnitude, and CP of F relative to the body) the goals of rehabilitation sessions.
The concept of neuroplasticity52 is important when considering the utility of this approach in injured populations as well as those with impairment due to neural insult such as stroke, SCI, or TBI, where aspects of standing and walking recovery are often viewed as motor learning.19,26,40,53,54 KIINCE relies on human’s ability to modify and develop human motor control,55–57 which persists after these neural insults (whether compensatory or true recovery of function).54,58,59 KIINCE provides an environment for exploring the different context-appropriate feedback types such that the most effective modes can be refined and applied to rehabilitation. Possibilities for feedback modes are discussed in “Feedback modes” section.
Similarly, the idea of task-specific training is central to modern understanding of effective rehabilitation methods24,26,60 and KIINCE’s approach. KIINCE can operate transparently or assist-as-needed,38,61 but assistance is always quantified so that the remaining deficit in the user is clear. While interventions such as Lokomat19 and BWS62 treadmill training cite task-specific practice as beneficial attributes of their methods, the task actually performed in these interventions is not representative of the requirements of actual walking and standing26 in a crucial way. It does not include practice at balancing oneself to produce appropriate force on the ground for remaining upright.27,63 In the case of BWS, the task may inadvertently alter the challenge of balance compared to that consistent with walking.27
Also from a motor learning perspective, active volitional effort from the user also must be encouraged and monitored for appropriateness.24,26,40 Interventions such as traditional use of Lokomat to drive kinematics have been criticized for the lack of necessary patient-initiated movement in their methods.64 Even if the user exerts effort and feels engaged using such intervention,19 he or she may not be engaging in the appropriate way to relearn standing and walking.26 Recent applications of Lokomat using a patient-cooperative controller to guide patient-driven kinematics yielded promising improvements over traditional Lokomat intervention.20,21,65 Those outcomes support the feasibility of promoting engagement and the potential for increased effectiveness of approaches that facilitate active patient involvement. KIINCE can enforce correct volitional effort by providing real-time, appropriate task-specific feedback for essential error correction of the forces needed for balance (e.g. visual feedback on correcting F), and the plates can be programmed in a motion-incentivized manner such that walking motion only occurs with correct coordination (i.e. correct F) to remain balanced. KIINCE provides highly accurate CP feedback, a metric that is believed to be critical for retraining walking.36
Where strength deficit is an identified patient issue, the device could also be used to challenge users with resistive training objectives while maintaining task specificity (i.e. muscle coordination, summarized by F). The stabilizing harness, handrail, or position-controlled force plates could all be used to inform feedback of the user’s performance (force magnitude, direction, and location). That feedback would guide increased force magnitude to promote muscle strength gains that contribute toward functional neuromuscular coordination goals. In addition, external devices that have been shown to provoke task-specific muscle force output during walking could be used, such as the lightweight resistive knee torque device developed by Washabaugh et al.66
Another critical design characteristic of KIINCE that is fundamental from a motor learning perspective is that it allows the user to fail while the objective task is being attempted26 while providing real-time feedback to guide error correction. This allows the user to test out various control strategies and make adjustments without dangerous consequences like falling, allowing the user to explore and train at the boundaries of their capabilities.
Feedback modes
The design of KIINCE enables the use of task-relevant real-time error correction feedback to train its users. It is widely accepted that feedback can enhance motor learning, though the mode of feedback and augmentation that is optimal for any particular task is controversial.67 CP, F direction and magnitude, handle force, and harness force are all candidates for useful kinetic feedback variables toward training proper balance and coordination (F). The addition of a motion capture system can supplement any of those measures with kinematic variables.
On KIINCE, these variables can be packaged in a number of ways as visual feedback presented on the screen in front of the user (Figure 1). Game-like interfaces, visual feedback,26 and virtual environments have been well received as motivational forms of motor control training, particularly in the upper limb.19,68,69 Further investigation is needed to explore possible confounds of visual feedback with optic flow.70 Tactile feedback, as alluded to above, is also possible on KIINCE. Motion-incentivized training where the plates move only with appropriate coordination (appropriate F) or a small vibration induced in the plate in response to user behavior are possibilities. The versatility of operation provides for infinite training possibilities. Someone who is not quite ready to walk but can support his or her weight could practice dynamic balance via a combination of these feedback modes.
Application to stroke
Hemiparesis following stroke is an example of impaired standing and walking for which evidence suggests a kinetic goal-based rehabilitation paradigm such as KIINCE is warranted. The supraspinal control essential for coordinating muscles to retain balance in unimpaired locomotion28 is likely disrupted following stroke. The substantial upright support provided by BWS treadmill training12 and robotic gait orthoses that drive kinematics,12 as well as non-task-specific strength training strategies,71 forego the need for the patient to practice balance during walking. Specifically, a shared balance (i.e. muscle coordination) impairment across walking and standing in this population72 is not addressed by these therapies.
Individuals’ poststroke has been shown to utilize asymmetric weight bearing and F production73,74 as well as atypical lower limb muscle coordination strategies manifesting as altered synergies50,75 and endpoint F direction.76,77 The anteriorly biased F revealed in the hemiparetic limb during a nonbalance task,77 if present in walking, is consistent with the impairment and functional compensatory behaviors observed in hemiparesis.78 Preliminary study of chronic stroke patients (protocol approved by the University of Wisconsin Institutional Review Board, 2014-0466-CP003) on KIINCE has captured miscoordination in a dynamic balance task similar to walking that may support this notion (Figure 5). From this diverse evidence of an F coordination problem and predictable ineffectiveness of conventional therapies, one can triangulate that exploiting brain plasticity58 to train F appropriate for balance is a promising approach. Preliminary studies with chronic stroke patients on KIINCE (see “Feedback modes”) demonstrated their ability to respond to visual and tactile feedback to modulate F location, direction, and distribution between limbs during standing and walking. Thus, KIINCE’s capabilities are primed to aid in better understating of impairment following stroke and yield promising approaches for novel rehabilitation interventions in this population.
Figure 5.

On KIINCE we observed three individuals with stroke that showed distinct coordination differences between their paretic and nonparetic legs while performing a task similar to walking. In the sagittal plane, the location of an intersection point (xi) of foot force lines of action was calculated after adjusting for foot rollover.36 The location of xi was lower and more anterior in the paretic leg (filled circles) compared to the nonparetic leg (open circles). The hip is located at the open square (0,1). CM: center of mass.
Preliminary experiments of visual and kinematic feedback
Preliminary experiments demonstrate the promise of some possible modes of operation. Ten individuals with chronic stroke (Table 1) improved weight-bearing asymmetry during a 30 s dynamic balance task while using the device with the following setups: In baseline trials, the plates moved fore and aft out of phase in a simplified “walking” motion and the user had to balance with no external aid. In a second condition, a visual display of vertical force distribution on each foot was provided to guide the user toward the objective of fully loading the stance limb (plate moving aft). In a third condition, the plates were programmed to incentivize stance limb loading by only moving when the user supported most of his or her weight with the stance foot. Vertical foot force (Fz) during the middle third of stance was averaged for the paretic limb (FzP) and nonparetic limb (FzNP). Asymmetry was quantified with the asymmetry index of |(FzP/FzNP) − 1|. A value of zero meant that both legs had the same vertical force, whereas the value was 0.5 when the paretic leg vertical force was half of the nonparetic leg force. A t-test was used to test for significant differences between conditions. Results are listed in Table 2. There was a significant reduction in asymmetry from baseline to both the motion-incentivized (p = 0.0395) and visual feedback (p = 0.0142) trials. Visual feedback has also been successfully used to guide the CP and/or direction of F during standing (Figure 6) in individuals with chronic stroke or no impairment.
Table 1.
Participant characteristics.
| Age (yrs) | Sex | Chronicity (yrs) | Type | Height (m) | Body mass (kg) | Paresis | F–M (/34) | BBS (/56) | TUG (s) | |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 72 | M | 2 | I | 1.66 | 66.4 | L | 14 | 32 | 82 |
| S2 | 61 | M | 2.8 | I | 1.78 | 91.8 | R | 16 | 36 | 23 |
| S3 | 37 | F | 3.8 | I | 1.65 | 91.3 | R | 28 | 56 | 9 |
| S4 | 79 | F | 5.6 | H | 1.53 | 58.1 | L | 28 | 48 | 21 |
| S5 | 64 | M | 1.8 | U | 1.71 | 91.1 | L | 25 | 54 | 10 |
| S6 | 57 | M | 19.5 | H | 1.83 | 98.1 | L | 32 | 46 | 13 |
| S7 | 86 | M | 8 | U | 1.73 | 59.5 | R | 32 | 41 | 21 |
| S8 | 56 | F | 5.8 | U | 1.51 | 64.5 | R | 7 | 36 | 32 |
| S9 | 62 | M | 6.2 | U | 1.82 | 115.6 | L | 27 | 46 | 27 |
| S10 | 73 | F | 1.6 | I | 1.64 | 72.4 | R | 34 | 35 | 42 |
Ten chronic stroke participants were included in the study with varied type of stroke (I=ischemic, H=hemorrhagic, U=Unspecified). Fugl–Meyer Lower Extremity (F–M), Berg Balance Scores (BBS), and Timed Up-and-Go (TUG) assessments were performed to assess various aspects of impairment.
Table 2.
Asymmetry index for three conditions.
| No feedback | Visual feedback | Motion incentivized | |
|---|---|---|---|
| S1 | 0.444 | 0.427 | 0.638 |
| S2 | 0.196 | 0.175 | 0.181 |
| S3 | 0.116 | 0.007 | 0.034 |
| S4 | 0.293 | 0.070 | 0.024 |
| S5 | 0.384 | 0.253 | 0.015 |
| S6 | 0.067 | 0.013 | 0.002 |
| S7 | 0.072 | 0.138 | 0.011 |
| S8 | 0.263 | 0.152 | 0.091 |
| S9 | 0.521 | 0.179 | 0.253 |
| S10 | 0.184 | 0.009 | 0.083 |
Figure 6.
Tasks to retrain control of foot force magnitude and direction were piloted in individuals affected by stroke. The first task (participant visual feedback shown in (a)) required standing participants to position their center of pressure (vertical line) within the target (box). A second task (participant visual feedback shown in (c)) required standing participants to direct their foot force (gray arrow) to match the target vector (white arrow). The recorded targets and performance variables (paretic limb CP target (b) and paretic limb F direction target (d)) show that the paretic limb was capable of adjusting both aspects of foot force toward a target. CP: center of pressure.
The device intends to use models of F during walking35,36 to prescribe the target F throughout the gait cycle that guides users with balance and walking impairments toward relearning appropriate kinetics for staying upright. However, determining the optimal variable choice(s) and feedback delivery method to encourage users while producing clinically significant improvements in performance is a challenging objective beyond the scope of this manuscript that requires further investigation. Ensuring useful feedback that results in improved clinical balance and walking metrics will require intricate experimentation and validation of each desired application.4 The unique deficits in sensory perception and cognition across populations and individuals may influence the ability of any particular feedback mode to engage and encourage users, but the versatility in options on KIINCE provides a platform from which those options can be surveyed.
Conclusion
This article has introduced the theory and design behind a new walking and standing platform called KIINCE that aims to characterize and guide training of walking and standing. The device focuses on using the kinetic metrics of ground-on-foot force (F), as well as the force of the user on the harness and handle, to quantify and guide patient recovery. Further evaluation needs to be carried out to assess transparency of an overground walking mode on the device and optimize the feedback modes and control algorithms best suited for training populations with specific impairments.
The device is suited to study and potentially rehabilitate a broad spectrum of impairments, as the physical requirements of standing and walking that it addresses are universal. Focus on the essential balance components of walking via measurement and feedback of the forces on the body, particularly at the feet, should fill the gap in existing methodologies. Stroke, TBI, SCI, cerebral palsy, and aging all contain neuromuscular elements of impaired standing and walking that can be studied from the kinetic perspective on KIINCE. Lower limb injury and adapted walking and standing with prostheses and orthoses can be characterized to inform improved interventions. Once understood, rehabilitation approaches designed around a better kinetic understanding of each impairment should produce more complete rehabilitation outcomes.
Supplemental Material
Supplemental material, sj-vid-1-jrt-10.1177_2055668318793585 for Development of KIINCE: A kinetic feedback-based robotic environment for study of neuromuscular coordination and rehabilitation of human standing and walking by Wendy L Boehm and Kreg G Gruben in Journal of Rehabilitation and Assistive Technologies Engineering
Acknowledgements
We would like to thank the volunteer undergraduate students at the University of Wisconsin that assisted in construction and data collection for this project.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KG has a patent 8,257,284 B2 issued (foot force biofeedback system), and both authors have patent P150292US01 (1512.530) pending (harness system). The authors are also owners of a startup company that aims to develop rehabilitation tools.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This development and research received grant support from the Discovery to Product Research Foundation in Madison, Wisconsin.
Guarantor
WLB.
Contributorship
WLB and KGG researched literature, conceived the design of, constructed, and programmed the device, and collected data on the device. WLB wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Supplemental Material
Supplemental material is available for this article online.
References
- 1.Lord SE, McPherson K, McNaughton HK, et al. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil 2004; 85: 234–239. [DOI] [PubMed] [Google Scholar]
- 2.O’Toole RV, Castillo RC, Pollak AN, et al. Determinants of patient satisfaction after severe lower-extremity injuries. J Bone Joint Surg Am 2008; 90: 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirshblum SC, Priebe MM, Ho CH, et al. Spinal cord injury medicine. 3. Rehabilitation phase after acute spinal cord injury. Arch Phys Med Rehabil 2007; 88: S62–S70. [DOI] [PubMed] [Google Scholar]
- 4.Shirota C, van Asseldonk E, Matjačić Z, et al. Robot-supported assessment of balance in standing and walking. J Neuroeng Rehabil 2017; 14: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sackley CM, Lincoln NB. Physiotherapy for stroke patients: a survey of current practice. Physiother Theor Pract 1996; 12: 87–96. [Google Scholar]
- 6.Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev 2012; 11: CD006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley AM, Stark A, Cameron ID, et al. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev 2003, pp. CD002840. [DOI] [PubMed] [Google Scholar]
- 8.Hawkes TD, Siu KC, Silsupadol P, et al. Why does older adults balance become less stable when walking and performing a secondary task? Examination of attentional switching abilities. Gait Posture 2012; 35: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belda-Lois JM, Mena-del HS, Bermejo-Bosch I, et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil 2011; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swinnen E, Duerinck S, Baeyens JP, et al. Effectiveness of robot-assisted gait training in persons with spinal cord injury: a systematic review. J Rehabil Med 2010; 42: 520–526. [DOI] [PubMed] [Google Scholar]
- 11.Werner C, Bardeleben A, Mauritz KH, et al. Treadmill training with partial body weight support and physiotherapy in stroke patients: a preliminary comparison. Eur J Neurol 2002; 9: 639–644. [DOI] [PubMed] [Google Scholar]
- 12.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair 2012; 26: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennycott A, Wyss D, Vallery H, et al. Towards more effective robotic gait training for stroke rehabilitation: a review. J Neuroeng Rehabil 2012; 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillingham TR, Pezzin LE, MacKenzie EJ, et al. Use and satisfaction with prosthetic devices among persons with trauma-related amputations: a long-term outcome study. Am J Phys Med Rehabil 2001; 80: 563–571. [DOI] [PubMed] [Google Scholar]
- 15.Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 1999; 126: 289–306. [DOI] [PubMed] [Google Scholar]
- 16.Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 2002; 5: 1226–1235. [DOI] [PubMed] [Google Scholar]
- 17.Berniker M, Jarc A, Bizzi E, et al. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci USA 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil 2009; 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riener R, Lünenburger L, Maier IC, et al. Locomotor training in subjects with sensori-motor deficits: an overview of the robotic Gait Orthosis Lokomat. J Healthcare Eng 2010; 1: 197–216. [Google Scholar]
- 20.Krishnan C, Ranganathan R, Kantak SS, et al. Active robotic training improves locomotor function in a stroke survivor. J Neuroeng Rehabil 2012; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan C, Ranganathan R, Dhaher YY, et al. A pilot study on the feasibility of robot-aided leg motor training to facilitate active participation. PLoS One 2013; 8: e77370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain S, Xie SQ, Liu G. Robot assisted treadmill training: mechanisms and training strategies. Med Eng Phys 2011; 33: 527–533. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt H, Hesse S, Bernhardt R, et al. HapticWalker – a novel haptic foot device. ACM Trans Appl Percept 2005; 2: 166–180. [Google Scholar]
- 24.Schmidt H, Werner C, Bernhardt R, et al. Gait rehabilitation machines based on programmable footplates. J Neuroeng Rehabil 2007; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neckel ND, Blonien N, Nichols D, et al. Abnormal joint torque patterns exhibited by chronic stroke subjects while walking with a prescribed physiological gait pattern. J Neuroeng Rehabil 2008; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hidler J, Sainburg R. Role of robotics in neurorehabilitation. Top Spinal Cord Inj Rehabil 2011; 17: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dragunas AC, Gordon KE. Body weight support impacts lateral stability during treadmill walking. J Biomech 2016; 49: 2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen JB. How we walk: central control of muscle activity during human walking. Neuroscientist 2003; 9: 195–204. [DOI] [PubMed] [Google Scholar]
- 29.Meuleman J, van Asseldonk E, van Oort G, et al. LOPES II-design and evaluation of an admittance controlled gait training robot with shadow-leg approach. IEEE Trans Neural Syst Rehabil Eng 2016; 24: 352–363. [DOI] [PubMed] [Google Scholar]
- 30.van Asseldonk EH, Veneman JF, Ekkelenkamp R, et al. The effects on kinematics and muscle activity of walking in a robotic gait trainer during zero-force control. IEEE Trans Neural Syst Rehabil Eng 2008; 16: 360–370. [DOI] [PubMed] [Google Scholar]
- 31.Blaya JA, Herr H. Adaptive control of a variable-impedance ankle-foot orthosis to assist drop-foot gait. IEEE Trans Neural Syst Rehabil Eng 2004; 12: 24–31. [DOI] [PubMed] [Google Scholar]
- 32.Herr HM, Grabowski AM. Bionic ankle-foot prosthesis normalizes walking gait for persons with leg amputation. Proc Biol Sci 2012; 279: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrarin M, Pedotti A. The relationship between electrical stimulus and joint torque: a dynamic model. IEEE Trans Rehabil Eng 2000; 8: 342–352. [DOI] [PubMed] [Google Scholar]
- 34.Veneman JF, Kruidhof R, Hekman EE, et al. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2007; 15: 379–386. [DOI] [PubMed] [Google Scholar]
- 35.Gruben KG, Boehm WL. Force direction pattern stabilizes sagittal plane mechanics of human walking. Hum Mov Sci 2012; 31: 649–659. [DOI] [PubMed] [Google Scholar]
- 36.Gruben KG, Boehm WL. Ankle torque control that shifts the center of pressure from heel to toe contributes non-zero sagittal plane angular momentum during human walking. J Biomech 2014; 47: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 37.McLeish RD and Arnold DA. A foot-ground reaction force plate. JBCSA conference, 1972.
- 38.Cao J, Xie SQ, Das R, et al. Control strategies for effective robot assisted gait rehabilitation: the state of art and future prospects. Med Eng Phys 2014; 36: 1555–1566. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler MD, Zhong H, Roy RR, et al. Why variability facilitates spinal learning. J Neurosci 2010; 30: 10720–10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li CL, Lin CL, Chen CK. Stabilizing postural control for emulated human balancing systems. Int J Eng Sci 2008; 46: 1120–1135. [Google Scholar]
- 41.Collins SH, Adamczyk PG, Ferris DP, et al. A simple method for calibrating force plates and force treadmills using an instrumented pole. Gait Posture 2009; 29: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G, Patten C, Kothari DH, et al. Gait deviations associated with post-stroke hemiparesis: improvement during treadmill walking using weight support, speed, support stiffness, and handrail hold. Gait Posture 2005; 22: 57–62. [DOI] [PubMed] [Google Scholar]
- 43.Kang KW, Lee NK, Son SM, et al. Effect of handrail use while performing treadmill walking on the gait of stroke patients. J Phys Ther Sci 2015; 27: 833–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yen SC, Schmit BD, Landry JM, et al. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res 2012; 216: 473–482. [DOI] [PubMed] [Google Scholar]
- 45.Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Trans Biomed Eng 2007; 54: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 46.Geurts AC, de Haart M, van Nes IJ, et al. A review of standing balance recovery from stroke. Gait Posture 2005; 22: 267–281. [DOI] [PubMed] [Google Scholar]
- 47.Winter DA, Robertson DG. Joint torque and energy patterns in normal gait. Biol Cybern 1978; 29: 137–142. [DOI] [PubMed] [Google Scholar]
- 48.Nederhand MJ, van Asseldonk EH, Van der Kooij H, et al. Dynamic Balance Control (DBC) in lower leg amputee subjects; contribution of the regulatory activity of the prosthesis side. Clin Biomech 2012; 27: 40–45. [DOI] [PubMed] [Google Scholar]
- 49.Termoz N, Halliday SE, Winter DA, et al. The control of upright stance in young, elderly and persons with Parkinson’s disease. Gait Posture 2008; 27: 463–470. [DOI] [PubMed] [Google Scholar]
- 50.Neckel N, Pelliccio M, Nichols D, et al. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. J Neuroeng Rehabil 2006; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gruben KG, Boehm WL. Mechanical interaction of center of pressure and force direction in the upright human. J Biomech 2012; 45: 1661–1665. [DOI] [PubMed] [Google Scholar]
- 52.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther 2006; 86: 1406–1425. [DOI] [PubMed] [Google Scholar]
- 53.Krebs HI, Volpe B, Hogan N. A working model of stroke recovery from rehabilitation robotics practitioners. J Neuroeng Rehabil 2009; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson AR, Huie JR, Crown ED, et al. Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury. Front Physiol 2012; 3: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci 2004; 22: 281–299. [PubMed] [Google Scholar]
- 56.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 2008; 21: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin TA, Keating JG, Goodkin HP, et al. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 1996; 119: 1199–1211. [DOI] [PubMed] [Google Scholar]
- 58.Schaechter JD. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol 2004; 73: 61–72. [DOI] [PubMed] [Google Scholar]
- 59.Forrester LW, Wheaton LA, Luft AR. Exercise-mediated locomotor recovery and lower-limb neuroplasticity after stroke. J Rehabil Res Dev 2008; 45: 205–220. [DOI] [PubMed] [Google Scholar]
- 60.Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair 2003; 17: 3–11. [DOI] [PubMed] [Google Scholar]
- 61.Emken JL, Benitez R, Reinkensmeyer DJ. Human-robot cooperative movement training: learning a novel sensory motor transformation during walking with robotic assistance-as-needed. J Neuroeng Rehabil 2007; 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seif-Naraghi AH, Herman RM. A novel method for locomotion training. J Head Trauma Rehabil 1999; 14: 146–162. [DOI] [PubMed] [Google Scholar]
- 63.Swinnen E, Beckwee D, Meeusen R, et al. Does robot-assisted gait rehabilitation improve balance in stroke patients? A systematic review. Top Stroke Rehabil 2014; 21: 87–100. [DOI] [PubMed] [Google Scholar]
- 64.Harvey RL. Improving poststroke recovery: neuroplasticity and task-oriented training. Curr Treat Options Cardiovasc Med 2009; 11: 251–259. [DOI] [PubMed] [Google Scholar]
- 65.Krishnan C, Kotsapouikis D, Dhaher YY, et al. Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor. Arch Phys Med Rehabil 2013; 94: 1202–1206. [DOI] [PubMed] [Google Scholar]
- 66.Washabaugh EP, Claflin ES, Gillespie RB, et al. A novel application of eddy current braking for functional strength training during gait. Ann Biomed Eng 2016; 44: 2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sigrist R, Rauter G, Riener R, et al. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: a review. Psychon Bull Rev 2013; 20: 21–53. [DOI] [PubMed] [Google Scholar]
- 68.Krebs HI, Hogan N, Aisen ML, et al. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng 1998; 6: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rand D, Givon N, Weingarden H, et al. Eliciting upper extremity purposeful movements using video games: a comparison with traditional therapy for stroke rehabilitation. Neurorehabil Neural Repair 2014; 28: 733–739. [DOI] [PubMed] [Google Scholar]
- 70.Warren WH, Jr, Kay BA, Zosh WD, et al. Optic flow is used to control human walking. Nat Neurosci 2001; 4: 213–216. [DOI] [PubMed] [Google Scholar]
- 71.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil 2004; 18: 27–39. [DOI] [PubMed] [Google Scholar]
- 72.Hendrickson J, Patterson KK, Inness EL, et al. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Posture 2014; 39: 177–181. [DOI] [PubMed] [Google Scholar]
- 73.Sackley CM. Falls, sway, and symmetry of weight-bearing after stroke. Int Disabil Stud 1991; 13: 1–4. [DOI] [PubMed] [Google Scholar]
- 74.Allen JL, Kautz SA, Neptune RR. Forward propulsion asymmetry is indicative of changes in plantarflexor coordination during walking in individuals with post-stroke hemiparesis. Clin Biomech 2014; 29: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark DJ, Ting LH, Zajac FE, et al. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 2010; 103: 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arene N, Hidler J. Understanding motor impairment in the paretic lower limb after a stroke: a review of the literature. Top Stroke Rehabil 2009; 16: 346–356. [DOI] [PubMed] [Google Scholar]
- 77.Rogers LM, Brown DA, Gruben KG. Foot force direction control during leg pushes against fixed and moving pedals in persons post-stroke. Gait Posture 2004; 19: 58–68. [DOI] [PubMed] [Google Scholar]
- 78.Boehm WL, Gruben KG. Post-stroke walking behaviors consistent with altered ground reaction force direction control advise new approaches to research and therapy. Transl Stroke Res 2016; 7: 3–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-vid-1-jrt-10.1177_2055668318793585 for Development of KIINCE: A kinetic feedback-based robotic environment for study of neuromuscular coordination and rehabilitation of human standing and walking by Wendy L Boehm and Kreg G Gruben in Journal of Rehabilitation and Assistive Technologies Engineering