Abstract
Background
Frequent practice of functional movements after stroke may optimise motor recovery; however, it is challenging for patients to remember to integrate an impaired limb into daily activities. We report the activity responses of stroke patients receiving a vibrating alert delivered by a tri-axial accelerometer wristband to prompt movement of the impaired arm if hourly activity levels fell.
Methods
Adults with upper limb impairment ≤28 days post-stroke wore the device for four weeks. Therapists and patients reviewed movement activity data twice weekly to agree ongoing rehabilitation activities and programme the wristband with a personalised prompt threshold (median baseline activity + 5%, 25% or 50%).
Results: Seven patients completed the programme (five males; mean ± standard deviation (age) 64 ± 5 years; days post-stroke 13 ± 7; baseline/four-week Action Research Arm Test median (Interquartile range (IQR)) 39 (8, 44)/56 (11, 57)). Wristbands were worn for 89% of programme duration. A total of 1,288 prompts were delivered, with a median of four (IQR 3,7) prompts per patient per day. Mean activity increases following a prompt ranged from 11% to 29%.
Conclusions
Feedback delivered by a programmable accelerometer increased impaired arm activity. Improvements are required in device reliability before conducting a pragmatic clinical trial to examine the impact upon recovery.
Keywords: Stroke rehabilitation, arm, accelerometry, feedback, activity
Introduction
Systematic reviews suggest that recovery of arm function post-stroke may be optimised through frequent practice of functionally related movements.1,2 Optimal therapy dosage is still unclear,3 but 60 h of practice over six weeks has recently been shown to be beneficial.4 It is unclear how best to support this level of intensity outside of therapy sessions or to encourage stroke patients to integrate impaired limb movement practice into daily routines. Learned non-use of the affected upper limb is common after stroke, and to prevent or reduce this, regular prompts may be required to remind the stroke survivor to use this limb during their usual daily activities.
Feedback from accelerometers may offer opportunities to encourage impaired arm use outside of formal therapy sessions, but it is challenging to provide ‘real-time’ feedback,5 and so this approach may not result in a behavioural change.6 The ‘CueS’ (‘Cues for Stroke’) wristband is a programmable wrist-worn cueing device incorporating a tri-axial accelerometer logging movement at 100 Hz and a miniature motor.6 It monitors arm movement relative to individually pre-set levels of hourly activity. When hourly arm movement falls below a predetermined personalised threshold, the wearer is prompted by a vibrating alert. This could be a useful way to encourage patients to attend to their impaired limb and prompt its use during daily activities, but this concept has not been previously evaluated in a clinical stroke population.
This proof of concept study evaluated the technical feasibility of using the CueS device to collect, download and display activity data whilst integrated into an upper limb stroke therapy programme and describes how patients responded to the prompting mechanism.
Methods
Participants within four weeks of acute stroke were recruited from two inpatient stroke units in North East England between June 2015 and February 2016. All participants had a new stroke-related upper limb motor deficit but retained enough movement to lift their hand off their lap. Patients were excluded if they had pre-existing upper limb limitations (e.g. frozen shoulder) or could not comply with a structured therapy programme as a result of significant cognitive, communication or visual impairment. The study was approved by the National Health Service Newcastle Central Research Ethics Committee. All participants gave written informed consent, and the study was conducted according to international standards for Good Clinical Practice.
Intervention
Participants commenced the intervention whilst still an inpatient on the stroke unit. They continued to receive usual clinical care from National Health Service therapists in addition to the twice-weekly review from the study therapist. Participants who were discharged from hospital during the study period were asked to continue the programme at home, and subsequent review sessions took place in the participants’ own homes.
Participants wore a CueS wristband on their impaired arm for 12 h (8 a.m.–8 p.m.) every day over the four-week programme. Accelerations detected by the CueS wristband were converted into signal vector magnitude (SVM) that summarises the intensity of activity across three dimensions relative to ‘g’ (9.8 m/s2) per minute as a single value.7 An initial sampling rate of 100 Hz was chosen because of concern about whether it would be possible to detect very low-amplitude upper limb movements which may occur after stroke. To personalise the intervention, the CueS device was worn for the first week without prompts being set. This provided a baseline of the participants’ upper limb activity levels against which to set the initial prompt threshold and frequency. The maximum frequency for receiving a prompt was specified by the wearer as every hour, 2 h, 3 h or 4 h according to their ability and motivation. The CueS wristband provided feedback in the form of a short vibration if activity during the selected fixed time interval between prompts did not meet the threshold target. If prompted, participants were instructed to increase impaired limb movements, ideally by performing pre-selected activities from a self-directed repetitive functional task practice programme.8 The programme had been previously developed to encourage practice of purposeful movements involving the affected arm which could be easily integrated into activities of daily living such as personal care, eating and drinking. This self-directed practice was recorded by the participant on a patient held log sheet and expanded throughout the four-week programme during twice weekly therapy reviews. As well as guiding self-directed practice, this generated a personalised menu of activities for each participant to choose from if prompted by the CueS device.
During subsequent twice weekly review sessions with a therapist, the CueS data were downloaded onto a portable computer interface where each day was represented by a clock face displaying hourly activity levels and prompt frequencies. The upper limb was re-assessed during these reviews and feedback requested from the participant on the number of prompts they had received, whether this amount had been acceptable and how they had responded to the prompt. The therapist and participant then used these data to discuss progress and maintenance of an appropriate balance of activity practise and rest periods. To accommodate changes in motor performance, CueS data accumulated since the previous review defined a new baseline activity pattern and prompt settings were agreed for the next three days. In order to encourage movement in the upper range of ability, prompt thresholds were set at 5%, 25% or 50% above the wearer's median hourly activity level according to individual preference.
As part of the feasibility assessment, we estimated the proportion of time that the CueS device was worn out of the possible maximum hours. If there was a continuous period of 30 min or more during each fixed hourly interval when the device recorded an SVM value of zero, then this hour was labelled as ‘device not worn’. Although an SVM > 0 may have been recorded for part of that hour, this definition was chosen to reflect the hourly timing of the prompt mechanism and provide a ‘count’ of how many whole hours that the CueS device appeared to be in use.
Outcome measures
Daily log sheets were completed to record the scheduled and prompted therapy programme content selected and practised by patients. This described what activities participants had practiced in order to either avoid a prompt or in response to receiving a prompt.
Data were collected at each therapy review session regarding the acceptability of the number and frequency of prompts received and participant preference for changes to the therapy programme and prompt settings.
Baseline and post-intervention assessments at week 4 included: Action Research Arm Test9 (ARAT) and the Motricity Index10 to measure upper limb motor function/impairment. Discomfort of the paretic arm was measured using a numeric rating scale of pain where a score of 0 indicated no pain up to a score of 10 indicating the worst possible pain. Patient fatigue was measured using a similar scale of 0 to 10 where 0 indicated ‘not tired at all’ and 10 indicated ‘extremely tired’. The tri-axial accelerometer in the CueS device objectively measured impaired upper limb activity levels.
Statistical analysis
Data were analysed using SPSS software (IBM Corp., Released 2013, IBM SPSS Statistics for Windows, Version 22.0, Armonk, NY).
For each participant, Student's t-test was used to compare the mean SVM over 60 min before a prompt was delivered by the CueS with the mean SVM over 60 min after a prompt was delivered.
Results
A total of 11 patients, mean time post stroke 13 days (standard deviation (SD) ± 9), were enrolled, but one withdrew prior to commencing the intervention for personal reasons and no data were collected. A further three participants' accelerometer data could not be used due to technical failures which reflect that the CueS device and interface were prototypes. Due to a CueS coding error for the first two participants (who were recruited in parallel), there was random deletion of data and no prompts were delivered despite changing the settings at each review. The reason for this only became apparent following a detailed review of their raw data, and the code was corrected. We did not include their data due to uncertainties about how well it reflected the full four-week programme and whether there were unrecognised times when a prompt could have been delivered.
For the last participant, the data interface software was modified with the intention of displaying the activity data in a style that could further facilitate prompt setting decisions. However, it became apparent during its use that the interface was not displaying the most recent activity on the same time axis as the previously downloaded data. Due to the geographical location of the patient relative to the research team, it was not possible to correct this before the end of the four-week programme. As the prompt setting process was then corrupted, we did not include this patient's data in the results as the impact of prompts would not have reflected the protocol used with the other participants.
Baseline characteristics of the remaining seven participants are shown in Table 1.
Table 1.
Baseline characteristics.
| Male/female | 5/2 |
| Age (y) | 64 ± 5 |
| Time since stroke onset (days) | 13 ± 9 |
| Impaired side (right/left) | 3/4 |
| Infarct/haemorrhage | 5/2 |
| Clinical stroke sub type | |
| Total anterior circulation | 2 |
| Partial anterior circulation | 1 |
| Lacunar | 2 |
| Posterior circulation | 2 |
| Baseline assessments | |
| NIHSS | 6 (3,12) |
| ARAT | 39 (8,44) |
| Motricity Index | 63 ± 26 |
| Fatigue NRS | 7 (5,8) |
| Pain NRS | 4 (0,6) |
Note: Values are presented as mean ± SD, median (IQR) or n. ARAT: Action Research Arm Test; NIHSS: National Institutes of Health Scale; NRS: numeric rating scale.
Prompting schedules for each participant are detailed in Table 2. With the exception of the first prompt schedule that was set by the research therapist, all settings were determined by the participant based on their experiences and preferences for being prompted.
Table 2.
Participant-selected prompting schedule.
| Participant | Prompt setting 1 |
Prompt setting 2 |
Prompt setting 3 |
Prompt setting 4 |
Prompt setting 5 |
Median number of prompts/day |
Influence of CueS prompt on arm
activity |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prompt frequency | Prompt threshold | Participant reported prompts | Prompt frequency | Prompt threshold | Participant reported prompts | Prompt frequency | Prompt threshold | Participant reported prompts | Prompt frequency | Prompt threshold | Participant reported prompts | Prompt frequency | Prompt threshold | Participant reported prompts | Reported by participants | Recorded by CueS device | Overall mean activity (95%CI) 1 h pre-prompt (g) | Overall mean activity (95%CI) 1 h post-prompt (g) | % Change in activity 1 h post-prompt | p-value | |
| 1 | 3 | Easy | 2 | 1 | Easy | 3 | 1 | Easy | 3 | 1 | Med. | 3 | 1 | Med. | 4 | 3 | 4 | 0.31 (0.26–0.36) | 0.38 (0.32–0.43) | +23 | 0.04 |
| 2 | 4 | Easy | 0 | 1 | Easy | 2 | 1 | Easy | 4 | 1 | Hard | 6 | 1 | Easy | 2 | 1 | 4 | 0.78 (0.59–0.98) | 0.94 (0.74–1.14) | +20 | 0.18 |
| 3 | 1 | Easy | 3 | 1 | Easy | 1 | 1 | Med. | 0 | 1 | Med. | 2 | 1 | Easy | n/a | 2 | 5 | 1.22 (0.99–1.45) | 1.37 (1.14–1.60) | +12 | 0.11 |
| 4 | 2 | Easy | 2 | 1 | Easy | 2 | 1 | Easy | 2 | 1 | Med. | 2 | 2 | Easy | 2 | 2 | 3 | 1.58 (1.32–1.83) | 2.03 (1.73–2.33) | +29 | 0.01 |
| 5 | 3 | Easy | 0 | 1 | Easy | 1 | 1 | Easy | 0 | 1 | Easy | 0 | 1 | Hard | 0 | 0 | 1 | 0.13 (0.11–0.14) | 0.15 (0.12–0.18) | +20 | 0.19 |
| 6 | 1 | Med. | 4 | 1 | Med. | 6 | 1 | Easy | 4 | 1 | Easy | 4 | 1 | Easy | 5 | 4 | 7 | 0.42 (0.36–0.48) | 0.52 (0.45–0.59) | +23 | 0.01 |
| 7 | 1 | Hard | 3 | 1 | Easy | 0 | 1 | Easy | 4 | 1 | Easy | 5 | 1 | Easy | 5 | 4 | 11 | 0.88 (0.81–0.95) | 0.98 (0.87–1.08) | +11 | 0.05 |
Note: Frequency in hours e.g. 1 = hourly, 2 = 2 hourly, 3 = 3 hourly and 4 = 4 hourly. Prompt threshold levels: easy = 105%; med. = 125% and hard = 150% of previous activity. CI: confidence interval.
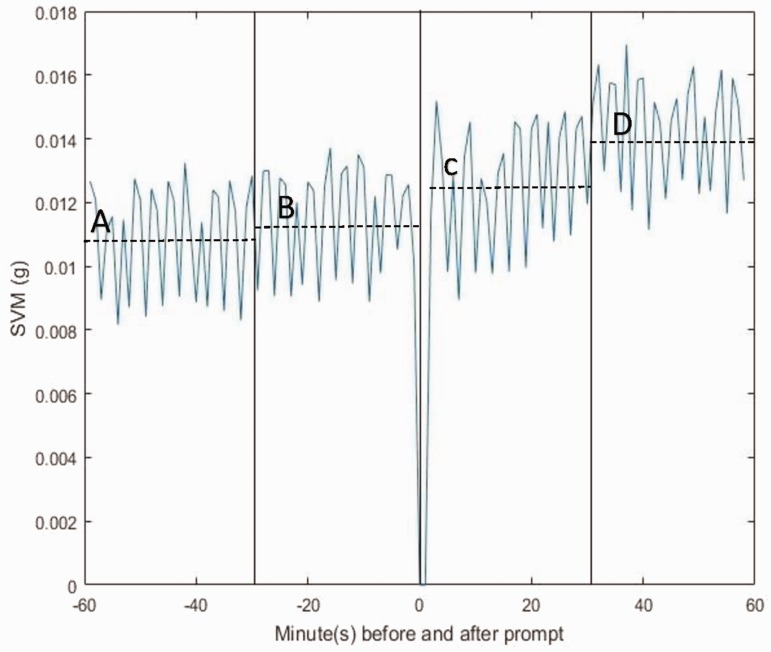
Participants wore the CueS device for an average of 299 out of a maximum of 336 h (89%). The study group received at total of 1,288 prompts from the wristband, an overall median of 4 (IQR 3,7) per day. Individual median number of prompts ranged from 1 to 11 per day. In the hour following a prompt, there were increases in mean activity levels from 11% to 29% compared to the previous hour, with a mean (SD) SVM increase across all recorded prompts of 0.15 g (0.03) or 19.8% (4.3%) (p = 0.03). Figure 3 represents the average activity per minute of the affected limb across all participants in the hour before and after delivery of a prompt. The increase appears greatest in the second half of the hour afterwards, increasing from 0.0111 in the half hour before the prompt to 0.0125 in the half hour directly afterwards, and then further increasing to 0.0139 at between 31 and 60 min. This could suggest a change in behaviour to avoid a further prompt rather than simply an immediate response to the device.
Figure 1.
CueS wristband. source: Axivity Wrist Band Data Sheet.
Figure 2.
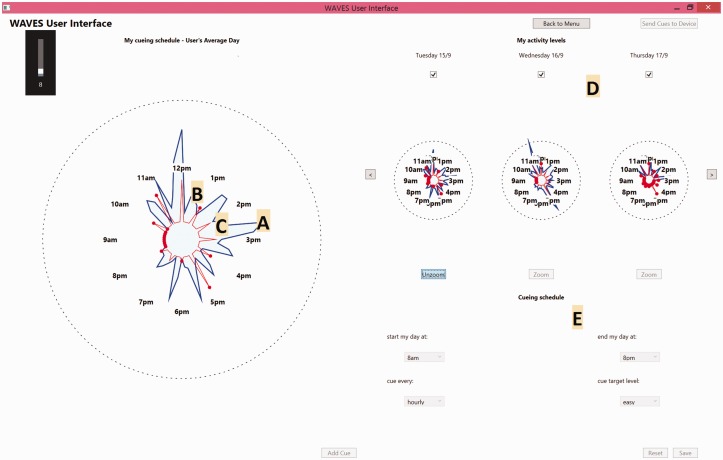
CueS computer interface. (A) Shaded area represents movement activity, (B) dots illustrate when a prompt was delivered, (C) point of line indicates activity threshold for that hour, (D) history of last three days data with option to scroll back to view earlier data and (E) options to set the CueS prompting schedule (start time; end time; maximum prompt frequency; prompt threshold).
Figure 3.
Distribution of SVM in minutes before and after prompt. Vertical solid lines represent 30-min time intervals. Dashed horizontal lines reflect the mean SVM/minute for each time interval as follows: (A) mean SVM/min −60 to −30 min before a prompt = 0.0109, (B) mean SVM/min −30 to −1 min before a prompt = 0.0111, (C) mean SVM/min + 1 to + 30 min after a prompt = 0.0125 and (D) mean SVM/min + 31 to + 60 min after a prompt = 0.0109. Note that data ± 1 min of a prompt were not included in the analysis to avoid possible SVM contamination by the CueS motor vibration. SVM: signal vector magnitude.
When agreeing prompt settings at therapy review sessions, participants mostly chose 1 hourly minimum intervals (96% reviews) rather than 2, 3 or 4 hourly. There was also a clear preference for the target threshold to be set at the lowest setting i.e. 5% above the previous median baseline activity (75% reviews). Participants were noted to report less prompts than the actual number delivered by the CueS device.
Table 3 shows individual participant clinical outcomes. There was no notable increase in pain or fatigue and no adverse events reported. The median increase in ARAT scores was 13 (IQR 3, 18).
Table 3.
Clinical outcome measures.
| Participant | Clinical stroke classification | Dominant hand affected? | ARAT baseline | ARAT four weeks | Motricity arm score baseline | Motricity arm score four weeks | Pain NRS baseline | Pain NRS four weeks | Fatigue NRS baseline | Fatigue NRS four weeks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TACS | Y | 3 | 4 | 10 | 29 | 4 | 4 | 6 | 4 |
| 2 | PACS | Y | 45 | 55 | 62 | 71 | 0 | 0 | 8 | 6 |
| 3 | TACS | N | 44 | 57 | 77 | 92 | 3 | 0 | 7 | 6 |
| 4 | POCS | N | 40 | 57 | 78 | 77 | 6 | 3 | 8 | 5 |
| 5 | POCS | N | 8 | 11 | 56 | 70 | 0 | 0 | 5 | 6 |
| 6 | LACS | N | 26 | 56 | 62 | 92 | 6 | 0 | 8 | 4 |
| 7 | LACS | N | 39 | 57 | 93 | 92 | 5 | 4 | 5 | 6 |
Note: ARAT: Action Research Arm Test; TACS: total anterior circulatory stroke; PACS: partial anterior circulatory stroke; POCS: posterior circulatory stroke; LACS: lacunar syndrome; NRS: numerical rating scale.
Discussion
Remembering to frequently perform exercises and integrate the impaired limb into daily activities after stroke is challenging, particularly for patients with perceptual difficulties who unintentionally fail to use their affected arm.11 This study supports the possibility of using a wrist-worn programmable accelerometer with personalised intermittent feedback to prompt upper limb activity throughout the day since participants wore the device for 89% of the four-week programme, responded to vibrating prompts and reported no related adverse events. Although the clinical implications are still unclear, the study indicated a statistically significant increase in upper limb activity during the hour after a prompt was delivered, thereby supporting the concept that constant monitoring and real-time feedback may positively influence independently initiated practice of functional activity. A person's intrinsic awareness of their movements can be compromised after a stroke necessitating the use of external feedback to support this loss.12 The indication in the CueS data that patients chose to delay their response to the prompt may suggest that they were starting to re-develop their own intrinsic awareness of their stroke arm which hopefully would reduce the need to continue to rely on the CueS prompt in the long term. Participants also engaged with the opportunity to adjust prompt settings according to their own preferences. For example, a participant with minimum movement of their affected upper limb and a sensory inattention requested frequent prompts at a medium setting knowing that this would ensure they received regular prompts to use their arm. Participants with a moderate to high level of arm function tended to opt for a low prompt threshold and described trying to avoid the hourly prompts by increasing use of the arm in activities. Patients and therapists are keen to embrace technology to support rehabilitation13 and wrist-worn monitoring with incremental prompting could offer a personalised, portable and affordable solution to optimise therapy practice in community settings.
Previous trial evidence supports selective use of constraint induced movement therapy (CIMT) to encourage high intensities of upper limb practice after stroke, but this has not been widely adopted due to the prohibitive costs of the associated therapy time and the high demands placed upon patients.11,14 In much the same way that the constraint component of CIMT ‘forces’ use of the affected arm after stroke, the CueS wristband draws the patients’ attention to their impaired upper limb. This may be a more acceptable and affordable approach, as it enables participants to carry out natural bi-manual upper limb activities without the restrictions of wearing a constraint sling or mitt. People with a broader range of upper limb motor impairments than featured in CIMT studies may benefit from live accelerometer feedback and our cohort included patients with severe weakness as well as unilateral ataxia.
It is important to acknowledge that the CueS wristband is also sensitive to general activity, such as arm swing whilst walking, and so the accelerometer data must be interpreted cautiously.12 Previous studies have found that data from wrist-worn accelerometers correlate well with longitudinal arm function changes,5 and the consistent nature of daily activity routines amongst community dwelling stroke patients helps to highlight data variability resulting from arm movement patterns in the context of a structured therapy programme.15,16 The CueS computer interface displays the accelerometer data as a 12-h clock, so that therapists can confirm with participants that a specific activity pattern represents changes in arm movement based upon their reported activities at the time e.g. making lunch, grocery shopping and so on. However, further research in the community is required to determine the upper and lower limb response to the vibration prompt.
It is important to acknowledge that this was an un-blinded observational study on a small number of volunteers. As participants were within four weeks of stroke onset, it is not surprising that arm power and function improved, and this cannot be ascribed to the intervention. A pragmatic trial would be required to determine the medium- and long-term impact of the CueS wristband compared to a therapy programme without prompts and data feedback, including patients with a range of motor impairments both early and late following disabling stroke. The devices used in this proof of concept study were prototypes, with three failures due to software errors, so there would first need to be improvements in reliability and quality assurance.
Conclusion
Participants in a four-week self-directed therapy programme with twice weekly therapist review showed an increase in upper limb activity following prompts by the CueS wristband, which were triggered when arm activity level fell below pre-set thresholds. Participants favoured an hourly prompt with prompt thresholds set at 5% above the median baseline activity level. After improvements in device reliability, this approach requires further evaluation to determine the impact upon functional recovery.
Acknowledgements
None
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project is funded by The Stroke Association (Ref: TSA 2014/01).
Guarantor
CP.
Contributorship
CP, RDS, LS, HR, FvW, LB, TP and MB conceived the study and were involved in protocol development. CP and RDS were involved in gaining ethical approval, patient recruitment and data analysis. CP, RDS, DJ, TP, KL and MB were involved in design and development of the patient–computer interface and prompting mechanism. RDS wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.French B, Thomas LH, Coupe J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2016; 11: CD006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. The Cochrane Database Syst Rev 2014; 11: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011; 377: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 4.Han C, Wang Q, Meng PP, et al. Effects of intensity of arm training on hemiplegic upper extremity motor recovery in stroke patients: a randomized controlled trial. Clinical Rehabil 2013; 27: 75–81. [DOI] [PubMed] [Google Scholar]
- 5.Hayward KS, Eng JJ, Boyd LA, et al. Exploring the role of accelerometers in the measurement of real world upper-limb use after stroke. Brain Impair 2016; 17: 16–33. [Google Scholar]
- 6.Holden A, McNaney R, Balaam M, et al. CueS: cueing for upper limb rehabilitation in stroke. In: British HCI 2015 conference proceedings, Lincoln, UK, 13–17 July 2015, pp. 18–25. New York, NY: ACM.
- 7.Karantonis DM, Narayanan MR, Mathie M, et al. Implementation of a real-time human movement classifier using a triaxial accelerometer for ambulatory monitoring. IEEE Trans Inf Technol Biomed 2006; 10: 156–167. [DOI] [PubMed] [Google Scholar]
- 8.Brkic L, Shaw L, van Wijck F, et al. Repetitive arm functional tasks after stroke (RAFTAS): a pilot randomised controlled trial. Pilot Feasibility Stud 2016; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res 1981; 4: 483–492. [DOI] [PubMed] [Google Scholar]
- 10.Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980; 19: 382–389. [DOI] [PubMed] [Google Scholar]
- 11.Kwakkel G, Veerbeek JM, van Wegen EE, et al. Constraint-induced movement therapy after stroke. Lancet Neurol 2015; 14: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molier BI, Van Asseldonk EHF, Hermens HJ, et al. Nature, timing, frequency and type of augmented feedback; does it influence motor relearning of the hemiparetic arm after stroke? A systematic review. Disabil Rehabil 2010; 32: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 13.Demain S, Burridge J, Ellis-Hill C, et al. Assistive technologies after stroke: self-management or fending for yourself? A focus group study. BMC Health Serv Res 2013; 13: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viana R, Teasell R. Barriers to the implementation of constraint-induced movement therapy into practice. Top Stroke Rehabil 2012; 19: 104–114. [DOI] [PubMed] [Google Scholar]
- 15.Bailey RR, Lang CE. Upper-limb activity in adults: referent values using accelerometry. J Rehabil Res Dev 2013; 50: 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tieges Z, Mead G, Allerhand M, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil 2015; 96: 15–23. [DOI] [PubMed] [Google Scholar]