Abstract
Introduction
A number of patients are excluded from electrical stimulation treatment because there is concern that electrical stimulation could cause electromagnetic interference with pacemakers and implanted cardioverter defibrillators. The decision to use electrical stimulation in these patients needs to be supported by an assessment of benefit and harm.
Methods
We conducted a systematic review of the risk of electromagnetic interference between electrical stimulation and pacemakers or implanted cardioverter defibrillators. We included the electronic databases MEDLINE and EMBASE in the time period between 1966 and 26 August 2016.
Results
18 papers fulfilled the inclusion criteria (eight safety studies and ten case studies). Although we were unable to accurately estimate the risk of electromagnetic interference, the studies revealed that patients having electrical stimulation of the lower limb are less susceptible to electromagnetic interference.
Conclusions
The results suggest that electrical stimulation could be used safely to help drop foot in patients with pacemakers or implanted cardioverter defibrillators. However, in order to obtain an accurate estimate of the risk of electromagnetic interference, a large, long-term, and intervention-specific safety study is required. Until such a study is undertaken, electrical stimulation should be used with caution in patients with pacemakers and implanted cardioverter defibrillators.
Keywords: Electrical stimulation, cardiac pacemaker, artificial, implantable defibrillators
Introduction
Drop foot is a major cause of disability. It is estimated that over 20% of stroke survivors have drop foot, as well as many patients with multiple sclerosis, incomplete spinal cord injury, and traumatic brain injury.1 Several different types of electrical stimulation (ES) can be used to treat motor dysfunction and pain. Functional electrical stimulation (FES), neuromuscular electric stimulation (NMES), and transcutaneous electric nerve stimulation (TENS) all deliver a current to muscles through electrodes. FES and NMES are functional interventions which use frequencies of 20–50 Hz in order to produce muscle tetany and a ‘functional’ contraction, whereas TENS is a therapeutic intervention, usually administered either at low frequencies (2–10 Hz) and high amplitude, or high frequencies (100 Hz) and low amplitude, which does not activate motor fibres.
FES is commonly used as an orthosis for drop foot. It has been shown to increase walking safety, speed, range, and independence, as well as reduce the effort of walking.2–4 However, it is relatively common for people with drop foot to have a pacemaker or implanted cardioverter defibrillator (ICD) and there is concern that applying FES could cause electromagnetic interference (EMI). Patients with pacemakers and ICDs are generally advised to avoid exposure to electrical currents and electromagnetic sources such as ES because EMI can lead to pacemaker and ICD malfunction, resulting in failure to detect arrhythmia or misinterpretation of EMI as a shockable arrhythmia.5
Modern pacemakers and ICDs are generally placed in a pectoral position and connected to one or two transvenous bipolar endocardial leads. Modern ICDs have pacing capabilities and many ICD patients have concurrent indication for cardiac pacing. What distinguishes an ICD from a pacemaker is the presence of a defibrillation electrode (generally combined on one transvenous lead with the pacing/sensing electrodes). All modern pacemakers sense the activity of the heart and pace when the intrinsic electrical activity of the heart rate falls below a programmed rate. Most patients are managed with one of three pacing modes: AAI (atrium paced, atrium sensed, pacemaker inhibited), VVI (ventricle paced, ventricle sensed, pacemaker inhibited), or DDD (both chambers paced, both chambers sensed, pacemaker triggered or inhibited). For further information on pacemaker nomenclature and selection, see Bernstein et al.6 Contemporary ICDs also have several options for arrhythmia detection and treatment.
The use of bipolar leads and improved sensing algorithms in pacemakers and ICDs are thought to have reduced the chance of EMI. However, the exact risk and what determines interaction between ES and modern pacemakers/ICDs is unknown. Therefore, studies investigating the benefits of FES for drop foot have excluded patients with pacemakers and ICDs because of the risk of EMI.2–4 A detailed evaluation of the determinants and likelihood of harm as a result of device interaction is required to help clinicians decide whether or not to start treatment.
Methods
We systematically reviewed the scientific literature including MEDLINE and EMBASE (in the time period between 1966 and 26 August 2016) to identify reports of pacemaker and ICD adverse events associated with ES. The search strategy was developed in conjunction with a Clinical Librarian. We searched using the free-text terms ‘functional electrical stimulation’, ‘FES’, ‘neuromuscular stimulation’, ‘NMES’, ‘electrical stimulation’, ‘transcutaneous electrical nerve stimulation’, ‘TENS’, ‘pacemaker’, ‘defibrillator’, ‘implanted cardioverter defibrillator’, ‘ICD’. Studies were deemed eligible for inclusion if they were original articles/safety studies or case reports on the topic of FES, NMES, or TENS in patients with a pacemaker or ICD. Author searches for key experts in the field were conducted and the references cited in relevant papers were considered for inclusion. Only studies with a full text available in English were included.
Results
A total of 951 publications were identified using the search strategy and then screened for eligibility by title and abstract. Nine hundred and thirty-two articles were rejected based on their title and abstract as they were unrelated, duplicates or the full text was not available in English. Seventeen publications were selected to be read in full (see Figure 1) and these consisted of eight safety studies and nine case studies. One advance online publication was identified by personal communication. The safety studies and case studies are summarised in tables 1 and 2, respectively.
Figure 1.
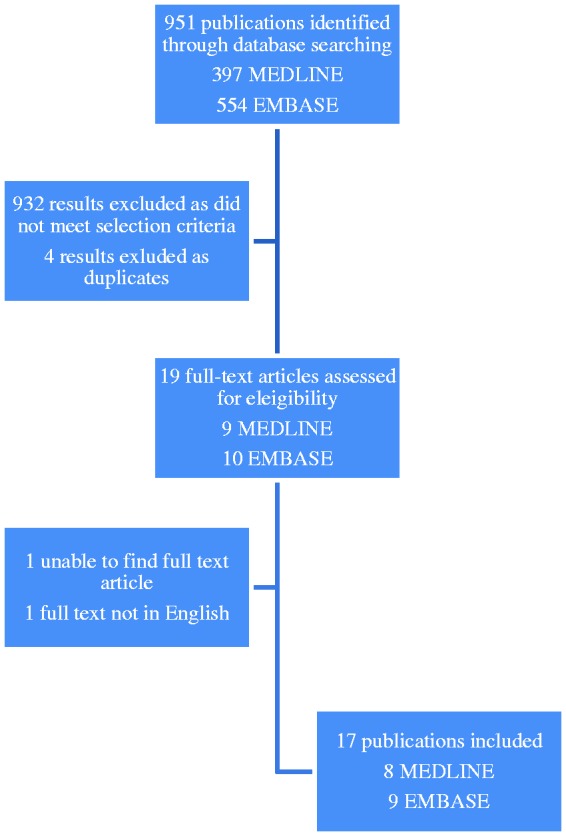
Study flow diagram.
Table 1.
Characteristics of included safety studies.
| Study | Intervention | Location of simulation | Objective | Device | N | Outcomes | EMI |
|---|---|---|---|---|---|---|---|
| Crevennaet al.7 | Functional and therapeutic | Torso, lower extremity | Test the safety of different electrical stimulation algorithms | ICD | 8 | ICD interrogation | Yes, torso and lower extremity |
| Crevenna et al.8 | Functional | Lower extremity | Evaluate the safety of NMES to increase strength and endurance capacity in patients with heart failure and implanted pacemakers | Pacemaker | 7 | Continuous ECG monitoring during safety procedure, heart rate, subjective and clinical condition during subsequent sessions. Pacemaker check at the end | No |
| Crevenna et al.9 | Functional | Lower extremity | Test the practicability and safety of a long-term NMES | ICD | 6 | ICD interrogation, adverse events | No |
| Eriksson et al.10 | Therapeutic | Torso, lower extremity | Identify the hazards associated with TENS in patients with pacemakers | Pacemaker | 8 | Continuous ECG monitoring | Yes, torso |
| Holmgren et al.11 | Therapeutic | Torso | Study the risk of interference between TENS and ICD function | ICD | 30 | Continuous intracardiac electrogram monitoring | Yes |
| Iwatsu et al.12 | Functional | Lower extremity | Determine safety and feasibility of NMES from post-operative days 1–5 after cardiovascular surgery | Temporary pacing | 10 | Continuous ECG monitoring | No |
| Kamiya et al.13 | Functional | Lower extremity | Investigate whether leg NMES causes EMI | ICD | 27 | Continuous ECG and intracardiac ECG monitoring | No |
| Wiesinger et al.14 | Functional | Lower extremity | Determine whether NMES caused pacemaker dysfunction in patients with chronic heart failure awaiting a donor heart | Pacemaker | 4 | Continuous ECG monitoring, pacemaker check | No |
ECG: electrocardiogram; EMI: electromagnetic interference; ICD: implantable cardioverter defibrillator; NMES: neuromuscular electrical stimulation; TENS: transcutaneous electrical nerve stimulation.
Functional interventions are those resulting in a muscle contraction whereas therapeutic interventions do not.
Table 2.
Included case studies.
| Study | Intervention | Location of simulation | Patient | Outcome |
|---|---|---|---|---|
| Curwin et al.15 | Therapeutic | Upper extremity | ICD | Inappropriate shock |
| Engelhardt et al.16 | Functional | Upper extremity | Pacemaker | Bradycardia |
| Glotzer et al.17 | Functional | Torso | ICD | Inappropriate shock |
| Nagele and Azizi18 | Therapeutic | Torso | ICD | Inappropriate shock |
| Philbin et al.19 | Therapeutic | Not known | ICD | Inappropriate shock |
| Pyatt et al.20 | Therapeutic | Torso | ICD | Dizziness, bradycardia |
| Siu et al.21 | Therapeutic | Torso | ICD | Inappropriate shock |
| Street et al.22 | Functional | Lower extremity | Pacemaker (n = 2) | No EMI or adverse events |
| Vlay23 | Therapeutic | Torso | ICD | Inappropriate shock |
| Wayar et al.24 | Functional | Torso | ICD (n = 2) | Inappropriate shock |
EMI: electromagnetic interference; ICD: implantable cardioverter defibrillator.
Functional interventions are those resulting in a muscle contraction whereas therapeutic interventions do not.
Safety studies
Crevenna et al.7 performed a safety study in eight clinically stable subpectoral ICD patients (implanted >6 months ago) with transvenous bipolar sensing leads. They sought to identify whether they could induce EMI by changing the stimulation algorithm and the location of the electrodes. The following therapeutic electrical stimulation algorithms were applied to the neck and shoulder:
Impulse galvanisation (IG50: an amplitude-modulated direct current impulse with a frequency of 200 Hz, an impulse duration of 400 µs, and a serial duration of 50 ms).
Frequency modulation (FM: individual impulses with a frequency of 3.33–33.3 Hz and an impulse duration of 400 µs alternated by tetanising impulse effects)
High-frequency transcutaneous electrical nerve stimulation (narrow rectangular impulses of 200 µs at a frequency of 100 Hz)
Low-frequency transcutaneous electrical nerve stimulation (LF-TENS: narrow rectangular impulses of 200 µs at a frequency of approximately 2 Hz).
The following functional interventions were applied to the thigh muscles:
‘E200’ (impulses of a rising ramp of 200 ms and pulse duration of 270 ms at a frequency of 0.44 Hz)
‘aS’ (pulse duration of 400 µs with a threshold duration of approximately 6.5 s at a frequency of 66.7 Hz)
‘aS1’ (pulse duration of 400 µs and a threshold duration of 3.6 Hz at a frequency of 66.7 Hz)
‘FIB’ which is a specially programmed current that mimics tachycardia (triangular impulses over 60 ms with an interval of 200 ms).
Two home therapy NMES devices for stimulating muscles were also tested:
Stiwell 1200 (biphasic symmetric constant voltage impulses of 500 µs pulse width at a frequency of 15 Hz over 2 s)
Compex 2 (biphasic, symmetric, constant voltage impulses of 250 µs pulse width at a frequency of 8, 15, 30, and 50 Hz and a rising ramp of 1 s, steady impulse over 8 s, and 1 s falling ramp).
The ICD was interrogated for EMI following each stimulation algorithm at the highest tolerable current. EMI of atrial sensing was detected in one patient during FM of the neck and two patients during TENS of the neck. EMI of ventricular sensing was detected in one patient during FM and two patients during LF-TENS. EMI was detected as ventricular under-sensing in two patients during FIB. None of the EMI detected fulfilled ICD detection criteria for a tachyarrhythmic ventricular episode. The authors suggest that a safety procedure to look for EMI is needed before starting ES in patients with ICDs.
In a different study, Crevenna et al.8 assessed the safety of NMES of the thigh muscles in seven patients with chronic heart failure and pacemakers (>6 months post-implantation) with bipolar sensing leads (VVI and DDD) over a series of 20 sessions lasting 40 min for strength and endurance training. The impulses were biphasic, symmetric, and rectangular. The pulse width was kept at 250 µs, but frequency was adjusted between 8 and 50 Hz and current was increased up to 100 mA in all patients. No adverse effects were detected during the safety procedure (which involved applying NMES under electrocardiogram (ECG) monitoring) or during subsequent sessions (which involved heart rate monitoring). They suggest that, after a safety procedure, NMES of the thigh is safe in patients with pacemakers. They recommend maximum burst duration of 3 s because if the stimulation causes the heart to pause, pre-syncope/syncope is only likely to occur after 3 s.
Crevenna et al.9 also carried out a safety study to test long-term NMES of the thigh in clinically stable patients with subpectoral ICDs (>6 months post-implantation) for increasing muscle strength and endurance capacity. Two outpatients had NMES for 4 h daily over 12 weeks (biphasic, symmetric pulses of 500 µs, pulse width at 15 Hz, 2 s on 4 s off), and four inpatients had up to sixteen 30 min sessions during their stay (biphasic, symmetric pulses with duration of 400 µs at 63.3 Hz, 3.5 s on, 4.5 s off). ICD interrogation showed no abnormal rhythm detection after an initial supervised stimulation session. There were no adverse events in the long term. They conclude that NMES of the thigh seems to be safe in the long term.
Eriksson et al.10 studied TENS at the thoracic, paravertebral, lumbar-sciatic, and distal lower extremity levels in eight patients with different types of pacemaker (four with ventricular inhibited, one with ventricular triggered, one with atrial triggered, and two with asynchronous). They monitored patients for blocking of the pacemaker using a surface ECG whilst changing the current and frequency of stimulation (constant current stimulator with 200 µs square pulses at a frequency of 10–100 Hz in short trains between 1 and 10 Hz and up to 40 mA). They found that the four ventricular-inhibited pacemakers were blocked with 1–6 Hz and up to 10 mA in the thoracic, paravertebral, and lumbar-sciatic positions but not in the lower extremity. The ventricular-triggered pacemaker was affected by frequencies above 2 Hz, which corresponds to a heart rate of up to 130/min, the upper limit for the pacemaker. The atrial synchronous pacemaker was triggered by stimulation up to the maximal rate of 150/min. The two asynchronous pacemakers were not blocked or triggered by the stimulator because these pacemakers have no sensing capability. The authors suggest that TENS is contraindicated in synchronous pacemakers but TENS can be given without risk to those with asynchronous pacemakers. An asynchronous mode is often used in patients undergoing surgical procedures because electrocautery can cause EMI with synchronous pacemakers. It can also be used as a backup mode if a synchronous pacemaker’s ability to sense is deactivated. However, asynchronous pacing modes are rarely used long term because they can be associated with competition between the physiologic and paced rhythm.
Holmgren et al.11 studied TENS at the mamilla and hip levels in patients with an ICD. The stimulator delivered an asymmetric biphasic current-compensated pulse with duration of 180 µs and the amplitude set at the highest comfortable level. The high-frequency setting delivers continuous impulses at 80 Hz whereas the low-frequency setting delivers impulses in 2 Hz bursts as a train of eight impulses. A surface ECG, intracardiac electrogram, and ICD marker channel were monitored continuously. EMI of the ICD sensing function was detected during all attempts, resulting in inappropriate interpretation of ventricular fibrillation (VF)/ventricular tachycardia (VT) in eight patients (27%) and premature ventricular beats in 14 patients (47%). EMI was interpreted as noise in two patients and under-sensing in two patients. They found that EMI occurred more frequently in patients with integrated, rather than bipolar leads; at the mamilla, rather than the hip level; and at 80 Hz, rather than 2 Hz. The authors suggest that TENS should not be recommended for any ICD patients.
Iwatsu et al.12 sought to determine the feasibility of NMES of the thigh following cardiovascular surgery. Sixty-one patients had 60 min NMES sessions, and 10 of these patients were having temporary epicardial pacing at the time of their first NMES session. Frequency was varied between 20 and 200 Hz, the waveform was symmetric and biphasic. The current was delivered for 0.4 s followed by a 0.6 s pause. Pulse groups consisted of 10 impulse trains delivered at 30 s intervals. Pulse width was 400 µs and current was varied according to muscle response. They looked at several outcome measures: compliance, systolic blood pressure, heart rate, and the incidence of pacemaker malfunction during NMES. They identified no pacemaker malfunction in response to NMES and no excessive changes in the other outcome measures. They suggest that NMES is safe in patients after cardiovascular surgery.
Kamiya et al.13 studied NMES of the lower limb in 27 heart failure patients with pectoral ICDs hospitalised for decompensation. Knee flexors and calf muscles were stimulated for 20 min with burst-modulated stimulation (alternating sinusoidal current (2.5 kHz) in bursts with a carrier frequency of 50 Hz at the highest intensity tolerable, 5 s on 5 s off). Patients were monitored with a continuous intracardiac electrocardiography and a surface ECG. EMI was defined as over-sensing, inappropriate tachycardia detection, or reprogramming of the device. There was no evidence of EMI throughout the study.
Wiesinger et al.14 performed a safety study investigating NMES of knee extensor muscles in four men with chronic heart failure awaiting a transplantation and implanted bipolar dual-chamber pacemakers (DDD) due to bradyarrhythmias. A stimulation exercise programme was applied for 20 min under continuous ECG. The NMES protocol consisted of biphasic, symmetric, constant voltage impulses of 700 µs pulse width at a frequency of 50 Hz, 2 s on and 6 s off. Pacemakers were then checked for changes in the programmed parameters. No clinical adverse effects were noted on the ECG or on pacemaker check. The authors suggest that, after a safety protocol, NMES of the knee extensor muscles is safe in patients with bipolar DDD pacemakers, but long-term safety data are needed.
Case studies
Curwin et al.15 followed a patient with an ICD who had right upper extremity TENS for 16 sessions but then experienced an inappropriate shock when the electrodes were moved. ICD intracardiac electrograms confirmed normal sinus rhythm, with additional high-frequency signals, prompting discharge at the time. They suggest that orientation of the TENS electrodes may be important in preventing EMI and that the balance of benefit and harm should be weighed on an individual basis.
Engelhardt et al.16 described a case of pacemaker inhibition during shoulder surgery in response to a peripheral nerve electrical stimulator. The size of shoulder muscle contraction guided placement of local anaesthetic for regional anaesthesia as the stimulating catheter was advanced towards the nerves of the brachial plexus. An interscalene brachial plexus stimulating catheter was used. A current of 1.4 mA, pulse duration 1 ms, and frequency 2 Hz resulted in immediate bradycardia which resolved as the current was decreased. An absence of pacing spikes was also noted on the ECG, followed by normal pacemaker function as the current was decreased.
Glotzer et al.17 described an ICD patient (abdominal, epicardial bipolar leads) who experienced an inappropriate discharge in response to electrical muscle stimulation (60 Hz, asymmetrical biphasic wave, 0–25 V) of the lower back. The intracardiac electrogram revealed normal sinus rhythm, whilst the timing channel sensed electrical activity at 152 ms intervals, resulting in a shock delivery. The second patient with an ICD (subpectoral, endocardial bipolar sensing leads) received electrical muscle stimulation (0–100 mA at 300 µs pulse width, 50 Hz) to the left forearm, left shoulder, left upper back, left upper abdomen, and back. The ICD intracardiac electrogram and marker channel was monitored continuously throughout the stimulation. There was no evidence of inappropriate sensing by the ICD. The authors suggest that there may be a lower risk of EMI with endocardial leads, although more data are needed. They also recommend deactivating ICDs before electrical muscle stimulation at any site on the body.
Nagele and Azizi18 report the case of a patient with an ICD who received an inappropriate shock whilst having TENS (40 mA, 50 Hz) of the lumbar region. ICD interrogation revealed false VF. The authors conclude that devices for ES should not be used in patients with pacemakers or defibrillators.
Philbin et al.19 describe a patient who had an inappropriate ICD shock in response to TENS (location of electrodes not described). Device interrogation revealed sinus rhythm on the intracardiac electrogram that was interpreted as VF on the disclosure channel. The authors suggest that an ICD should be considered a relative contraindication to TENS.
Pyatt et al.20 describe a patient who had a pacing ICD device and developed bradycardia with the application of TENS (100 Hz± 20%, pulse duration 200 µs, delivered in bursts of 7 ± 1 ms at a frequency of 2 Hz) over the chest wall. The TENS device was tested for EMI by altering its frequency, position, and output whilst monitoring the intracardiac electrogram and marker signals for sensing problems or artefacts. No interaction was observed. Six months later the patient complained about dizziness and bradycardia so the TENS device was tested for EMI again, using the same method as before. Testing revealed inappropriate sensing during a symptomatic episode. Pyatt et al. suggest that despite initial negative testing, patients with a biventricular ICD should be cautioned about the potential for device interaction and followed up closely, retesting for EMI at regular intervals if using TENS.
Siu et al.21 describe the case of a patient with an ICD (subpectoral, endocardial bipolar leads) receiving an inappropriate discharge whilst using TENS (1–250 Hz, maximal intensity) to the mid back. On device interrogation, there was sinus rhythm and a TENS artefact which the device interpreted as VF. The authors suggest that ICD wearers should avoid TENS.
Street et al.22 carried out a cohort study investigating the training effect of FES on the lower limb in 133 stroke patients. Two participants had implanted pacemakers and underwent pacemaker monitoring whilst FES was set up. No EMI was detected at set up and there were no adverse events reported in the 20-week follow-up period.
Vlay23 describe a case of inappropriate ICD discharge in response to sacral TENS stimulation 12 in. away from the ICD pulse generator. The patient had no symptoms leading up to the discharge. ICD interrogation revealed a sinusoidal wave that was misinterpreted by the ICD. The authors suggest that ICD patients should be instructed to avoid TENS.
Wayar et al.24 report two consecutive cases where patients with subpectoral ICDs experienced interference when using NMES for abdominal training (5.0–11.0 V, 7.3–10 mA, 55–75 Hz/biphasic), resulting in inappropriate shock delivery. On ICD interrogation, EMI was incorrectly interpreted as a shockable rhythm. The authors conclude that abdominal muscle stimulation should be avoided by patients with an ICD.
Discussion
The risk of utilising lower limb FES for drop foot in patients with pacemakers and ICDs is unclear. This is because there are few studies to date that investigate the use of ES in patients with pacemakers and ICDs, only one of which included patients using FES for drop foot.22 Several studies investigated the safety of applying NMES to the thigh in order to improve muscle strength and endurance capacity; whilst others examined TENS of the torso and lower extremity. However, there is likely to be a number of systematic differences between these interventions and applying FES for drop foot, such as the location of the electrodes, stimulation parameters, and duration of treatment.
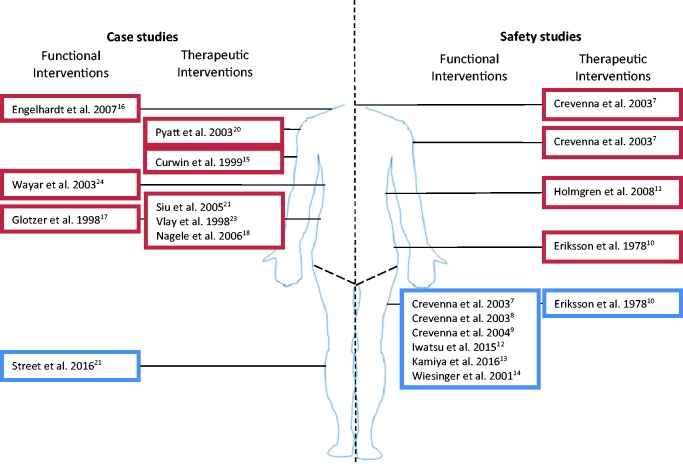
Although the case reports and safety studies cannot be used to identify the risk of FES for drop foot in patients with pacemakers and ICDs, they do suggest that the location of the electrodes is a susceptibility factor for EMI. For instance, all but one of the studies in which ES caused EMI involved applying ES to the torso and upper extremity. In contrast, safety studies where ES was applied to the lower extremity concluded that ES is probably safe. However, EMI cannot be ruled out when ES is applied to the lower extremity, as demonstrated by Crevenna et al.7 who deliberately used a low-frequency (3–4 Hz) triangular waveform ES to the thigh in order to mimic VT waveforms. Although this did induce EMI, very low-frequency ES is generally not used in clinical practice so Crevenna et al.7 conclude that NMES can be applied safely to ICD patients so long as standard waveforms and frequencies are used and patients are monitored during the initial ES set-up. Figure 2 is a summary of the electrode positions used in studies investigating the effect of ES on ICDs and pacemakers.
Figure 2.
A summary of studies investigating the safety of ES on patients with pacemakers or ICDs. The blue boxes contain studies investigating the effect of ES of the lower limb. They all conclude that ES is likely to be safe in patients with pacemakers and ICDs. The red boxes represent studies where ES caused EMI and all of which involved ES above the lower limb. Case studies are to the left of the picture and safety studies are on the right. Functional interventions are those resulting in motor activation, whereas therapeutic interventions do not.
Although several lower extremity safety studies concluded that NMES of the thigh is probably safe, these investigations are all limited by small participant numbers. The largest safety study investigating lower extremity ES included 27 patients.13 A much larger sample size is needed to exclude the risk of EMI. In order to demonstrate that risk of EMI is less than one in 100 using the rule of threes, for example, a safety study involving 300 participants and no adverse events would be required.25 Such a study is yet to be undertaken. The safety studies that have been conducted could not be combined to produce a summary statistic because of divergent interventions, outcome measures, and durations of treatment. The type of ES was variable, often poorly defined, and in many cases the stimulation parameters were not fully detailed. The outcome measures varied from patient-reported adverse events to real time monitoring of intrinsic cardiac rhythm and marker channel (Table 1). The duration of the safety studies also varied from a single session of ES to using the device for 4 h every day for 12 weeks.9 Due to the small sample sizes and diverse range of interventions, it is not possible to give a numerical estimate of the risk of EMI between ES and pacemakers or ICDs.
Cenik et al.26 conducted a systematic review of the literature to investigate the safety of NMES for increasing exercise capacity and reducing cardiac cachexia in patients with bipolar ICDs. They included in their results original articles, safety studies, and case studies relating to NMES and EMI in ICDs. They excluded pacemakers and TENS from their search. The outcome of interest was EMI defined as over-sensing or inappropriate shocks. They identified four studies with the full text published in English or German,7,9,13,24 all of which were included in this review. Cenik et al. concluded that NMES can be applied to the thigh and gluteal muscles in ICD patients if the individual risks of pacemaker malfunction are acceptable before starting NMES, patients are compliant, the treatment is regularly supervised by a doctor, and the ICD device is examined for evidence of EMI after first use. They suggest that large sample sizes are needed to identify the risk of adverse effects. We not only included patients with different types of ES but also included studies investigating pacemakers. This is important because a pacemaker and ICD is often combined in one unit for people who need both.
Conclusion
This systematic review indicates that FES for drop foot could be considered safe in patients with pacemakers and ICDs. Several short-term safety studies investigating lower limb NMES have concluded that it is safe to use in patients with pacemakers and ICDs; and there have been no case studies describing EMI when ES is applied to the lower limb. However, a definitive safety study that is intervention specific, long term, and high powered is needed to identify the exact risk. Until such a study is realised, FES should be used with caution in patients with pacemakers and ICDs. We recommend that pacemaker and ICD function should be monitored for EMI when ES is first used.
Acknowledgement
The findings within this paper were previously presented at the 21st Annual Conference of the International Functional Electrical Stimulation Society, IFESS, London 2017.
Declaration of conflicting interests
Author JB has no competing financial interests. Authors PT and ID hold shares in a the company Odstock Medical Limited, a manufacture of FES devices.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Southampton National Institute of Health Research Academic Foundation Programme.
Guarantor
JB
Contributorship
All authors contributed to the conception and design of this systematic review. JB developed the search strategy with the assistance of a librarian, performed the searches and drafted the paper. IS and PT read, provided feedback and approved the manuscript.
References
- 1.Wade DT, Wood VA, Heller A, et al. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med 1987; 19: 25–30. [PubMed] [Google Scholar]
- 2.Robbins SM, Houghton PE, Woodbury MG, et al. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil 2006; 87: 853–859. [DOI] [PubMed] [Google Scholar]
- 3.Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil 1999; 80: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PN, Burridge JH, Dunkerley AL, et al. Patients’ perceptions of the Odstock Dropped Foot Stimulator (ODFS). Clin Rehabil 1999; 13: 439–446. [DOI] [PubMed] [Google Scholar]
- 5.McIvor ME, Reddinger J, Floden E, et al. Study of pacemaker and implantable cardioverter defibrillator triggering by electronic article surveillance devices (SPICED TEAS). Pacing Clin Electrophysiol 1998; 21: 1847–1861. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein AD, Daubert JC, Fletcher RD, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol 2002; 25: 260–264. [DOI] [PubMed] [Google Scholar]
- 7.Crevenna R, Stix G, Pleiner J, et al. Electromagnetic interference by transcutaneous neuromuscular electrical stimulation in patients with bipolar sensing implantable cardioverter defibrillators: a pilot safety study. Pacing Clin Electrophysiol 2003; 26: 626–629. [DOI] [PubMed] [Google Scholar]
- 8.Crevenna R, Mayr W, Keilani M, et al. Safety of a combined strength and endurance training using neuromuscular electrical stimulation of thigh muscles in patients with heart failure and bipolar sensing cardiac pacemakers. Wien Klin Wochenschr 2003; 115: 710–714. [DOI] [PubMed] [Google Scholar]
- 9.Crevenna R, Wolzt M, Fialka-Moser V, et al. Long-term transcutaneous neuromuscular electrical stimulation in patients with bipolar sensing implantable cardioverter defibrillators: a pilot safety study. Artif Organs 2004; 28: 99–102. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson M, Schuller H, Sjolund B. Hazard from transcutaneous nerve stimulation in patients with pacemakers. Lancet 1978; 1: 1319. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren C, Carlsson T, Mannheimer C, et al. Risk of interference from transcutaneous electrical nerve stimulation on the sensing function of implantable defibrillators. Pacing Clin Electrophysiol 2008; 31: 151–158. [DOI] [PubMed] [Google Scholar]
- 12.Iwatsu K, Yamada S, Iida Y, et al. Feasibility of neuromuscular electrical stimulation immediately after cardiovascular surgery. Arch Phys Med Rehabil 2015; 96: 63–68. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya K, Satoh A, Niwano S, et al. Safety of neuromuscular electrical stimulation in patients implanted with cardioverter defibrillators. J Electrocardiol 2016; 49: 99–101. [DOI] [PubMed] [Google Scholar]
- 14.Wiesinger GF, Crevenna R, Nuhr MJ, et al. Neuromuscular electric stimulation in heart transplantation candidates with cardiac pacemakers. Arch Phys Med Rehabil 2001; 82: 1476–1477. [DOI] [PubMed] [Google Scholar]
- 15.Curwin JH, Coyne RF, Winters SL. Inappropriate defibrillator (ICD) shocks caused by transcutaneous electronic nerve stimulation (TENS) units. Pacing Clin Electrophysiol 1999; 22: 692–693. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt L, Grosse J, Birnbaum J, et al. Inhibition of a pacemaker during nerve stimulation for regional anaesthesia. Anaesthesia 2007; 62: 1071–1074. [DOI] [PubMed] [Google Scholar]
- 17.Glotzer TV, Gordon M, Sparta M, et al. Electromagnetic interference from a muscle stimulation device causing discharge of an implantable cardioverter defibrillator: epicardial bipolar and endocardial bipolar sensing circuits are compared. Pacing Clin Electrophysiol 1998; 21: 1996–1998. [DOI] [PubMed] [Google Scholar]
- 18.Nagele H, Azizi M. Inappropriate ICD discharge induced by electrical interference from a physio-therapeutic muscle stimulation device. Herzschrittmacherther Elektrophysiol 2006; 17: 137–139. [DOI] [PubMed] [Google Scholar]
- 19.Philbin DM, Marieb MA, Aithal KH, et al. Inappropriate shocks delivered by an ICD as a result of sensed potentials from a transcutaneous electronic nerve stimulation unit. Pacing Clin Electrophysiol 1998; 21: 2010–2011. [DOI] [PubMed] [Google Scholar]
- 20.Pyatt JR, Trenbath D, Chester M, et al. The simultaneous use of a biventricular implantable cardioverter defibrillator (ICD) and transcutaneous electrical nerve stimulation (TENS) unit: implications for device interaction. Europace 2003; 5: 91–93. [DOI] [PubMed] [Google Scholar]
- 21.Siu CW, Tse HF, Lau CP. Inappropriate implantable cardioverter defibrillator shock from a transcutaneous muscle stimulation device therapy. J Interv Card Electrophysiol 2005; 13: 73–75. [DOI] [PubMed] [Google Scholar]
- 22.Street T, Swain I, Taylor P. Training and orthotic effects related to functional electrical stimulation of the peroneal nerve in stroke. J Rehabil Med 2017; 49: 113–119. [DOI] [PubMed] [Google Scholar]
- 23.Vlay SC. Electromagnetic interference and ICD discharge related to chiropractic treatment. Pacing Clin Electrophysiol 1998; 21: 2009. [DOI] [PubMed] [Google Scholar]
- 24.Wayar L, Mont L, Silva RM, et al. Electrical interference from an abdominal muscle stimulator unit on an implantable cardioverter defibrillator: report of two consecutive cases. Pacing Clin Electrophysiol 2003; 26: 1292–1293. [DOI] [PubMed] [Google Scholar]
- 25.Eypasch E, Lefering R, Kum CK, et al. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ 1995; 311: 619–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cenik F, Schoberwalter D, Keilani M, et al. Neuromuscular electrical stimulation of the thighs in cardiac patients with implantable cardioverter defibrillators. Wien Klin Wochenschr 2016; 128: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]