Abstract
This review describes the effects of two control strategies – used in robotic gait-training devices for chronic stroke survivors – on gait speed, endurance and balance. Control strategies are classified as ‘patient-in-charge support’, where the device ‘empowers’ the patient, and ‘device-in-charge support’, where the device imposes a pre-defined movement trajectory on the patient. Studies were collected up to 24 June 2015 and were included if they presented robotic gait training in chronic stroke survivors and used outcome measures that were indexed by the International Classification of Functioning, Disability and Health. In total, 11 articles were included. Methodological quality was assessed using the PEDro scale. Outcome measures were walking speed, endurance and balance. Pooled mean differences between pre and post measurements were calculated. No differences were found between studies that used device-in-charge support and patient-in-charge support. Training effects were small for both groups of control strategies, and none were considered to be clinically relevant as defined by the Minimal Clinically Important Difference. However, an important confounder is the short training duration among all included studies. As control strategies in robotic gait training are rapidly evolving, future research should take the recommendations that are made in this review into account.
Keywords: Stroke rehabilitation, robot-assisted rehabilitation, rehabilitation devices, gait rehabilitation, assistive technology
Introduction
One of the many important aspects in motor learning is believed to be the principle of error-based training.1,2 This principle holds that subjects do not improve on a task when they are not allowed to make errors. Subjects use their sensory system to detect movement errors and they consequently use this information to update their upcoming motor actions, thereby continually learning from their mistakes.3 Physical therapists intuitively apply this principle during conventional gait training of, among others, stroke patients. They provide patients with more or less support, depending on the capabilities and needs of the patients. However, applying this principle demands high physical effort and awareness by the therapists due to the constant interaction between patient and therapist. In addition, the number of (chronic) stroke survivors is expected to grow rapidly due to a demographically changing human population.4 With an increase of 35% stroke patients, predicted by the year 2050,5 the workload of each individual physical therapist (and the resulting healthcare costs) will greatly increase in the near future. This puts great pressure on the ability of therapists to provide patients with sufficient training intensity. Kwakkel6 indicated in a meta-analysis that rehabilitation therapy leads to better results when more hours were spent training by the patient (often regarded as the ‘more is better’ principle). Training frequency and physical effort by the patient are the two other factors that determine training intensity7 and affect training outcome to a great extent.
Both (chronic) stroke survivors and therapists would benefit from having a good alternative to conventional gait-training. Gait-training in stroke rehabilitation is characterized by a high level of repeatability in terms of movements performed by the patient. The repetitive character of gait training makes this type of therapy in particular suitable for training through the use of robotic devices,8 thereby enabling patients to train with a sufficient level of training intensity. However, there is a large variability in robotic gait-training devices, for instance in terms of mechatronic design or because they are based on various control strategies. Several devices ‘empower’ the patients in their movements, thereby mimicking the working principle of physical therapists and the principle of error-based training. Other devices do not use these principles, and impose a pre-defined movement trajectory on the patient by use of guidance forces. As robotic gait training is a rapidly evolving field, this systematic review will investigate the reported effects of control strategy on clinical training effects.
Two categories are distinguished, based on the control strategy that is used in the robotic gait-training device: (1) devices that provide ‘device-in-charge support’; and (2) devices that provide ‘patient-in-charge support’ to the patient. For patient-in-charge robotic support, the movement of the device is primarily controlled by the patient. This is commonly referred to as ‘Patient Empowerment’.9 Devices that apply patient-in-charge support mimic the way that physical therapists provide training to patients: they assist the patient during the training and let the patient be in charge of their movements as much as possible. This category is not only restricted to strategies such as ‘Patient-Cooperative Control’10 or ‘Assist-As-Needed’,11 but includes all types of robotic devices in which the device reacts to the movements of the patient. Patient-in-charge support can be seen as the opposite of device-in-charge support, which imposes a pre-defined movement trajectory on the patient. No deviation from this trajectory is allowed by the device and the robot fully controls the movements of the patient. Device-in-charge support does not use the principles of error-based training: patients are not challenged to learn from their mistakes as the robot continuously provides support to patients, regardless of their performance.
To date, it has not been possible to determine the clinical relevance of the various control strategies. Even though two papers11,12 have indicated that the control strategy could make a difference in determining which robotic device leads to the most optimal training effects, a descriptive approach is used in both papers to present the results, rather than to provide the reader with actual numbers that indicate clinical relevance. Moreover, both (sub)-acute and chronic stroke survivors are included in the descriptive analysis,12 which confounds the results. By solely including chronic stroke survivors, training effects will not be biased by natural recovery effects as it is believed that no spontaneous neurological recovery takes place after 6 months post-stroke.13 Furthermore, upper and lower extremity training effects are assessed together, with no specific reference to lower extremity.12 Since training goals and training methods are different for the upper and lower extremities, it might be more beneficial to separate these two.
This systematic review will focus on the clinical effects of two types of control strategies during robotic gait training: device-in-charge and patient-in-charge support. Results will be related to training effects after conventional physical therapy. A specific focus lies on walking ability and balance in chronic stroke survivors, as both aspects are prerequisites for community walking and other Activities of Daily Living (ADL). Outcome measures are the 10 metre walking test (10MWT), 6 minute walking test (6MWT) and Berg Balance Scale (BBS), as these are considered to be clinical tests that provide information about walking independence.14 Clinically relevant training effects will be identified by comparing training results to the Minimal Clinically Important Differences (MCID) found in the literature. These values reflect changes in a clinical outcome measure that is meaningful to the patient.15
Methods
Literature search
A systematic search of articles was conducted in NCBI PubMed, Center for International Rehabilitation Research Information and Exchange (CIRRIE), National Rehabilitation Information Center for Independence REHABDATA, PEDro and Cochrane Controlled Trials register up to 24 June 2015. Keywords included stroke, CVA, cerebral vascular disorders, hemiplegic, training, therapy, treatment, robot, assistive device, assistive technology, training apparatus, interface, gait, balance, walking and locomotion. A detailed description of our search strategy can be found as an online supplement. In addition to searching the databases, the reference lists of relevant publications (i.e. fitting the inclusion criteria or closely related to it) were checked for articles that satisfied the search criteria.
Study selection
Studies were included if they met the following criteria: (1) chronic stroke survivors (>6 months); (2) training with the intention to improve gait function by use of a robotic device that is specifically designed for this purpose; (3) focus on lower limb motor control; (4) use of functional outcome measures that measure human performance as classified by the International Classification of Functioning, Disability and Health; and (5) full-length English publication in a peer-reviewed journal. No limitation was set on the year of publication. As robotic gait training with patient-in-charge support is a new research field, reports of both controlled and uncontrolled trials were included in this review. Studies were excluded if: (1) functional electrical stimulation was used complementary to the intervention; (2) the study was a case report and/or had fewer than five subjects within a subgroup; (3) the training consisted of one single session; or (4) the study was part of a larger trial in which the same subjects were used. The first round of article selection was based on title and abstract. In case of doubt, articles were included into the next round of selection. After full-text selection, results were compared by two reviewers (JAM Haarman and J Reenalda). In case of disagreement, a third reviewer (JS Rietman) was consulted.
Methodological quality judgment
The PEDro scale,16 comprising 11 items (Table 1)17, was used to assess the methodological quality of the included studies. Studies were rated according to three subcategories: external validity (item 1), internal validity (items 2–9) and interpretability (items 10 and 11). Each item scored one point when it could be answered positively. The maximum total score was 11 points. Studies with 4 points or more were considered as having sufficient quality18,19 for further analysis. Methodological quality was assessed by two reviewers independently (JAM Haarman and J Reenalda) and compared afterwards. If no consensus was reached about the final score, a third reviewer (JS Rietman) was consulted.
Table 1.
Methodological quality of the studies. Y = yes (1 point), N = No (0 points),? = Not clarified (0 points).
| 1 = Westlake et al. (2009) | 2 = Hornby et al. (2008) | 3 = Kelley et al. (2013) | 4 = Tanaka et al. (2012) | 5 = Dias et al. (2007) | 6 = Peurala et al. * (2005) | 7 = De Luca et al. (2013) | 8 = Ucar et al. (2014) | 9 = Wu et al. (2011) | 10 = Kubota et al.† (2013) | 11 = Kawamoto et al. (2013) | 12 = Stein et al. (2014) | 13 = Wu et al. (2014) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Eligibility criteria were specified | y | y | y | n | n | n | y | n | n | y | n | y | n |
| 2. Subjects were randomly allocated to groups | y | y | y | y | y | y | n | y | n | n | n | y | y |
| 3. Allocation was concealed | y | y | y | y | y | y | n | n | n | n | n | y | ? |
| 4. The groups were similar at baseline regarding the most important prognostic indicators | y | y | y | y | y | ? | n | n | n | ? | n | y | y |
| 5. There was blinding of all subjects | n | n | n | n | n | n | n | n | n | n | n | n | n |
| 6. There was blinding of all therapists who administered the therapy | n | n | n | n | n | n | n | n | n | n | n | n | n |
| 7. There was blinding of all assessors who measured at least one key outcome | n | n | y | n | y | n | y | y | n | n | n | y | n |
| 8. Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups | y | n | y | y | n | y | y | n | y | y | y | y | y |
| 9. All subjects for whom outcome measures were available received the treatment or control condition as allocated | y | y | y | y | n | y | y | n | y | y | y | y | y |
| 10. The results of between-group statistical comparisons are reported for at least one key outcome | y | y | y | y | y | y | n | y | n | n | y | y | y |
| 11. The study provides both point measures and measures of variability for at least one key outcome | y | y | y | y | y | y | y | n | y | y | y | y | y |
| Total | 8 | 7 | 9 | 7 | 6 | 6 | 5 | 3 | 3 | 4 | 4 | 9 | 6 |
Only the control group and the gait training group were included in the analysis.
Only the stroke patients were included in the analysis.
Note that it was decided to include all items in the total score of the PEDro scale (max = 11 points), instead of leaving the first item out as is usually the case. This was done in order to take all 11 aspects equally into account for the final score, especially as it is not possible with this type of research to comply with the blinding criteria (blinding of patients and blinding of therapist) as robotic devices are used and compared with conventional therapy (no use of robotic devices).
Data extraction
Data were extracted from the studies and categorized into: study design; patient characteristics; intervention; and outcome measures. A section of ‘Robotics’ was included to provide information about the training systems. Categories were formed within this section, on the basis of the type of control strategy that was used: (1) studies assessing robotics with device-in-charge support and (2) studies assessing patient-in-charge support. To relate the results to conventional therapy, a third category was formed in which conventional therapy was assessed. This third category included all patients from the included articles that served as control subjects during the studies.
Outcome measures that were chosen in this review are walking speed, as measured by the 10MWT, endurance, as measured by the 6MWT and balance, as measured by the BBS. These outcome measures are considered to be clinical tests that provide information about walking independence,14 and those activities that are important in performing ADL,20 the main focus point in conventional therapy.14,21
Data analysis
Studies assessing device-in-charge support were compared with studies assessing patient-in-charge robotic support for each outcome measure. Categorization of devices in one group or another was based on the classification that was defined in the Introduction section. In addition, results from the robotic treatment were compared with results from participants who acted as control groups in the included studies. Conventional therapy was often used as a control group in these studies. Mean differences were calculated for each individual study, by using the reported mean for pre- and post-measures. In addition, data were pooled for each control strategy and outcome measure, by using the sample size and mean values of each individual study. Inter-rater agreement after study selection was assessed by Cohen’s kappa. MCID were presented in the literature for two outcome measures for a general population of stroke patients. The reported MCID value for the 10MWT was 0.18 m/s for stroke patients.22 A value of 50 m23 was reported for the 6MWT. No MCID value for stroke survivors on BBS was documented in the literature. The Minimal Detectable Change (MDC) for this outcome measures was reported as 4.66 points.24
Results
Study selection
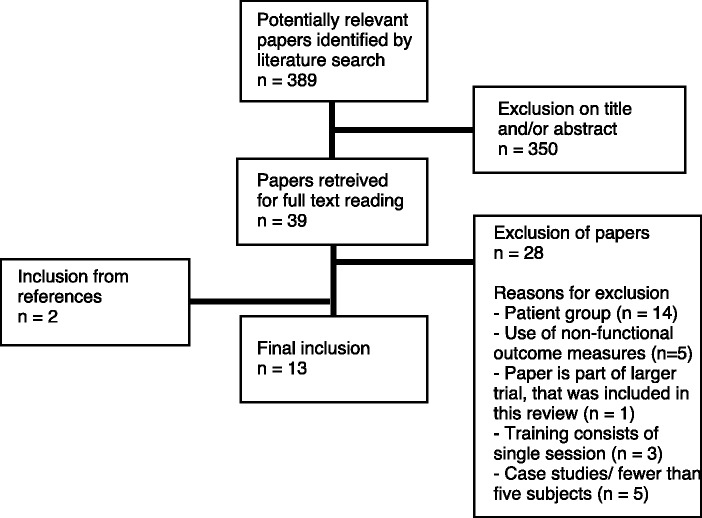
The systematic search strategy resulted in 389 articles. Based on title and abstract, 39 studies were included for full text reading. From these, 14 studies25-38 were found not to include chronic stroke patients. Five studies39-43 were excluded because they did not use functional outcome measures as classified by the International Classification of Functioning, Disability and Health, and one study44 was excluded because it was part of a larger trial already included in this review. Three studies45-47 described training that consisted of just a single session and five studies48-52 were case studies and/or used fewer than five subjects. Searching the references of relevant publications led to two53,54 additional articles. As a result, 13 articles matched the selection criteria;53-65 eight studies assessed device-in-charge support, and five assessed patient-in-charge support. The flow diagram of the article retrieval process is presented in Figure 1. Inter-rater agreement after full-text selection was ‘very good’66 (Cohen’s κ = 0.82). Consensus was reached in all cases of disagreement.
Figure 1.
Flow diagram of article retrieval.
Methodological quality judgement
Methodological quality scores ranged between 3 and 9 points. Details are presented in Table 1. Two studies60,61 were excluded from further analysis in this review because they had a quality score lower than 4. As a result, 11 studies were eligible for data extraction in the present review. Studies assessing robotics with device-in-charge support53-59 had, on average, a quality score of 6.9 ± 1.4 points and studies assessing robotics with patient-in-charge support62-65 had, on average, a quality score of 5.8 ± 2.4 points. The Mann–Whitney test indicated that this difference was non-significant (p = 0.25). Inter-rater agreement assessing the methodological quality of the studies was ‘very good’66 (Cohen’s κ = 0.81). Consensus was reached in all cases of disagreement.
Characteristics of included studies
Study details
All studies had been conducted between 2005 and 2014. Seven studies were identified as randomized controlled trials (RCTs),53-57,64,65 one study as a cross-over trial,58 and three studies were identified as effect studies without a control group.59,62,63 The study by Wu et al.64 was identified as an RCT as it compared two types of robotic training with patient-in-charge support, but it did not include a control group following conventional therapy. Details of the individual studies are presented in Table 2.
Table 2.
Characteristics of the included studies.
| Study |
Patients |
Robotics |
Intervention |
Outcome measures |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author(s) | Study design | Quality score | N | Age in years (mean ± SD) | Time poststroke in years (mean ± SD) | Baseline velocity (m/s) | Apparatus | Body Weight Support (Y/N) | Treadmill training (Y/N) | Interaction with human body | Study duration | Follow-up | Use of 10MWT, 6MWT and/or BBS | Use of other outcome measures |
| Device-in-charge support | ||||||||||||||
| Kelley et al. (2013) | RCT | 9 | E: 11 C: 9 | E: 66,9 ± 8,5 C:64,3 ± 10,9 | E: 3,7 C: 1,4 | E: 0,20 ± 0,10 C: 0,18 ± 0,12 | Lokomat | E: Y C: N | E: Y C: N | Exoskeleton | 150 min/wk for 8 wk Total = 20 h | 3 months | 10MWT, 6MWT | BI, FMA-L, SIS |
| Hornby et al. (2008) | RCT | 7 | E: 27 C: 21 | E: 57 ± 10 C: 57 ± 11 | E: 4,1 ± 4,2 C: 6,1 ± 7,3 | E: 0,59 ± 0,3 C: 0,6 ± 0,33 | Lokomat | E: Y C: Y | E: Y C: Y | Exoskeleton | 90 min/ wk for 4 wk Total = 6 h | 6 months | 6MWT, BBS | FAI, SF36 |
| Westlake et al. (2009) | RCT | 8 | E: 8 C: 8 | E: 58,6 ± 16,9 C: 55,1 ± 13,6 | E: 3,7 ± 2,2 C: 3,1 ± 1,7 | E: 0,87 ± 0,55 C: 0,72 ± 0,37 | Lokomat | E: Y C: Y | E: Y C: Y | Exoskeleton | 90 min/wk for 4 wk Total = 6 h | _ | 6MWT,BBS | SPPB, FMA-L, LLFDI |
| Tanaka et al. (2012) | Cross-Over | 7 | E1: 7 E2: 5 | E1: 63 ± 10 E2: 60 ± 8,5 | E1: 4,6 ± 3,1 E2: 5,5 ± 5,6 | E1: 0,75 ± 0,42 E2: 0,86 ± 0,16 | Gait-Master4 | E: N C: N | E: N C: N | End-Effector | 40 min/wk for 6 wk Total = 4 h | 1 month | 10MWT | TUG |
| Dias et al. (2007) | RCT | 6 | E: 20 C: 20 | E: 70,4 ± 7,4 C: 68,0 ± 10,7 | E: 3,9 ± 5,3 C: 4,0 ± 2,5 | E: 0,42 ± 0,25 C: 0,53 ± 0,33 | Gait Trainer (GT1) | E: Y C: N | E: N E: N | End-Effector | 216 min/wk for 5 wk Total = 18 h | 3 months | 10MWT, 6MWT | TMS, RMI, FMA-L, MI |
| Peurala et al. (2005) | RCT | 6 | E: 15 C: 15 | E: 51,2 ± 7,9 C: 52,3 ± 6,8 | E: 2,4 ± 2,6 C: 4,0 ± 5,8 | E: 0,25 ± 0,28 C: 0,25 ± 0,39 | Gait Trainer | E: Y C: N | E: N C: N | End-Effector | 100 min/wk for 3 wk Total = 5 h | 6 months | 10MWT, 6MWT | MMAS, FIM |
| De Luca et al. (2013) | Before/After | 5 | E: 6 | E: 56,6 ± 13,2 | E: 5,1 ± 2,7 | E: 0,41 ± 0,04 | G-EO System | E: N | E: N | End-Effector | 225 min/wk for 4 wks Total = 15 h | _ | 10MWT, 6MWT | TUG |
| Patient-in-charge support | ||||||||||||||
| Kawamoto et al. (2013) | Before/After | 4 | E: 15 | E: 61 ± 14,8 | E: 3.9 ± 3,1 | E: 0,41 ± 0,26 | HAL (Hybrid Assistive Limb) | E: Y | E: N | Exoskeleton | 50 min/wk for 8 wk Total = 6,6 h | _ | 10MWT, BBS | TUG, |
| Kubota et al. (2013) | Before/After | 4 | E: 9 | E: 56,8 ± 15,9 | E: 6,4 ± 6,3 | E: 0.39 ± 0.37 | HAL (Hybrid Assistive Limb) | E: Y | E: N | Exoskeleton | 40 min/wk for 8 wk Total = 5.3 h | _ | 10MWT,BBS | TUG |
| Stein et al. (2014) | RCT | 9 | E: 12 C: 12 | E: 57,6 ± 10,7 C: 56,6 ± 15,1 | E: 4,1 ± 3,2 C: 7,4 ± 12,8 | E: 0,44 ± 0,45 C: 0,36 ± 0,47 | RLO (Robotic Leg Orthosis) | E: N C: N | E: N C: N | Exoskeleton | 150 min/wk for 6 wk Total = 15 h | 1 month, 3 months | 10MWT, BBS, 6MWT | FTSTS, TUG, CAFE40, Romberg |
| Wu et al. (2014) | RCT* | 6 | E1: 14 E2: 14 | E1: 53,6 ± 8,9 E2: 57,4 ± 9,8 | E1: 7,3 ± 5,6 E2: 7,1 ± 6,0 | E1: 0,65 ± 0,38 E2: 0,72 ± ,036 | Cable-Driven Robotic Gait Trainer | E: Y | E: Y | End-Effector | 135 min/wk for 6 wk Total = 13.5 h | 2 wk | 10MWT, BBS, 6MWT | MAS, ABC, SF-36 |
E: experimental group; C: control group; BI: Barthel Index; FMA-L: Fugl Meyer Assessment Lower Extremity; SIS: Stroke Impact Scale; FAI: Frenchay Activity Index; SF-36: Short Form 36; SPPB: Short Physical Performance Battery; LLFDI: Late Life Function and disability Instrument; TUG: Timed Up and Go Test; TMS: Toulouse Motor Scale; RMI: Rivermead Mobility Index; MI: Motricity Index; MMAS: Modified Motor Assessment Scale; FIM: Functional Independence Measure; FTSTS: Five Times Sit-to-Stand; Romberg: Romberg’s Test; CAFE40: California Functional Evaluation; MAS: Modified Ashworth Scale; ABC: Activities-specific Balance Confidence.
Two experimental groups were compared with each other in this study.
Patient characteristics
The number of subjects within the included studies ranged between 6 and 48. The cumulative number of subjects in the studies assessing device-in-charge support was 99, and in the studies assessing patient-in-charge was 64. The pooled mean age was 60.6 years in the device-in-charge group and 57.4 years in the patient-in-charge group. Time since stroke onset ranged between 1.4 and 7.4 years, with a pooled mean of 4.6 years (pooled mean of 3.9 years for the studies assessing device-in-charge support and a pooled mean of 5.7 years for the studies assessing patient-in-charge support). The baseline level indicated by walking speed varied among the individual studies between 0.18 m/s and 0.87 m/s, with a pooled mean of 0.51 m/s (pooled mean of 0.50 m/s for all studies assessing device-in-charge support and a pooled mean of 0.53 m/s for all studies assessing patient-in-charge support). Note that all pooled mean values are based on the mean values reported in the included studies. Training speed was based on individual baseline speeds and the progression that was made by each patient individually. Individual study details are presented in Table 2.
Robotics
Seven types of robotic devices were used across the 11 studies. Four devices used a control strategy with device-in-charge support: Lokomat, GaitMaster4, Gait Trainer and G-EO system. All four types of devices imposed a pre-programmed, high-stiffness, reference trajectory onto the lower legs of the subject.55,56,67-70 This position-controlled pattern could either be imposed on the entire leg (exoskeleton; Lokomat) or only on the feet of the patient (end-effector; GaitMaster4, Gait Trainer, G-EO system). The level of guidance force does not depend on user input. Three devices used a control strategy with patient-in-charge support, in which the movement of the device is driven by the subject: Hybrid Assistive Limb (exoskeleton), Robotic Leg Orthosis (exoskeleton), Cable-Driven Robotic Gait Trainer (end-effector). The level of guidance force is variable within a training session and depends on user input.
Device-in-charge robotic support
Lokomat
Lokomat consists of a powered gait orthosis with linear actuators at the hip and knee joints, a treadmill and a Body Weight Support (BWS) system.71 The device is attached to the patient at the location of the trunk, pelvis, upper and lower legs. Hip and knee joints of the Lokomat are aligned with the corresponding joints of the patient. Elastic straps are used to assist toe clearance. Lokomat is position controlled and imposes a pre-programmed reference trajectory to the lower legs of the patient, based on the walking pattern of a general group of healthy subjects.55 No deviations from this reference trajectory are allowed by the device. The reference trajectory can be scaled to match patient characteristics such as step length.
GaitMaster4
GaitMaster4 is a footpad-type training device, each footpad having 2 Degrees-of-Freedom (DoF).67 Back and forth movements are allowed by use of a slider crank mechanism. A movable linear actuator at the end of the mechanism allows for up and down movements of the feet. Patients are therefore able to walk without the use of a treadmill. A double-hinge mechanism is used between foot and footpad such that plantar and dorsal flexion of the foot is possible. GaitMaster4 is position controlled: it uses a pre-defined reference trajectory, based on the movement trajectory of healthy subjects (pre-recorded with a motion capture system). In contrast to Lokomat, GaitMaster4 only takes the foot trajectory into account and not the trajectory of the entire leg. This is done under the assumption that restrictions in the movement of the feet lead to restrictions (to some extent) in the movement of the rest of the legs (due to restrictions in the range of motion of human joints). Furthermore, the reference trajectory of GaitMaster4 is adjustable to match specific (physical) patient characteristics. No BWS is used in this training set-up.
Gait Trainer
Gait Trainer is also a high-stiffness, position-controlled, footpad-type training device, based on a commercially available fitness trainer (Fast Track).72 Gait Trainer consists of two footplates that are positioned on two bars, two rockers and two cranks that provide propulsion. Crank propulsion and rocker dimensions are chosen such that they mimic foot movements during stance and swing without the use of a treadmill. To adapt the device to individual (physical) patient characteristics, such as stride length and phase, gear sizes can be varied. Gait Trainer additionally controls the centre of mass of the patient. This is done by connecting the planetary gear system (with, among others, a vertical and horizontal crank) to the waist of the patient. The repetitive movements and circular motions of the footplates are thus (directly) used to constrain the movements of the centre of mass of the subject. Note that the centre of mass oscillates sinusoidally in the vertical and horizontal directions during normal gait, meaning that additional parts, such as transmission gears, are needed for the correct transmission of one subsystem to another. A BWS system is used to secure the subject in the training device.
G-EO
G-EO is similar to the above two devices: a footpad-type training device (end-effector), and also consists of two footplates, connected to a pivoting arm and two moving sledges.34 Each footplate has 3 DoF and again, no treadmill is needed in this training set-up. The patient’s centre of mass is controlled in the vertical and horizontal directions, not as a direct consequence of the footpad motions as is the case with Gait Trainer, but its trajectory is programmable by the computer. G-EO is position controlled and uses reference trajectories of the feet and the centre of mass of healthy subjects’ data reported in the literature. The device has a BWS system (though not used in the study by De Luca et al.59).
Note that both the Gait Trainer and the G-EO system introduce a mode in which the patient can control the speed of the device when the active contribution of the patient is above a selected threshold. However, this mode is only used in the study by Dias et al.53 As only the speed of the device is adjustable and not the amount and the timing of the support, this device is categorized in the group of robotics providing device-in-charge support.
Patient-in-charge robotic support
Hybrid Assistive Limb
Hybrid Assistive Limb (HAL) is an exoskeleton that is attached to the pelvis and lower limbs of the subject.73 The joints of the exoskeleton are aligned to the joints of the patient, and both hip and knee joints of the device are actuated (torque controlled). HAL is able to measure several bioelectric signals such as muscle activity (by skin surface electromyography (EMG) electrodes placed over muscles for hip and knee flexion and extension), ground reaction forces (from force pressure sensors in the shoes, to measure weight shift) and joint angles that are generated by the patient. In a complete voluntary mode, HAL uses the patient’s EMG signals (which are believed to represent the patient’s intentions) to assist the patient, so that the patient is able to execute the desired movements. HAL thus interactively provides motion assistance to the patient. The amount of assistive force is adjustable (in a configuration mode, before the start of a training session) to meet the individual needs of the patient and variable within a gait cycle (swing/stance phase). Torque signals are used as input signal to control for the desired output level of mechanical impedance; for instance, by keeping the impedance low when no support from HAL is needed in a specific phase. When patients have little muscle activity (meaning that the system is not able to identify user intentions based on EMG signals), ground reaction forces and joint angles are additionally used to support the movements of a patient. In that case, user intentions are inferred from comparison of ground reaction force and joint angles to a reference pattern recorded from healthy subjects. This mode is available for both legs and an entire training session, but can also be used for a specific interval or on one leg or one joint only. Both Kawamoto et al.63 and Kubota et al.62 used this mode for several patients in their study. HAL can be used overground without a treadmill. A movable BWS system is used to secure the patient while walking.
Robotic Leg Orthosis
Robotic Leg Orthosis (RLO) is an exoskeleton and is attached to the upper and lower leg of a patient so that sensing (angle and torque sensors) and actuation is possible at the knee.51 A foot (force) sensor with ankle support is added to the orthosis to measure ground reaction forces and weight shift. Two small motors (one high-torque/low-speed motor and one low-torque/high-speed motor) are incorporated in the exoskeleton. Like HAL, this device can be used overground. Each individual user needs to configure the system to set up subject specific settings (i.e. set up the torque limits, range of motion limits and tuning parameters). After that, the system is ready to be used. The control algorithm combines this configuration information with real-time information of angle, torque and force to detect the movement intentions of the subject; for instance, is the knee flexed or extended, is the weight on the ball or the heel of the foot? Then, support is given to the leg (with high-torque/low-speed motor), with the amount of support depending on the output of the control algorithm. Support is only given to the subject during stance phase, no actuation is provided to the leg during the swing phase, thereby not impeding the patient’s movements (low-torque/high-speed motor is used to be able to monitor the movements, yet allow free-swinging of the leg). The patient is thus always in control of the system, as he or she has to initiate the movement. Moreover, configuration settings can be adjusted as the patient improves. No BWS system is used during training with this device.
Cable-Driven Robotic Gait Trainer
The Cable-Driven Robotic Gait Trainer (CaLT) is a training device that attaches four cables, driven by four motors, pulleys and cable spools to the ankles of the patient.74 Each ankle is thus connected to two cables: one anterior cable that pulls on the ankle (assisting the movement, i.e. making the movement easier) and one posterior cable that pulls on the ankle (resisting the movement, i.e. making the movement harder). While walking on a treadmill, custom 3D position sensors at the ankle are used to record kinematics (rotational angular and linear position), and the measured data are used as input for the control algorithm. This algorithm compares the measured ankle trajectory with a reference (ankle) trajectory that is recorded in healthy subjects. The tolerance level of deviation between the desired (reference) and the measured path of the ankle is adjustable for each individual patient and training session. The control algorithm determines whether the patient needs assistance (anterior cable pulls on the ankle) or resistance (posterior cable pulls on the ankle) in its movements. Study results published by Wu et al.74 suggest that the cable system is highly backdrivable, not impeding the movements of the patients at times that this is not desired. A BWS system is used during training with this device.
As mentioned previously, not all studies used BWS during the training, nor did all studies use a treadmill to assist the training. In total, eight studies used BWS53-57,62-64 and four studies used a treadmill.55-57,64 The level of BWS that was used was patient specific but often as low as possible (the highest reported value was 40%). The level of BWS was decreased as patients progressed through the various training sessions. Additional information about the robotic devices is listed in Table 2.
Intervention
Table 2 shows that training duration ranged between 3 and 8 weeks, with a total training time of 4–20 hours. The studies assessing device-in-charge support had, on average, a training duration of 10.6 hours, and the studies assessing patient-in-charge support had an average training duration of 10.1 hours.
Seven of the studies included a follow-up measurement. Of these, three studies performed a short-term (≤2 months) follow-up measurement and five studies performed a long-term (>2 months) follow-up measurement. Both long-term and short-term follow-up measurements were performed in the study reported by Stein et al.65
Outcome measures
Nine studies used the 10MWT as the outcome measure; eight used the 6MWT and six studies used the BBS. A total of 18 additional outcome measures were used among the various studies and these are listed in Table 2.
Walking speed (10MWT)
The 10MWT was used as an outcome measure in five studies that assessed device-in-charge support (see Table 3). The pooled mean difference between pre and post training was 0.09 m/s (n = 64 patients). Four studies (five experimental training groups) assessing patient-in-charge support used the 10MWT as the outcome measure, resulting in a pooled mean increase of 0.05 m/s (n = 64 patients). The studies assessing conventional therapy demonstrated a pooled mean difference between pre and post measurement of 0.04 m/s (n = 48 patients). Wide deviations are observed in the individual data within each subcategory, ranging from –0.07 m/s to +0.14 m/s. The greatest improvement was obtained by Tanaka et al.58 with GaitMaster4, providing device-in-charge support (increase of 0.14 m/s). The second highest increases of 0.11 m/s were obtained by Dias et al.53 with Gait Trainer (device-in-charge support) and Wu et al.64 with CaLT-assistive-support (patient-in-charge support). The lowest improvement was obtained by Stein et al.65 with RLO, providing patient-in-charge support (decrease of 0.07 m/s).
Table 3.
(Pooled) mean differences between pre and post measurements for gait speed (10MWT).
| Group size | Pre (mean (±std), m/s) | Post (mean (±std), m/s) | Mean difference (m/s) | |
|---|---|---|---|---|
| Device-in-charge support | ||||
| Kelley | 11 | 0.20 (± 0.10) | 0.20 (± 0.10) | 0.00 |
| Tanaka* | 12 | 0.84 (N.D.) | 0.98 (N.D.) | 0.14 |
| Peurala | 15 | 0.25 (± 0.28) | 0.33 (± 0.42) | 0.08 |
| De Luca | 6 | 0.39 (± 0.37) | 0.49 (± 0.05) | 0.10 |
| Dias* | 20 | 0.42 (± 0.25) | N.D. (N.D.) | 0.11 |
| Pooled data | 64 | 0.42 | N.C. | 0.09 |
| Patient-in-charge support | ||||
| Kubota | 9 | 0.39 (± 0.37) | 0.46 (± 0.40) | 0.07 |
| Kawamoto | 15 | 0.41 (± 0.26) | 0.45 (± 0.24) | 0.04 |
| Stein | 12 | 0.44 (± 0.50) | 0.36 (± 0.47) | –0.07 |
| Wu - assistance group | 14 | 0.65 (± 0.38) | 0.76 (± 0.45) | 0.11 |
| Wu - resistance group | 14 | 0.72 (± 0.36) | 0.82 (± 0.39) | 0.10 |
| Pooled data | 64 | 0.53 | 0.58 | 0.05 |
| Conventional therapy | ||||
| Kelley | 9 | 0.18 (± 0.12) | 0.27 (± 0.27) | 0.09 |
| Tanaka | 12 | 0.80 (± 0.13) | N.D. (N.D.) | –0.03 |
| Peurala | 15 | 0.25 (± 0.39) | 0.31 (± 0.63) | 0.06 |
| Stein | 12 | 0.49 (± 0.67) | 0.52 (± 1.10) | 0.03 |
| Pooled data | 48 | 0.44 | N.C. | 0.04 |
Only the data that were presented in the paper are presented in the table.
N.D. (No Data) This value was not presented in the paper.
N.C. (Not Calculated) This value could not be calculated due to missing data.
The pooled mean baseline level was found to be higher for the studies assessing patient-in-charge support, compared with the studies assessing either conventional therapy or therapy with device-in-charge support. None of the three categories indicated a pooled mean difference that represented a clinically relevant improvement as indicated by the MCID.
Endurance (6MWT)
The 6MWT was an outcome measure cited in six studies assessing device-in-charge support (see Table 4). The pooled mean difference was 17 m for this category (n = 86 patients). Two studies (involving three experimental training groups) assessing patient-in-charge support analysed this outcome measure. Also, a pooled mean increase of 17 m was observed in these studies (n = 40 patients). Conventional therapy demonstrated a pooled mean increase of 22 m (n = 85 patients). The greatest improvement after training was obtained by Hornby et al.,56 in a conventional therapy group (increase of 34.0 m). This was followed by De Luca et al.59 with GEO (device-in-charge support; increase of 33.2 m) and Stein et al.65 with RLO (patient-in-charge support; increase of 27.5 m). The lowest improvement was also obtained in the conventional therapy group, reported by Westlake et al.55 (decrease of 21.9 m).
Table 4.
(Pooled) mean differences between pre and post measurements for endurance (6MWT).
| Group size | Pre (mean (±std), m) | Post (mean (±std), m) | Mean difference (m) | |
|---|---|---|---|---|
| Device-in-charge support | ||||
| Westlake | 8 | 267.30 (±187.20) | 278.10 (±176.50) | 10.80 |
| Hornby | 27 | 170.00 (±86.00) | 186.00 (±88.00) | 16.00 |
| Kelley | 11 | 53.94 (±30.53) | 57.02 (±25.50) | 3.08 |
| Peurala | 14 | 152.30 (±89.60) | 177.50 (±111.5) | 25.20 |
| De Luca | 6 | 196.00 (±53.8) | 229.20 (±64.90) | 33.20 |
| Dias* | 20 | 140.20 (±90.07) | N.D. (N.D.) | 18.92 |
| Pooled data | 86 | 156.21 | N.C. | 17.24 |
| Patient-in-charge support | ||||
| Stein | 12 | 185.90 (±95.90) | 213.40 (±108.20) | 27.50 |
| Wu – assistance group | 14 | 177.40 (±99.90) | 197.50 (±109.50) | 20.10 |
| Wu – resistance group | 14 | 201.00 (±84.00) | 207.00 (±80.00) | 6.00 |
| Pooled data | 40 | 188.21 | 205.60 | 17.39 |
| Conventional therapy | ||||
| Westlake | 8 | 234.30 (±141.20) | 212.40 (±113.50) | −21.90 |
| Hornby | 21 | 170.00 (±86.00) | 204.00 (±96.00) | 34.00 |
| Kelley | 9 | 48.77 (±21.04) | 70.26 (±60.4) | 21.49 |
| Peurala | 15 | 111.80 (±57.30) | 135.10 (±67.9) | 23.30 |
| Stein | 12 | 169.30 (±89.70) | 194.80 (±83.2) | 25.50 |
| Dias* | 20 | 141.48 (±102.22) | N.D. (N.D.) | 23.28 |
| Pooled data | 85 | 146.14 | N.C. | 21.80 |
Only the data that were presented in the paper are presented in the table.
N.D. (No Data) This value was not presented in the paper.
N.C. (Not Calculated) This value could not be calculated due to missing data.
Again, the baseline level for the studies assessing patient-in-charge support was found to be higher than for the other two categories. Individual study results did not demonstrate improvements that were indicated to be clinically relevant by the MCID.
Balance (BBS)
BBS was assessed in three studies that used device-in-charge support (see Table 5). The pooled mean difference between pre and post measurement was 2.1 points for these studies (n = 55 patients). Five studies assessing patient-in-charge support measured this outcome measure. The pooled mean difference was found to be 3.1 points (n = 64 patients). Conventional therapy demonstrated an increase of 2.3 points (n = 61 patients). The greatest improvement after training was obtained by Kubota et al.62 with HAL (patient-in-charge support, increase of 5.4 points). This was followed by Kawamoto et al.,63 also using HAL (patient-in-charge support, increase of 4.8 points). The lowest improvement was obtained by Stein et al.,65 in the conventional therapy group (decrease of 0.2 points).
Table 5.
(Pooled) mean differences between pre and post measurements for balance (BBS).
| Group size | Pre (mean (±std), points) | Post (mean (±std), points) | Mean difference (points) | |
|---|---|---|---|---|
| Device-in-charge support | ||||
| Westlake | 8 | 46.90 (±7.50) | 48.30 (±6.80) | 1.40 |
| Hornby | 27 | 43.00 (±10.00) | 44.00 (±10.00) | 1.00 |
| Dias* | 20 | 36.85 (±6.53) | N.D. (N.D.) | 3.90 |
| Pooled data | 55 | 41.33 | N.C. | 2.11 |
| Patient-in-charge support | ||||
| Kubota | 9 | 36.30 (±15.70) | 41.70 (±8.80) | 5.40 |
| Kawamoto | 15 | 40.60 (±13.60) | 45.40 (±8.02) | 4.80 |
| Stein | 12 | 46.10 (±11.70) | 48.60 (±10.20) | 2.50 |
| Wu - assistance group | 14 | 43.60 (±9.00) | 45.50 (±8.80) | 1.90 |
| Wu - resistance group | 14 | 44.10 (±8.80) | 45.60 (±9.30) | 1.50 |
| Pooled data | 64 | 42.45 | 45.55 | 3.10 |
| Conventional therapy | ||||
| Westlake | 8 | 47.00 (±7.00) | 51.00 (±5.40) | 4.00 |
| Hornby | 21 | 42.00 (±10.00) | 44.00 (±11.00) | 2.00 |
| Stein | 12 | 49.40 (±5.80) | 49.20 (±5.80) | −0.20 |
| Dias* | 20 | 34.60 (±13.85) | N.D. (N.D.) | 3.42 |
| Pooled data | 61 | 41.69 | N.C. | 2.30 |
Only the data that were presented in the paper are presented in the table.
N.D. (No Data) This value was not presented in the paper.
N.C. (Not Calculated) This value could not be calculated due to missing data.
Studies assessing device-in-charge support, patient-in-charge support and conventional therapy all demonstrated baseline levels that were of the same order of magnitude. Note, however, a score below 45 points indicates high fall risk. No MCID values were known for this outcome measure, but results of the individual studies could be compared with the MDC value. The two studies62,63 that used the HAL device demonstrated training effects larger than was indicated by the MDC value.
Discussion
The goal of this systematic review was to investigate the effects of ‘device-in-charge’ versus ‘patient-in-charge’ gait training on walking ability and balance in chronic stroke survivors. Devices were categorized based on the method they used to provide support to the patients: training by use of patient-in-charge support uses the principle of error-based training. Patients are challenged to learn from their mistakes as the robot only interferes with/supports the patient at times when that is needed. Otherwise, patients are in control of their own movements. This is opposite to device-in-charge support, which does not use this principle and provides the patients with continuous support throughout a training session. No preference for one type of control strategy over another was found in this review. In addition, training effects of individual robotic devices were compared with each other. Again, no preference for one device over another was found in the general sense. However, interesting observations regarding the training intensity and the effect of baseline levels were found that inform the conclusions in this review.
None of the studies indicates clinically relevant improvements, as indicated by the MCID (expect for HAL in terms of MDC for balance). The most important aspect affecting the interpretation of the results in this review is the short training duration in all the individual studies: training duration varied between 4 hours58 and 20 hours.57 Time post-stroke was, on average, 4.6 years for the included patients; thus it is questionable whether significant and relevant training effects would or could be observed after only short periods of training. Therefore, it is likely that training duration was too short,6 especially since the literature7,75,76 indicates that physical effort, and its interaction with training duration and frequency, affects training outcome to a great extent. Physical effort describes the effort it takes for patients to execute and complete a training session. The articles included in this review only mention generally that treadmill speed was adapted to the progress of individual patients, but they do not provide details on the physical effort of patients, in terms of (for instance) oxygen uptake or Heart Rate Reserve (HRR).75 The ideal combination between physical effort, duration and frequency depends on the baseline characteristics of the patient and should be adapted when the patient improves.7,77
In addition, the baseline level of patients is of great importance in predicting training outcome. It is an indicator for the severity of the disorder and the ability of stroke survivors to recover. Patients in the studies assessing patient-in-charge support generally had higher baseline velocity (10MWT) and endurance (6MWT) levels than patients in the studies assessing device-in-charge support and conventional therapy. Yet all groups showed the same training effects (even though no clinical relevant results were observed). Physical effort in the form of the control strategy of the robotic device might have affected this: patients with lower baseline levels (indicating low walking ability) might have benefitted from device-in-charge support, as this requires less physical effort from the patient, whereas patients with a higher baseline level might have benefitted from patient-in-charge support, as this requires higher physical effort.
In terms of balance (BBS), no difference in baseline level was observed between the three categories. However, only studies using the HAL device demonstrated improvements that were larger than was indicated by the MDC values (even though this does not automatically indicate a clinically significant improvement, and BBS still indicates a high fall risk in one study). The fact that devices within a category do not all use the same working principle might support this observation. For example, the CaLT uses a reference trajectory of healthy subjects and defines a band around this path in which the patient can move freely.74 The HAL uses the patient’s EMG signals, which are believed to represent the patient’s movement intentions.73 Both devices provide patient-in-charge support, but the way that assistance is provided varies widely. Furthermore, some evidence is found that the mechanical design of the devices affects training outcome in terms of independent walking.78-80 A distinction is made between ‘end effectors’, that only control the trajectory of a specific end point of the legs (e.g. the position of the patient’s feet), and ‘exoskeletons’, that control the trajectory of the entire lower leg. This review does not take this difference into account: end effectors in which the distal joints are exposed to a pre-defined trajectory are classified in the category of device-in-charge support: patients are not empowered and the working principle of physical therapists is not taken into account. However, the difference in the control of legs during the training might affect the way that balance is trained.
Many studies used BWS and/or a treadmill during the training sessions. Note that both are attributes of the device and the training setting rather than of the control strategy. Mehrholz et al.81 reported on this topic and its training effects in terms of walking independence, and did not find evidence that training with BWS in stroke rehabilitation is more effective than training without BWS. Moreover, treadmill training (whether with or without BWS) was also not found to be more effective than training without a treadmill in terms of regaining walking independence.
Although individual results in this study do not seem to show a clear benefit of one type of training device over another, this does not mean that no differences in terms of training effects exist between them. The factors mentioned previously might have contributed to this, but other factors, such as the motivation of the patient, or the use of (bio-) feedback might also be of importance. Therefore, it would be useful if future research further categorizes the robotic devices, instead of using only two categories as is done currently. This would provide more information on the precise type of interaction of the device and the patient. It would eliminate the effects of the variability in control strategies and mechanical designs among the robotic devices within the current categories. In addition, measuring EMG might be a useful tool to classify the systems, as it provides more insight into the actual training that is provided by the robotic device (amount of assistance) and the amount of contribution that is required from the patient during the training session.
Moreover, it is very important to classify the baseline level of patients, not only to identify the severity of the impairment and the ability to recover, but also to determine the most suitable training session for patients in terms of physical effort, training duration and frequency. Pang et al.82 indicated in a systematic review that stroke patients with moderate baseline characteristics and low cardiovascular risk should train at 40–50% of their HRR (progressing to 60–80% of their HRR) for 20–40 minutes and 3–5 times per week in order to improve their walking speed and endurance. Patients at higher risk should decrease their HRR to 30%.83 Furthermore, it might be useful for such patients to include short 30-second bursts of rest during the training session.75 These numbers must be interpreted as guidelines rather than prescriptive numbers, but they do indicate that each training session must be adapted to the individual needs of a patient and the specific training environment: for instance, to determine a suitable treadmill speed during training. Moreover, these guidelines could additionally be used to classify the progress of patients.
Study limitations
Although this systematic review was conducted with care, there were some potential limitations. Even though the methodological quality was not statistically significantly different between the categories, the categories included both high and low-quality studies, In general, the methodological quality of the included studies was moderate. A low methodological quality indicates that the internal validity, external validity and interpretability of a study are low according to the PEDro scale.
No statistical tests could be performed on the data that were collected for this review, as no standard deviation for the paired difference could be calculated. This would require all the individual patient data from each study, which were not available. However, results from the data analysis in this review still provide insight into the effects of various control strategies: the analysis demonstrates the current status of research on this topic and enables the reader to appreciate the clinical relevance of the results. Furthermore, many studies that were included in this review only included a pre/post measurement with an experimental group but did not include a control group. The low number of RCTs was the reason that no meta-analysis could be performed in this review. Stronger conclusions could have been drawn if there had been more RCTs.
Conclusion
This systematic review investigated training effects in terms of walking ability and balance, after robotic gait training with ‘device-in-charge support’ and after ‘patient-in-charge support’, for chronic stroke survivors. This classification focuses on the effects of training with using devices that follow the principle of error-based training and thus ‘empower’ the patient (patient-in-charge support) in their movement during gait-training and training with devices that do not (device-in-charge support).
No differences were found between the two control strategies, in terms of training effects. All primary outcome measures demonstrated small differences that were not considered clinically relevant, as indicated by the MCID. In addition, training effects for individual robotic devices also did not indicate a preference for one type of device over the other. An important confounder affecting the results in this review was the short training duration among all included studies, specifically in relation to the long time post-stroke of the included patients. Also, the included studies did not objectify the physical effort of the patients during the training sessions. Future research should focus on these aspects: baseline levels of patients should be identified and the training (in terms of, among others, control strategy, training duration, frequency and intensity) should be adapted to that level in order to obtain training conditions that suit the patient.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a research grant from ZonMw (grant number: 10-10400-98-005, http://www.zonmw.nl/nl/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hidler J, Sainburg R. Role of robotics in neurorehabilitation. Top Spinal Cord Inj Rehabil 2011; 17: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diedrichsen J, White O, Newman D, et al. Use-dependent and error-based learning of motor behaviors. J Neurosci 2010; 30: 5159–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidler RD, Kwak Y, Fling BW, et al. Neurocognitive mechanisms of error-based motor learning. Adv Exp Med Biol 2013; 782: 39–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martini EM, Garrett N, Lindquist T, et al. The boomers are coming: A total cost of care model of the impact of population aging on health care costs in the United States by Major Practice Category. Health Serv Res 2007; 42: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truelsen T, Piechowski-Jozwiak B, Bonita R, et al. Stroke incidence and prevalence in Europe: A review of available data. Eur J Neurol 2006; 13: 581–598. [DOI] [PubMed] [Google Scholar]
- 6.Kwakkel G. Intensity of practice after stroke: More is better. Schweizer Archiv Neurol Psychiatrie 2009; 160: 295–298. [Google Scholar]
- 7.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med 1986; 3: 346–356. [DOI] [PubMed] [Google Scholar]
- 8.Pennycott A, Wyss D, Vallery H, et al. Towards more effective robotic gait training for stroke rehabilitation: A review. J Neuroeng Rehabil 2012; 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung J, Malouin F, McFadyen BJ, et al. Locomotor rehabilitation in a complex virtual environment. Conf Proc IEEE Eng Med Biol Soc 2004; 7: 4859–4861. [DOI] [PubMed] [Google Scholar]
- 10.Duschau-Wicke A, von Zitzewitz J, Caprez A, et al. Path control: A method for patient-cooperative robot-aided gait rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2010; 18: 38–48. [DOI] [PubMed] [Google Scholar]
- 11.Cai LL, Fong AJ, Otoshi CK, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci 2006; 26: 10564–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil 2009; 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaechter JD. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol 2004; 73: 61–72. [DOI] [PubMed] [Google Scholar]
- 14.van de Port IG, Kwakkel G, Lindeman E. Community ambulation in patients with chronic stroke: How is it related to gait speed? J Rehabil Med 2008; 40: 23–27. [DOI] [PubMed] [Google Scholar]
- 15.Cook CE. Clinimetrics corner: The Minimal Clinically Important Change Score (MCID): A necessary pretense. J Man Manip Ther 2008; 16: E82–E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhogal SK, Teasell RW, Foley NC, et al. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J Clin Epidemiol 2005; 58: 668–673. [DOI] [PubMed] [Google Scholar]
- 17.Physiotherapy Evidence Database (PEDro). PEDro scale. 1999.
- 18.Peppen RPS van KG, Harmeling-van der Wel BC, Kollen BJ, et al. KNGF Clinical Practice Guideline for physical therapy in patients with stroke. Review of the evidence. [Translation 2008]. Nederlands Tijdschrift voor Fysiotherapie. 2004, p.114. [Google Scholar]
- 19.Mazerolle SM, Ganio MS, Casa DJ, et al. Is oral temperature an accurate measurement of deep body temperature? A systematic review. J Athl Train 2011; 46: 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Port IG, Wood-Dauphinee S, Lindeman E, et al. Effects of exercise training programs on walking competency after stroke: A systematic review. Am J Phys Med Rehabil 2007; 86: 935–951. [DOI] [PubMed] [Google Scholar]
- 21.Fulk GD, Reynolds C, Mondal S, et al. Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil 2010; 91: 1582–1586. [DOI] [PubMed] [Google Scholar]
- 22.Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke 1995; 26: 982–989. [DOI] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 24.Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil 2012; 93: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson A, Vreede KS, Haglund V, et al. Gait training early after stroke with a new exoskeleton – the hybrid assistive limb: A study of safety and feasibility. J Neuroeng Rehabil 2014; 11: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochi M, Wada F, Saeki S, et al. Gait training in subacute non-ambulatory stroke patients using a full weight-bearing gait-assistance robot: A prospective, randomized, open, blinded-endpoint trial. J Neurol Sci 2015; 353: 130–136. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, Yang L, Park IJ, et al. Effects of innovative WALKBOT robotic-assisted locomotor training on balance and gait recovery in hemiparetic stroke: A prospective, randomized, experimenter blinded case control study with a four-week follow-up. IEEE Trans Neural Syst Rehabil Eng 2015; 23: 636–642. [DOI] [PubMed] [Google Scholar]
- 28.van Nunen MP, Gerrits KH, Konijnenbelt M, et al. Recovery of walking ability using a robotic device in subacute stroke patients: A randomized controlled study. Disabil Rehabil Assist Technol 2015; 10: 141–148. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H, Tanaka N, Inuta T, et al. Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients – a randomized controlled pilot study. Arch Phys Med Rehabil 2014; 95: 2006–2012. [DOI] [PubMed] [Google Scholar]
- 30.Chisari C, Bertolucci F, Monaco V, et al. Robot-assisted gait training improves motor performances and modifies motor unit firing in post-stroke patients. Eur J Phys Rehabil Med 2015; 51: 59–69. [PubMed] [Google Scholar]
- 31.Chang WH, Kim MS, Huh JP, et al. Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: A randomized controlled study. Neurorehabil Neural Repair 2012; 26: 318–324. [DOI] [PubMed] [Google Scholar]
- 32.Fisher S, Lucas L, Thrasher TA. Robot-assisted gait training for patients with hemiparesis due to stroke. Top Stroke Rehabil 2011; 18: 269–276. [DOI] [PubMed] [Google Scholar]
- 33.Hesse S, Tomelleri C, Bardeleben A, et al. Robot-assisted practice of gait and stair climbing in nonambulatory stroke patients. J Rehabil Res Dev 2012; 49: 613–622. [DOI] [PubMed] [Google Scholar]
- 34.Hesse S, Waldner A, Tomelleri C. Innovative gait robot for the repetitive practice of floor walking and stair climbing up and down in stroke patients. J Neuroeng Rehabil 2010; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeshima S, Osawa A, Nishio D, et al. Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: A preliminary report. BMC Neurol 2011; 11: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuck A, Labruyere R, Vallery H, et al. Feasibility and effects of patient-cooperative robot-aided gait training applied in a 4-week pilot trial. J Neuroeng Rehabil 2012; 9: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esquenazi A, Lee S, Packel AT, et al. A randomized comparative study of manually assisted versus robotic-assisted body weight supported treadmill training in persons with a traumatic brain injury. PM R 2013; 5: 280–290. [DOI] [PubMed] [Google Scholar]
- 38.Delussu AS, Morone G, Iosa M, et al. Physiological responses and energy cost of walking on the Gait Trainer with and without body weight support in subacute stroke patients. J Neuroeng Rehabil 2014; 11: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal SK, Banala SK, Fattah A, et al. Assessment of motion of a swing leg and gait rehabilitation with a gravity balancing exoskeleton. IEEE Trans Neural Syst Rehabil Eng 2007; 15: 410–420. [DOI] [PubMed] [Google Scholar]
- 40.Banala SK, Kim SH, Agrawal SK, Scholz JP. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst Rehabil Eng 2009; 17: 2–8. [DOI] [PubMed] [Google Scholar]
- 41.Coenen P, van Werven G, van Nunen MP, et al. Robot-assisted walking vs overground walking in stroke patients: An evaluation of muscle activity. J Rehabil Med 2012; 44: 331–337. [DOI] [PubMed] [Google Scholar]
- 42.Neckel ND, Blonien N, Nichols D, et al. Abnormal joint torque patterns exhibited by chronic stroke subjects while walking with a prescribed physiological gait pattern. J Neuroeng Rehabil 2008; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan C, Ranganathan R, Dhaher YY, et al. A pilot study on the feasibility of robot-aided leg motor training to facilitate active participation. PLoS One 2013; 8: e77370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewek MD, Cruz TH, Moore JL, et al. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: A subgroup analysis from a randomized clinical trial. Phys Ther 2009; 89: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt RR, Simpson D, Jenner JR, et al. Ground reaction force after a sideways push as a measure of balance in recovery from stroke. Clin Rehabil 2000; 14: 88–95. [DOI] [PubMed] [Google Scholar]
- 46.Bonnyaud C, Zory R, Boudarham J, et al. Effect of a robotic restraint gait training versus robotic conventional gait training on gait parameters in stroke patients. Exp Brain Res 2014; 232: 31–42. [DOI] [PubMed] [Google Scholar]
- 47.Bonnyaud C, Pradon D, Boudarham J, et al. Effects of gait training using a robotic constraint (Lokomat(R)) on gait kinematics and kinetics in chronic stroke patients. J Rehabil Med 2014; 46: 132–138. [DOI] [PubMed] [Google Scholar]
- 48.Calabro RS, Reitano S, Leo A, et al. Can robot-assisted movement training (Lokomat) improve functional recovery and psychological well-being in chronic stroke? Promising findings from a case study. Funct Neurol 2014. Early view: 1–3. [PMC free article] [PubMed] [Google Scholar]
- 49.Wong CK, Bishop L, Stein J. A wearable robotic knee orthosis for gait training: A case-series of hemiparetic stroke survivors. Prosthet Orthot Int 2012; 36: 113–120. [DOI] [PubMed] [Google Scholar]
- 50.Byl NN. Mobility training using a bionic knee orthosis in patients in a post-stroke chronic state: A case series. J Med Case Rep 2012; 6: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horst RW. A bio-robotic leg orthosis for rehabilitation and mobility enhancement. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 5030–5033. [DOI] [PubMed] [Google Scholar]
- 52.Bortole M, Venkatakrishnan A, Zhu F, et al. The H2 robotic exoskeleton for gait rehabilitation after stroke: Early findings from a clinical study. J Neuroeng Rehabil 2015; 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dias D, Lains J, Pereira A, et al. Can we improve gait skills in chronic hemiplegics? A randomised control trial with gait trainer. Eura Medicophys 2007; 43: 499–504. [PubMed] [Google Scholar]
- 54.Peurala SH, Tarkka IM, Pitkanen K, et al. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch Phys Med Rehabil 2005; 86: 1557–1564. [DOI] [PubMed] [Google Scholar]
- 55.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil 2009; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hornby TG, Campbell DD, Kahn JH, et al. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: A randomized controlled study. Stroke 2008; 39: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 57.Kelley CP, Childress J, Boake C, et al. Over-ground and robotic-assisted locomotor training in adults with chronic stroke: A blinded randomized clinical trial. Disabil Rehabil Assist Technol 2013; 8: 161–168. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka N, Saitou H, Takao T, et al. Effects of gait rehabilitation with a footpad-type locomotion interface in patients with chronic post-stroke hemiparesis: A pilot study. Clin Rehabil 2012; 26: 686–695. [DOI] [PubMed] [Google Scholar]
- 59.De Luca A, Lentino C, Vernetti H, et al. Functional evaluation of robot end-point assisted gait re-education in chronic stroke survivors. IEEE Int Conf Rehabil Robot 2013; 2013: 1–7. [DOI] [PubMed] [Google Scholar]
- 60.Ucar DE, Paker N, Bugdayci D. Lokomat: A therapeutic chance for patients with chronic hemiplegia. NeuroRehabilitation 2014; 34: 447–453. [DOI] [PubMed] [Google Scholar]
- 61.Wu M, Landry JM, Yen SC, et al. A novel cable-driven robotic training improves locomotor function in individuals post-stroke. Conf Proc IEEE Eng Med Biol Soc 2011; 2011: 8539–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota S, Nakata Y, Eguchi K, et al. Feasibility of rehabilitation training with a newly developed wearable robot for patients with limited mobility. Arch Phys Med Rehabil 2013; 94: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 63.Kawamoto H, Kamibayashi K, Nakata Y, et al. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol 2013; 13: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu M, Landry JM, Kim J, et al. Robotic resistance/assistance training improves locomotor function in individuals poststroke: A randomized controlled study. Arch Phys Med Rehabil 2014; 95: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein J, Bishop L, Stein DJ, et al. Gait training with a robotic leg brace after stroke: A randomized controlled pilot study. Am J Phys Med Rehabil 2014; 93: 987–994. [DOI] [PubMed] [Google Scholar]
- 66.Altman DG. Practical Statistics for Medical Research, Taylor & Francis, 1990. [Google Scholar]
- 67.Iwata H, Yano H, Nakaizumi F. GaitMaster: A versatile locomotion interface for uneven virtual terrain. Proceedings of the IEEE Virtual Reality Conference. Yokohama, Japan, 2001, pp.131–137. [Google Scholar]
- 68.Yano H, Masuda T, Nakajima Y, et al. Development of a gait rehabilitation system with a spherical immersive projection display. J Robotics Mechatron 2008; 12: 836–845. [Google Scholar]
- 69.Hesse S, Uhlenbrock D, Sarkodie-Gyan T. Gait pattern of severely disabled hemiparetic subjects on a new controlled gait trainer as compared to assisted treadmill walking with partial body weight support. Clin Rehabil 1999; 13: 401–410. [DOI] [PubMed] [Google Scholar]
- 70.Hesse S, Uhlenbrock D, Werner C, et al. A mechanized gait trainer for restoring gait in nonambulatory subjects. Arch Phys Med Rehabil 2000; 81: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 71.Colombo G, Joerg M, Schreier R, et al. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev 2000; 37: 693–700. [PubMed] [Google Scholar]
- 72.Uhlenbrock D, Sarkodie-Gyan T, Reiter F, et al. [Development of a gait trainer with regulated servo-drive for rehabilitation of locomotor disabled patients]. Biomed Tech (Berl) 1997; 42: 196–202. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki K, Mito G, Kawamoto H, et al. Intention-based walking support for paraplegia patients with Robot Suit HAL. Adv Robot 2007; 21: 1441–1469. [Google Scholar]
- 74.Wu M, Hornby TG, Landry JM, et al. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture 2011; 33: 256–260. [DOI] [PubMed] [Google Scholar]
- 75.Boyne P, Dunning K, Carl D, et al. Within-session responses to high-intensity interval training in chronic stroke. Med Sci Sports Exerc 2015; 47: 476–484. [DOI] [PubMed] [Google Scholar]
- 76.King AC, Haskell WL, Young DR, et al. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation 1995; 91: 2596–2604. [DOI] [PubMed] [Google Scholar]
- 77.Sun J, Ke Z, Yip SP, et al. Gradually increased training intensity benefits rehabilitation outcome after stroke by BDNF upregulation and stress suppression. Biomed Res Int 2014; 2014: 925762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang WH, Kim YH. Robot-assisted therapy in stroke rehabilitation. J Stroke 2013; 15: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehrholz J, Elsner B, Werner C, et al. Electromechanical-assisted training for walking after stroke: Updated evidence. Stroke 2013; 44: e127–e128. [DOI] [PubMed] [Google Scholar]
- 80.Mehrholz J, Pohl M. Electromechanical-assisted gait training after stroke: A systematic review comparing end-effector and exoskeleton devices. J Rehabil Med 2012; 44: 193–199. [DOI] [PubMed] [Google Scholar]
- 81.Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev 2014; 1: CD002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pang MY, Charlesworth SA, Lau RW, et al. Using aerobic exercise to improve health outcomes and quality of life in stroke: Evidence-based exercise prescription recommendations. Cerebrovasc Dis 2013; 35: 7–22. [DOI] [PubMed] [Google Scholar]
- 83.ACSM. ACSM’s Guidelines for Exercise Testing and Prescription, Wolters Kluwer Health, 2013. [DOI] [PubMed] [Google Scholar]