Abstract
Introduction
Electrical stimulation could provide an alternative method for preventing venous thromboembolism in stroke patients. The purpose of this preliminary study was to explore the effects of electrical stimulation and intermittent pneumatic compression on enhancing lower limb venous return in healthy and chronic stroke patients and also to evaluate patient and nurse satisfaction.
Methods
We investigated the effectiveness of two electrical stimulation devices: Geko (Firstkind Ltd, High Wycombe, UK) and Orthopaedic Microstim 2V2 (Odstock Medical Ltd, Salisbury, UK); and one intermittent pneumatic compression device: Huntleigh Flowstron Universal (Huntleigh Healthcare Ltd, Cardiff, UK). We recruited 12 healthy and 5 chronic stroke participants. The devices were fitted sequentially, and Doppler ultrasound measurements were taken. Eight patients and nurses were also recruited for a separate usability evaluation.
Results
The electrical stimulation devices emulated the blood flow characteristics of intermittent pneumatic compression in both healthy and stroke participants provided that the intensity of electrical stimulation was sufficient. Patients and nurses also felt that the electrical stimulation devices were acceptable.
Conclusions
Electrical stimulation may offer benefit as an alternative method for venous thromboembolism prevention in stroke patients. The apparent benefit is sufficient to warrant further investigation in a full powered randomised controlled trial.
Keywords: Electrical stimulation, venous thromboembolism, venous thromboprophylaxis, deep vein thrombosis, stroke
Introduction
Venous thromboembolism (VTE) is a major problem in the acute phase of a stroke. Deep vein thrombosis (DVT) has been detected in up to 42% of stroke patients in hospital, and pulmonary embolism accounts for up to 25% of early mortality after an acute stroke.1–3 Graduated compression stockings are not an effective method of reducing the risk of DVT after stroke, and guidelines suggest that anticoagulants should only be used for patients with a low risk of bleeding and a high risk of thromboembolism.4,5 Intermittent pneumatic compression (IPC) has been shown to be effective, but patient compliance is low.2,6 IPC consists of stockings which are secured around the calf, or the calf and thigh, and then inflate and deflate to compress the legs. This is thought to lower the risk of DVT by reducing venous stasis.7 A multi-centre, randomised, controlled trial demonstrated that thigh length IPC is an effective method of reducing the risk of DVT in the acute phase after stroke, but adherence was a major issue.2 Poor adherence to IPC has been recognised as a major limitation for some time.6
Electrical stimulation (ES) could be used as an alternative method of reducing the risk of DVT after stroke. Intermittent contraction of the muscles that dorsiflex the foot through ES of the common peroneal nerve is thought to compress the leg veins and improve venous return in a similar way to the skeletal-muscle pump action whilst walking. Several studies have demonstrated that ES is able to increase venous velocity and flow in healthy participants.8–15 Two recent systematic reviews demonstrated that there is moderate quality evidence to support ES over no prophylaxis in a clinical setting for preventing VTE.16,17 However, how patient selection influences efficacy and the most effective device and protocol is unknown. In particular, there is a dearth of experimental and clinical studies in the stroke population, who may have important differences in vasculature but could potentially benefit from ES as a method of VTE prophylaxis.
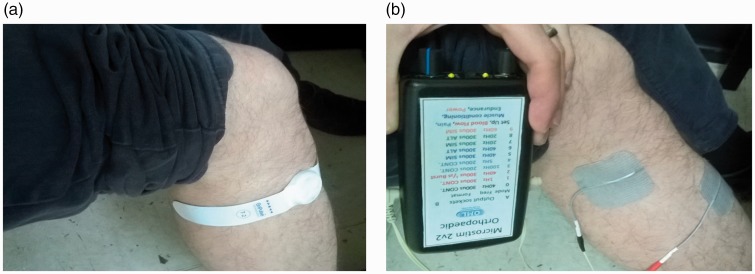
ES devices are battery powered and attach to the patient’s leg with self adhesive electrodes. Previous studies have shown that Geko (FirstKind Medical Ltd, UK), a single-use disposable ES device with an integrated battery, significantly increases venous blood flow in the lower limb of healthy participants (Figure 1(a)).18 An alternative reusable ES device, the Orthopaedic Microstim 2V2 (Odstock Medical Ltd, UK), has a reusable powered controller component with disposable surface electrodes and would be a less expensive option if used for multiple days (Figure 1(b)).
Figure 1.
ES devices (a) Geko and (b) Orthopaedic Microstim 2V2.
ES: electrical stimulation.
This preliminary study aimed to demonstrate the effect of Geko, Orthopaedic Microstim 2V2 and IPC on lower limb venous return in healthy and stroke-affected participants. We also sought to evaluate patient and nurse satisfaction with the ES devices and determine whether there would be any benefit over existing stroke VTE prophylaxis strategies.
Methods
Blood flow study
Participants
We recruited 12 healthy volunteers and a convenience sample of five existing users of ES for the correction of dropped foot caused by a stroke. All participants were over the age of 18, mobile enough to transfer to a bed safely and able to provide informed consent. The healthy participants had no history of cardiovascular disease whereas the existing users of ES were >6 months post stroke. This part of the study was approved by the University of Southampton Research Ethics committee (ERGO reference numbers 10652 and 23157).
ES interventions
We used two different types of ES: Geko (FirstKind Medical Ltd, UK) and Orthopaedic Microstim 2V2 (Odstock Medical Ltd, UK). Geko is a small, disposable self-adhesive band containing two electrodes and a stimulator with an integral battery which provides approximately 24 h of operation before the whole unit is replaced. It is applied to the lower leg on the skin overlying the common peroneal nerve (Figure 1(a)). It pulses a current of 27 mA at a frequency of 1 Hz, which results in a single ankle dorsiflexor muscle twitch every second. Pulse width can be varied between 7 settings (70, 100, 140, 200, 280, 400 and 560 µs) and is adjusted to produce a slight visible movement of the foot. The Orthopaedic Microstim 2V2 consists of a stimulator box and two leads which are connected to two multiple use self-adhesive electrodes placed over the common peroneal nerve (Figure 1(b)). Current intensity, pulse width and frequency can be varied. We used a pulse width of 300 µs and a frequency of 40 Hz, delivering 20 pulses within half a second, every two seconds. We observed, during our preparatory work for this study, that stroke-affected participants required higher levels of ES to increase blood flow than healthy participants. Therefore, two different Orthopaedic Microstim 2V2 levels of stimulation were used in the stroke-affected group. A low setting, Microstim low (MSL), defined as the minimum current required to produce a slight visible movement of the foot; and a high setting, Microstim high (MSH), defined as the minimum current required to reproduce the patient’s full range of passive dorsiflexion. Taking the ankle through passive range of dorsiflexion is closer to the movement achieved while walking. Walking is believed to cause the muscle pump effect and so it is likely that stretching of the calf muscle in this way pushes blood out of the compartment. Passive range of dorsiflexion was determined by the researcher at the start of the test using a manual goniometer. The two different levels of stimulation were used to provide dose-ranging information and inform future work.
IPC intervention
The IPC comparator was the Huntleigh Flowtron Universal™ (Huntleigh Healthcare Ltd, UK). The device runs a compression cycle of 1 min, which consists of 13 s of compression and 47 s of relaxation. The inflation pressure was 40 mmHg. Knee length stockings were used in the healthy group, in accordance with previous studies comparing IPC and ES haemodynamics,15,18 whilst thigh length stockings were used in the stroke-affected group because of subsequent clinical evidence demonstrating that thigh length IPC reduces the risk of VTE in the acute stroke.2 The devices were applied unilaterally (to the affected leg in stroke participants).
Study design
Lower limb venous flow was the primary outcome as measured by duplex ultrasound of the superficial femoral vein. Three interventions were applied to each subject: Geko, Microstim 2V2 and IPC. The order of interventions was randomised according to a pre-determined randomisation schedule (online Appendix D). There was a 5-min rest period before each intervention in order to allow venous flow to return to baseline. Measurements were taken at the end of this period in order to identify any baseline variability. Each intervention was applied for eight minutes, during which the first five minutes were ‘conditioning’ and measurements were taken in the final three minutes. After each intervention, participants were asked to rate discomfort levels using a visual analogue scale (VAS), which involved marking the level of pain on a 100 mm line marked with ‘no pain’ at one end and ‘worst pain imaginable’ at the other end. VAS is a standard method for assessing pain and its application was standardised within this study. A Likert scale was also devised to score perceived discomfort where 0 = ‘no sensation’, 1 = ‘mild sensation’, 2 = ‘moderate sensation’, 3 = ‘mild discomfort’, 4 = ‘moderate discomfort’ and 5 = ‘severe discomfort’. Several similar studies have used VAS and Likert scales to measure pain and discomfort associated with ES and IPC.14,18
Ultrasound measurements
All ultrasound measurements were taken at the superficial femoral vein by a single accredited vascular sonographer. Participants were in a sitting position with their knees extended on a two-section examination couch with the back at 45° (Fowler’s position). This is a position that may be used for nursing a patient in hospital following an acute stroke. Their head was supported by a pillow, and their legs were exposed to the upper thigh. Measurements were taken at the mid thigh superficial femoral vein (short axis view) using a 4–9 MHz linear array transducer and pulsed wave (4 MHz) colour-flow duplex ultrasound (Siemens Acuson S2000; Siemens AG, Erlangen, Germany). The instrument output was 70 dB and, with this setup, the maximum depth of measurement was 9 cm. The position of the ultrasound probe was marked on the skin for consistency.
Data analysis
We studied vessel diameter, volume flow, time-averaged mean velocity (TAMV) and peak velocity over a 14 s period. Volume flow (ml/min) is the product of the TAMV and the cross-sectional area of the blood vessel. TAMV (cm/s) is the weighted mean velocity during each time interval averaged out over the trace as an estimate of true mean velocity. Peak velocity (cm/s) is the maximum velocity reached during the trace. For the IPC device, measurements were taken three times in both the inflation and deflation period in order to calculate the average volume flow over one minute.
Statistical analysis
The baseline measurements acted as a reference range. Repeat measurements were averaged. The sample size was small, and so the results were assumed not to be normally distributed. The raw data was described using medians and inter-quartile ranges. We tested the null hypothesis that the distributions of volume flow, TAMV and peak velocity were the same by using the Kruskal–Wallis test with a post hoc Mann–Whitney test and Bonferroni correction. A p value of ≤ 0.05 was considered statistically significant. The outcome measures were also expressed as a percentage change from baseline.
User satisfaction study
Participants
We recruited eight patients and eight nurses on the stroke ward at Salisbury District Hospital for the ward evaluation. Patients were admitted with an acute stroke, over the age of 18 and able to provide informed consent. Exclusion criteria were presence of a cardiac pacemaker or implanted defibrillator, and uncontrolled epilepsy. There was no overlap between the participants in the blood flow and user satisfaction studies. This part of the study was approved by the Salisbury District Hospital Patient and Public Involvement Committee.
We asked the patients to wear each activated device (Geko, Orthopaedic Microstim 2V2 and IPC) sequentially on one leg for 20 min whilst reclining in bed. The stimulation parameters were the same as those used in the blood flow study. We asked for feedback in the form of a five-question, semi-structured interview/questionnaire regarding comfort, mobility and overall satisfaction. The order of interventions was randomised according to a predetermined randomisation schedule (online Appendix D). There was a time interval of up to 5 min between interventions.
We asked the nurses to observe a demonstration of all three devices and give feedback in the form of a nine-question, semi-structured interview/questionnaire regarding ease of use, safety concerns and anticipated effect on rehabilitation and care. Responses were in the form of a Likert Scale and attributed a value between 1 and 5 (1 being the least desirable response and 5 being the most desirable response). The difference from the worst possible score for each question was used to generate a satisfaction score, which was expressed as a percentage of the best possible score. The questionnaires were developed ‘in house’. For details of individual questions, see online appendices A and B.
Results
Blood flow study
We recruited 12 healthy (nine male and three female, age range 24–68) and five stroke-affected participants (two male and three female, median age 81, age range 58–87). Flow volume, TAMV and peak flow velocity at baseline and in response to each intervention are shown in Table 1. MSL produced the greatest median volume flow and TAMV and a significantly greater TAMV and peak flow compared to baseline in healthy participants. MSH produced the greatest median flow volume, TAMV and peak velocity, and both MSH and IPC produced a significantly greater peak flow velocity compared to baseline in stroke-affected participants. There were no significant differences between devices.
Table 1.
Blood flow volumes, TAMV and peak flow velocities in healthy (n = 12) and chronic stroke participants (n = 5).
| Baseline | Geko | MSL | MSH | IPC | p-Value | Pairwise comparisons | |
|---|---|---|---|---|---|---|---|
| Flow volume (ml/min) | |||||||
| Stroke | 94 (81–100) | 76 (69–110) | 87 (81–89) | 150 (120–260) | 98 (92–98) | 0.076 | – |
| Healthy | 92 (79–98) | 100 (92–130) | 130 (100–160) | – | 110 (81–140) | 0.080 | – |
| TAMV (cm/s) | |||||||
| Stroke | 3.2 (3.0–3.4) | 3.3 (3.0–3.6) | 3.4 (3.2–3.4) | 5.6 (4.4–7.3) | 3.1 (2.2–4.1) | 0.073 | – |
| Healthy | 2.3 (2.2–2.5) | 3.0 (2.6–3.6) | 3.5 (2.8–3.9) | – | 3.1 (2.2–3.7) | 0.034 | MSL-baseline (p = 0.045) |
| Peak flow velocity (cm/s) | |||||||
| Stroke | 10 (9.4–11) | 13 (11–14) | 13 (13–14) | 32 (26–61) | 31 (29–34) | <0.01 | MSH-baseline (p < 0.01) |
| IPC-baseline (p < 0.01) | |||||||
| Healthy | 8.0 (8.2–9.1) | 13 (10–16) | 19 (15–23) | – | 25 (15–30) | <0.01 | MSL-baseline (p < 0.01) |
| IPC-baseline (p < 0.01) | |||||||
Values are presented as median (inter-quartile range). p-Values represent the results of six separate Kruskal–Wallis tests. Pairwise comparisons refer to post hoc Mann–Whitney tests. Significance values have been adjusted by the Bonferroni correction for multiple tests. Significant results only are displayed.
MSL, Orthopaedic Microstim 2V2 low setting; MSH, Orthopaedic Microstim 2V2 high setting; IPC, intermittent pneumatic compression; TAMV, time average mean velocity.
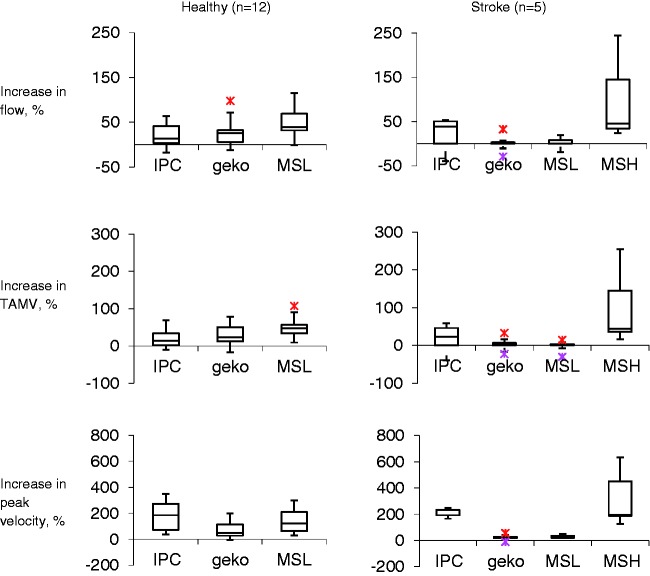
There were no discernible differences in baseline measurements before each intervention. The highest percentage change in flow volume and TAMV was achieved by MSH in the stroke group and MSL in the healthy group (Figure 2). IPC produced the greatest percentage increase in peak velocity in both groups. There was a much lower percentage increase in flow volume, TAMV and peak velocity in response to MSL and Geko in the stroke group compared to the healthy group.
Figure 2.
Percentage increase in blood flow from baseline. The bottom of the box represents the 25th percentile, the centre of the box represents the median and the top of the box represents the 75th percentile. The vertical lines represent the extremes of values.
TAMV, time average mean velocity; MSL, Orthopaedic Microstim 2V2 low setting; MSH, Orthopaedic Microstim 2V2 high setting; IPC, intermittent pneumatic compression.
Responses showed no significant difference in discomfort between the devices using the VAS or Likert Scale devised for this study.
User satisfaction survey
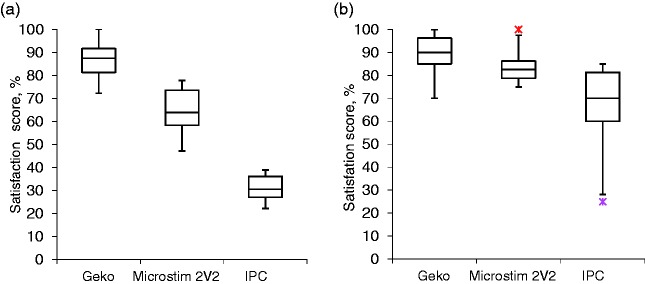
Eight patients (four male and four female) and eight nurses (two male and six female) completed the device evaluation. The median patient age was 77 (range 31–94). The results of the evaluation are presented in Figure 3. We found that the Geko had the best average patient satisfaction score (89%), followed by the Orthopaedic Microstim 2V2 (84%) and then IPC (66%). Similarly, the Geko had the best nurse satisfaction score (85%), followed by the Orthopaedic Microstim 2V2 (65%) and then IPC (31%). There were significant differences between devices in both patient and nurse satisfaction scores (p < 0.01). For patients, post hoc analysis showed significantly lower satisfaction with IPC, but no difference between the ES devices; whereas for nurses, there was a significant difference in satisfaction between all three devices.
Figure 3.
Satisfaction score distribution. Figure 2(a) represents the nurse’s average satisfaction score, and Figure 2(b) represents the patient’s average satisfaction score. The bottom of the box represents the 25th percentile, the centre of the box represents the median and the top of the box represents the 75th percentile. The vertical lines represent the extremes of values within 1.5 times the inter-quartile range. The red crosses represent outliers.
IPC, intermittent pneumatic compression.
Effect on mobility seemed to be the main area where nurses and patients felt that Geko outperformed the other two devices. None of the patients or nurses felt that Geko interfered with their ability to move or engage in rehabilitation, and the majority of nurses felt that it would not increase the risk of falls. In contrast, several patients and nurses felt that Orthopaedic Microstim 2V2 and IPC would interfere with their ability to move or engage in rehabilitation. The majority of nurses also felt that both devices would increase the risk of falls. Nurses felt that Geko was easier to apply than the other two devices and would have less of an impact on personal care. To see the individual questions and breakdown of the scores, please see online appendices A to C.
Discussion
It is thought that IPC reduces the risk of VTE in stroke-affected patients by preventing venous stasis.2 As such, venous blood flow is often used as a surrogate for the prevention of VTE and to add value to new VTE prevention technologies. Our small preliminary study suggests that ES is as effective as IPC at improving blood flow, TAMV and peak velocity in both healthy individuals and those with a chronic stroke, but the intensity of ES must be sufficient. The level of contraction produced by Geko and MSL seemed to produce little increase in blood flow in chronic stroke participants from baseline, whereas higher intensity MSH produced a greater increase in blood flow, TAMV and peak velocity. On the other hand, Geko and MSL seemed to be sufficient to increase blood flow in healthy participants. Changes in ES intensity and biological variation may explain conflicting evidence on the relationship between ES and the incidence of VTE reported in the review by Hajibandeh et al.19
We observe that the ES devices had a greater impact on venous flow and TAMV than IPC, whereas IPC has more of an impact on peak velocity, although there were no significant differences between devices. This finding is compatible with that of Jawad et al.18 who compared the efficacy of Geko against two different IPC devices (below knee Huntleigh Flowtron Universal and Kendall SCD Express) at enhancing lower limb blood flow in the superficial femoral vein in 10 healthy people. At threshold setting (the minimum setting to elicit a minor muscular contraction in both the calf and the foot), Geko increased venous flow by 14% whereas the Huntleigh Flowtrons decreased flow by 4%. The optimal blood flow characteristics for preventing VTE are unknown but there is some suggestion that IPC devices with the highest peak velocities also have the highest incidence of VTE.20
There is a dearth of data comparing the compliance and tolerability of ES with other forms of mechanical VTE prophylaxis in stroke rehabilitation therapy.17 This study is the first to evaluate patient and nurse satisfaction of ES and IPC to our knowledge. Our preliminary results show that both patients and nurses have positive initial impressions of ES devices, highlighting several advantages over IPC. Comfort, adherence, mobility and independence are all important factors for stroke rehabilitation, so choice of VTE prevention could have a major impact on adherence and recovery.
Study limitations
Whilst the results are promising, there are several methodological limitations to this preliminary study. We used a very small convenience sample of healthy and chronic stroke participants who were existing users of ES. No power calculations were used in the design of this study, so the results should be interpreted with caution. We did not attempt to match the baseline characteristics of healthy and stroke participants which limits comparison between the two groups. Moreover, the existing users of ES would have had some conditioning, which may have increased muscle strength compared to unconditioned users. They were also >6 months post stroke and therefore were likely to have variable levels of ankle stiffness or calf spasticity, which would require different levels of muscle contraction to produce dorsiflexion in comparison to those who have had an acute stroke. The generalisibility of the results to the acute stroke population is therefore questionable. We did not assess the effect of fatigue during ES, which may also affect its efficacy. We did not study blood flow in the period following ES.
The device evaluation was conducted within a single stroke unit, and the number of patients and nurses completing the clinical comparison were small. Nurse’s views may have been biased by previous IPC experience, and it is possible that only patients and nurses with a very positive or negative experience volunteered to take part in the study. The questionnaires were not validated and may be subject to error.
Conclusion
This study indicates that ES may be an effective and acceptable intervention for preventing VTE in healthy and chronic stroke patients. However, for ES to have an effect on blood flow comparable to IPC in those with a chronic stroke, the intensity should be sufficient to move the ankle through all or part of its passive range of dorsiflexion. There appears to be sufficient benefit to warrant further investigation in a full powered randomised controlled trial.
Supplemental Material
Supplemental material for Electrical stimulation devices for the prevention of venous thromboembolism: Preliminary studies of physiological efficacy and user satisfaction by James Badger, Paul Taylor, Neil Papworth and Ian Swain in Journal of Rehabilitation and Assistive Technologies Engineering
Acknowledgements
We would like to thank Linda Harris for her help taking ultrasound measurements.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JB and NP have no competing financial interests. PT and IS hold shares in the company Odstock Medical Limited.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Southampton National Institute of Health Research Academic Foundation Programme.
Guarantor
JB.
Contributorship
All authors contributed to the conception and design of this study. JB, NP and IS collected the data with the assistance of a vascular sonographer. JB analysed the data and drafted the paper. IS, PT and NP read, provided feedback and approved the manuscript.
Supplemental Material
Online appendices for this article is available online.
References
- 1.Kelly J, Rudd A, Lewis R, et al. Venous thromboembolism after acute stroke. Stroke 2001; 32: 262–267. [DOI] [PubMed] [Google Scholar]
- 2.Dennis M, Sandercock P, Reid J, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet 2013; 382: 516–524. [DOI] [PubMed] [Google Scholar]
- 3.Skaf E, Stein PD, Beemath A, et al. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol 2005; 96: 1731–1733. [DOI] [PubMed] [Google Scholar]
- 4.Dennis M, Sandercock PA, Reid J, et al. Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373: 1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteley WN, Adams HP, Jr, Bath PM, et al. Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurol 2013; 12: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comerota AJ, Katz ML, White JV. Why does prophylaxis with external pneumatic compression for deep vein thrombosis fail? Am J Surg 1992; 164: 265–268. [DOI] [PubMed] [Google Scholar]
- 7.Morris RJ, Woodcock JP. Evidence-based compression: prevention of stasis and deep vein thrombosis. Ann Surg 2004; 239: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen PP, Galvin O, Quondamatteo F, et al. Comparison of single- and two-channel neuromuscular electrical stimulation sites for enhancing venous return. IEEE Trans Neural Syst Rehabil Eng 2012; 20: 389–394. [DOI] [PubMed] [Google Scholar]
- 9.Broderick BJ, O’Briain DE, Breen PP, et al. A pilot evaluation of a neuromuscular electrical stimulation (NMES) based methodology for the prevention of venous stasis during bed rest. Med Eng Phys 2010; 32: 349–355. [DOI] [PubMed] [Google Scholar]
- 10.Griffin M, Nicolaides AN, Bond D, et al. The efficacy of a new stimulation technology to increase venous flow and prevent venous stasis. Eur J Vasc Endovasc Surg 2010; 40: 766–771. [DOI] [PubMed] [Google Scholar]
- 11.Izumi M, Ikeuchi M, Mitani T, et al. Prevention of venous stasis in the lower limb by transcutaneous electrical nerve stimulation. Eur J Vasc Endovasc Surg 2010; 39: 642–645. [DOI] [PubMed] [Google Scholar]
- 12.Lyons GM, Leane GE, Grace PA. The effect of electrical stimulation of the calf muscle and compression stocking on venous blood flow velocity. Eur J Vasc Endovasc Surg 2002; 23: 564–566. [DOI] [PubMed] [Google Scholar]
- 13.Tucker A, Maass A, Bain D, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol 2010; 19: e31–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warwick DJ, Shaikh A, Gadola S, et al. Neuromuscular electrostimulation via the common peroneal nerve promotes lower limb blood flow in a below-kneecast: a potential for thromboprophylaxis. Bone Joint Res 2013; 2: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broderick BJ, O’Connell S, Moloney S, et al. Comparative lower limb hemodynamics using neuromuscular electrical stimulation (NMES) versus intermittent pneumatic compression (IPC). Physiol Meas 2014; 35: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 16.Ravikumar R, Williams KJ, Babber A, et al. Neuromuscular electrical stimulation for the prevention of venous thromboembolism. Phlebology 2018; 33: 367–378. [DOI] [PubMed] [Google Scholar]
- 17.Hajibandeh S, Antoniou GA, Scurr JR, et al. Neuromuscular electrical stimulation for the prevention of venous thromboembolism. Cochrane Database Syst Rev 2017; 11: CD011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jawad H, Bain DS, Dawson H, et al. The effectiveness of a novel neuromuscular electrostimulation method versus intermittent pneumatic compression in enhancing lower limb blood flow. J Vasc Surg Venous Lymphat Disord 2014; 2: 160–165. [DOI] [PubMed] [Google Scholar]
- 19.Hajibandeh S, Antoniou GA, Scurr JR, et al. Neuromuscular electrical stimulation for thromboprophylaxis: a systematic review. Phlebology 2015; 30: 589–602. [DOI] [PubMed] [Google Scholar]
- 20.Proctor MC, Greenfield LJ, Wakefield TW, et al. A clinical comparison of pneumatic compression devices: the basis for selection. J Vasc Surg 2001; 34: 459–463. discussion 63–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Electrical stimulation devices for the prevention of venous thromboembolism: Preliminary studies of physiological efficacy and user satisfaction by James Badger, Paul Taylor, Neil Papworth and Ian Swain in Journal of Rehabilitation and Assistive Technologies Engineering