Abstract
Macrophage migration inhibitory factor (MIF) is an important cytokine for which an increasing number of functions is being described in the pathogenesis of inflammation and cancer. Nevertheless, the availability of potent and druglike MIF inhibitors that are well-characterized in relevant disease models remains limited. Highly potent and selective small molecule MIF inhibitors and validation of their use in relevant disease models will advance drug discovery. In this review we provide an overview of recent advances in the identification of MIF as a pharmacological target in the pathogenesis of inflammatory diseases and cancer. Based on that we give an overview of the current developments in the discovery and design of small molecule MIF inhibitors and define future aims in this field.
Keywords: macrophage migration inhibitory factor (MIF), inflammatory diseases, cancer, inhibitors
Introduction
Despite its discovery over 50 years ago in 1966 [1][2], the functions of the cytokine macrophage migration inhibitory factor (MIF) are still not fully elucidated. Initially, MIF was identified as a T cell-derived mediator that inhibits random movement of macrophages. Its activity was found to correlate with delayed-type hypersensitivity reactions, a prominent feature of several chronic diseases in humans [2]. In addition, MIF is released at sites of infection, causing macrophages to concentrate and carry out antigen processing and phagocytosis [3]. Today, MIF is recognized as a critical player in innate immune responses that play a role in multiple diseases [4][5]. Therefore, the development of small molecule MIF inhibitors that inferfere with its functions is quickly gaining importance.
The human MIF gene has been cloned and expressed for the first time in 1989 [6]. MIF is a relatively small protein that consists of 114 amino acids and has a molecular mass of 12,345 Da. Structural analysis of MIF revealed its striking similarities to bacterial enzymes from the tautomerase superfamily. Searching the human genome indicated that D-dopachrome tautomerase (D-DT) is the other gene with marked homology to MIF. Due to this similarity, D-DT is also referred to as MIF2 and an overlapping functional spectrum for MIF and D-DT has been suggested [7]. This should be taken into account in evaluation of MIF cytokine activities and in the development of small molecule MIF modulators.
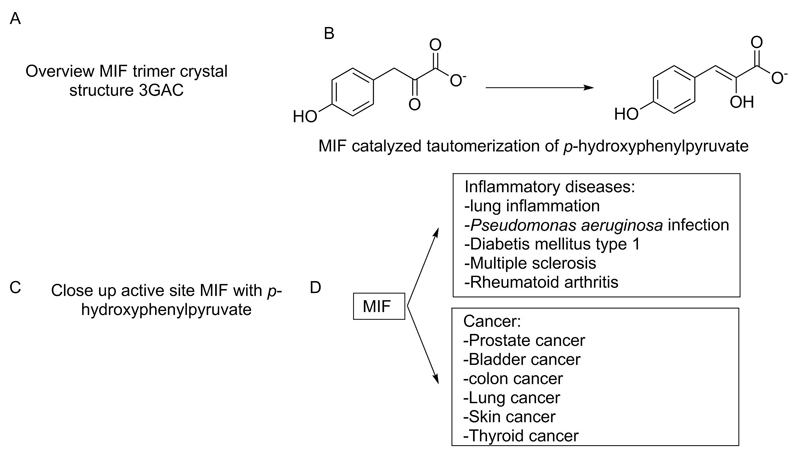
MIF, a member of the tautomerase superfamily [8], is found across various organisms including bacteria, mice, plants, protozoa, helminths, molluscs, arthropods, and fish [9–11]. These tautomerase family members have similar enzyme activity involving an amino acid-terminal proline that acts as general base in keto-enol tautomerisation reactions of α-keto-carboxylates. In addition to its cytokine activity, MIF harbours keto-enol tautomerase and low-level dehalogenase activity, providing a functional link to other members of the tautomerase family [10]. MIF is a homotrimeric protein in which three monomers associate to form a symmetrical trimer (Figure 1A). Each MIF trimer has three tautomerase active sites at the interfaces of the monomer subunits. Characteristic for this family, MIF has a N-terminal proline (Pro-1), which is located within a hydrophobic pocket [12]. The residue Pro-1 was shown to be conserved between MIF and its bacterial homologues. Moreover, other invariant residues were identified as being clustered around the N-terminal proline. The evolutionary preservation of this region suggests its importance in the biological function of MIF [13]. Despite the lack of a known physiological substrate, it was shown that D-dopachrome (a stereoisomer of naturally occurring L-dopachrome), phenylpyruvate and p-hydroxyphenylpyruvate are accepted as substrates by MIF (Figure 1B) [14] [15]. A crystal structure of MIF in complex with p-hydroxyphenylpyruvate demonstrated that Pro-1 functions as a catalytic base in the tautomerase reaction [16]. It is well recognized that MIF’s currently defined substrates either do not exist naturally in vivo, or do not exist at significant concentrations required for biological activity [17]. Nevertheless, small molecule modulators of MIF tautomerase activity may have an impact on MIF cytokine activity due to modulation of its conformation and/or ability to interact with other proteins.
Figure 1.
A. Crystal structure of MIF, showing that three monomers associate to form a symmetrical homotrimer (PDB 1CA7), B. MIF catalyses the tautomerisation of α-keto-carboxylates, C. Diseases for which a role of MIF has been described.
Although MIF tautomerase activity may not be directly linked to its cytokine activity, it provides an opportunity for efficient screening of compound collections that could provide molecules that interfere with MIF cytokine activity. One of the best known targets to mediate MIF cytokine activity upon binding is the cluster of differentiation 74 (CD74) receptor [18] Interestingly, recent findings demonstrate that MIF binding to CD74 occurs in the vicinity of the MIF tautomerase active site, which supports the idea that MIF tautomerase inhibitors may have potential to interfere with MIF cytokine function [19]. In this perspective, robust assays to test the ability of MIF tautomerase inhibitors to interfere with MIF cytokine functions in vitro and in vivo are highly important.
Functional cytokine roles of MIF and D-DT have been described in innate and adaptive immune responses [7][13]. It has been shown that MIF stimulates the production of pro-inflammatory mediators such as tumor necrosis factor α (TNFα, interferon-γ (IFNγ), interleukins 1β, 2, 6 and 8 (IL-1β, IL-2, IL-6 and IL-8) and other effector cytokines [13]. The MIF-CD74 interaction is well known to initiate subsequent signaling cascades leading to cellular responses [18]. The biological functions of the CD74 receptor in immune diseases has recently been reviewed by Su et al. [20]. With respect to CD74 binding it is interesting to note that a difference has been reported between MIF and D-DT in a study by Merk et al. [21]. The same study indicates that MIF has a steeper dose-response ratio for macrophage migration inhibition and glucocorticoid overriding. This suggests an immune downregulatory role for D-DT in the presence of MIF. On the contrary, a recent study demonstrated that both MIF and D-DT are connected to disease progression of multiple sclerosis subjects [22]. This study and other studies as reviewed by Merk et al. [7] and O’Reilly et al. [23] indicate an overlapping activity spectrum for both cytokines.
Apart from the CD74 receptor MIF can also bind to chemokine receptors such as CXCR2, CXCR4 and CXCR7 to induce inflammatory and immune cell chemotaxis [24][25]. Given the pro-inflammatory activities, MIF is implicated in acute and chronic inflammatory diseases such as asthma, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis, sepsis, diabetes, atherosclerosis and cardiovascular diseases [13]. Many studies have investigated MIF as a biomarker for various diseases that have an inflammatory component, including systemic infections and sepsis, cancer, autoimmune diseases and different metabolic disorders, suggesting its important role in these diseases [26].
It should also be noted that MIF has been demonstrated to be post-translationally modified and that these modifications affect its biological functions [27]. MIF, but not D-DT, has a CXXC motif that can be oxidized to an intramolecular disulfide-bond. Oxidised MIF has also been proposed as a biomarker for different diseases [28]. Other studies indicate that oxidized MIF is the disease-related conformational isoform that could be employed for diagnosis and therapy [29][30]. It is to be expected that MIF redox behavior interferes with binding of small molecule inhibitors. However, little is known about the structural consequences of these modifications in relation to inhibitor binding. Therefore, this represents an interesting and novel line of investigation.
Despite extensive studies on the functional role of MIF in multiple disease models, the identification and validation of the functional consequences of small molecule MIF tautomerase inhibitors is still in an early stage. The effects of such inhibitors have been investigated using the long-known standard MIF antagonist ISO-1 (Table 1) in in vitro assays and animal models [31]. Research on MIF antagonists is still in the preclinical stage and data on human disease are still observational [32], which indicates a need for further validation of MIF as a drug target.
Table 1. MIF inhibitors with a phenol functionality as the key structural element that presumably binds to the active site residue Asn-97 of MIF.
| Class | Compound | Structure | Activity, μM Reference(s) |
|---|---|---|---|
| chromen-4-one | Orita-13 |  |
Ki = 0.04 [39] (TA), Ki = 17 [49] (TA), Ki = 13-22 [40] (TA) |
| isoxazoline | ISO-1 | 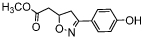 |
IC50 = 7 [41] (TA), IC50 = 24 [40] (TA); Max. 40% inhibition [42] (BA) |
| Alam-4b | 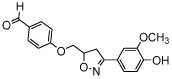 |
IC50 = 7.3 [46] (TA) | |
| ISO-66 | 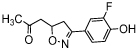 |
IC50 = 1.5 [47] (TA) | |
| 1,2,3-triazole | Jorgensen-3g |  |
IC50 = 0.75 [48] (TA); IC50 = 0.9 [48] (BA) |
| Jorgensen-3h |  |
IC50 = 1 [48] (BA) | |
| Dziedzic-3bb (Cisneros-3i) |
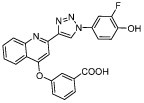 |
Dziedzic-3bb: Ki = 0.057 [49] (TA) Cisneros-3i: Ki = 0.057 [50] (TA); Kd = 0.071 [50] (BA) |
|
| Cisneros-3j |  |
Ki = 0.034 [50] (TA); Kd = 0.063 [50] (BA) |
TA, tautomerase assay; BA, binding assay MIF-CD74
In this review we aim to provide an overview of recent advances in the identification of MIF as a pharmacological target in the pathogenesis of inflammatory diseases and cancer (Figure 1C). Based on that we will provide an overview of the current developments in the disovery and design of small molecule MIF inhibitors and define future goals in this field.
The role of MIF in pathogenesis of inflammatory diseases
Acute inflammation is a protective, beneficial, and self-limiting process during innate immune responses, but chronic inflammation is maladaptive and may result in tissue injury and dysfunction. For instance, some studies have shown that MIF plays an important role in the pathology of bladder inflammation. In bladder tissue substance P, an important inflammatory mediator, increases the levels of MIF. The important role of MIF was subsequently shown by administration of an anti-MIF antibody that could decrease substance P-induced inflammatory changes in bladder [33]. MIF was also shown to play a role in worsening of lung inflammation. High levels of MIF were reported to be detrimental for survival in a mouse model of pneumococcal pneumonia. Treatment of mice with a small-molecule inhibitor of MIF, designated MIF098 (Alissa-5) (Table 2), improved survival by reducing inflammatory responses [34]. In addition, higher MIF levels were produced by alveolar macrophages in a mouse model for COPD as compared to those from healthy mice, and inhibition of MIF function by ISO-1 could block the corticosteroid-insensitive lung inflammation [35].
Table 2. Covalent MIF inhibitors and MIF inhibitors with other structures.
| Class | Compound | Structure | Activity, μM Reference(s) |
|---|---|---|---|
| phenyl-pyrimidine | 4-IPP | 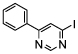 |
IC50 = 0.2-0.5 [51] (TA) |
| isothiocyanate | BITC | 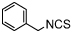 |
IC50 = 0.79 [53] (TA) |
| benzoxazinone | NVS-2 |  |
IC50 = 0.020 [55] (TA); Ki = 0.027 [50] (TA); Kd = 0.055 [50] (BA) |
| benzoxazol-2-one | MIF098 (Alissa-5) | 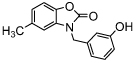 |
IC50 = 0.01 [56] (TA) |
| pyrimidazole | K664-1 | 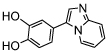 |
IC50 = 0.11 [59] (TA); Ki = 45 [40] (TA), Ki = 0.16 [58] (TA) |
| chromene | T-614 | 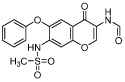 |
IC50 = 6.81 [58] (TA) |
| Kok-10 | 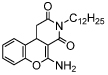 |
IC50 = 18 [60] (TA) | |
| Kok-17 | 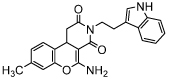 |
IC50 = 6.2 [60] (TA) | |
| isoxazoline | CPSI-2705 | 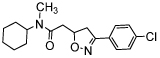 |
2-10-fold more potent than ISO-1 [35] (TA) |
| CPSI-1306 | 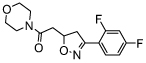 |
100-fold more potent than ISO-1 [35] (TA) | |
| isocoumarin | SCD-19 | 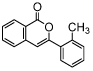 |
100% inhibition at concentration of 100 μM [37] (TA) |
TA, tautomerase assay; BA, binding assay MIF-CD74k
MIF also acts as an essential mediator of host immunity against various bacterial infections, however, its persistent or recurrent expression during chronic inflammatory disease stages can lead to loss of function and mortality. The involvement of MIF in the enhancement of biofilm formation by Pseudomonas aeruginosa was shown by the use of SCD-19, a small molecule inhibitor targeting MIF tautomerase activity. Application of this inhibitor resulted in lower bacterial burden in a mouse model of this infection as compared to untreated mice [36]. As another example, MIF promotor polymorphisms resulting in high MIF levels in cerebrospinal fluid of patients with streptococcal meningitis correlated with systemic complications and death [37]. Moreover, the authors of this study showed a reduction in bacterial load in a mouse model of pneumococcal pneumonia and sepsis after treatment with an anti-MIF antibody. From these studies it becomes clear that MIF plays a role in biofilm formation and mortality during bacterial infections, and it provides initial evidence that targeting MIF with small molecule inhibitors has potential to interfere with such pathological conditions.
A relationship of MIF with autoimmune inflammatory disease has been observed in experimental autoimmune myocarditis. Early treatment of this disease with an anti-MIF antibody markedly delayed the onset of, and significantly reduced the severity of, this disease in rats [38]. The importance of MIF in the autoimmune inflammatory process has also been demonstrated for rheumatoid arthritis. It was observed that treatment with an anti-MIF antibody before immunization with type II collagen leads to delayed onset of arthritis in a mouse model of collagen-induced arthritis [39]. Altogether, these studies indicated that MIF correlates with disease severity in autoimmune inflammatory diseases and that treatment with anti-MIF antibodies has beneficial effects.
The role of MIF in pathogenesis of cancer
Inflammation and immunity play key roles in the onset and progression of cancer. Persistent or recurrent inflammation is related to development of cancer. In contrast, anti-cancer immune responses counteract the development and progression of cancer. From this perspective, the functional output of MIF as a key cytokine of the immune system can be connected to various aspects of oncology [40].
High serum levels of MIF are seen in cancer patients and tissues and MIF has consequently been proposed as a biomarker. In addition, high MIF levels correlate with poor prognosis in various carcinomas. Besides being involved in angiogenesis and thus indirectly in promoting tumour growth, the interaction of MIF with its receptors has been shown to initiate cancer promoting signal transduction pathways. For example, binding of MIF to CD74 can lead to stimulation of the ERK1/2 but also PI3K/AKT pathways and binding of MIF to the CXCR4 receptor was suggested to induce metastases [41].
Involvement of MIF in the development of prostate cancer was shown by studies with an androgen-independent prostate cancer cell line. In these studies, inhibition of MIF by ISO-1, anti-MIF antibody, or MIF siRNA resulted in decreased cell proliferation. ISO-1 significantly decreased tumor volume and tumor angiogenesis [42]. In another study with a prostate cancer cell line, treatment with anti-MIF antibodies was reported to reduce cell growth and in a xenograft mouse model of prostate cancer, anti-MIF antibodies were shown to limit tumor growth [43].
The involvement of MIF in the development of bladder cancer has also been described. MIF was reported to promote in vitro and in vivo bladder cancer progression via increasing cell proliferation and angiogenesis. The orally available MIF inhibitors, CPSI-2705 and -1306 (Table 2), have been shown to effectively decrease the growth and progression of bladder cancer in vivo [44].
A study on the role of MIF in the development of colon cancer in patients reported a positive correlation between MIF serum concentrations and colorectal cancer severity [45], thus indicating a potential use of MIF as biomarker. The same study demonstrated in a mouse model with colon carcinoma cell transplants that treatment with MIFinhibitor ISO-1 or anti-MIF antibodies resulted in significant reduction in the tumor burden, thus indicating a potential use of MIF directed therapeutics in cancer.
Furthermore, MIF has been indicated to be involved in the progression of lung cancer. Blocking the hydrophobic pocket that harbours MIF tautomerase activity, by a small molecule inhibitor of the isocoumarin class, SCD-19 (Table 2), significantly attenuated lung cancer growth [46].
Taken together, these studies demonstrate a positive correlation between MIF and the progression of cancer. Application of small molecule MIF inhibitors or anti-MIF antibodies attenuated cancer growth, thus indicating the potential of anti-MIF therapeutics in cancer [47][48][49].
Small molecule inhibitors of MIF
Because of the essential involvement of MIF in the progression of numerous disorders with an inflammatory component, it is not surprising that attempts were made to find MIF directed therapeutics. One line of development is the application of biologicals, such as anti-MIF antibodies, as novel therapeutics. This approach has been used in many proof of concept studies [38] [39] [33] [42] [43] [45]. One clinical trial with an anti-MIF antibody has been reported, however no results have been revealed yet [50]. The other line of development is to generate MIF-binding small molecules with the aim to interfere with MIF functions. To develop MIF inhibitors, many studies resort to evaluating interference with MIF tautomerase activity. In this perspective, the evaluation of MIF tautomerase inhibitors for their interference with MIF cytokine functions in relevant disease models is highly important. Compared with biologicals, small molecule MIF inhibitors offer advantages such as lower manufacturing costs, non-immunogenic reaction and the possibility of oral administration. Therefore, this route of exploration gained tremendous interest. Here, we summarize the currently identified MIF tautomerase inhibitors and discuss their structure-activity relationships.
Inhibitors containing a chromen-4-one scaffold were identified in 2001 and their Ki values range between 0.04 and 7.4 μM. The most potent compound of this class is Orita-13 with a Ki of 0.04 μM [51]. However, a later investigation reported Ki values in the range of 13-22 μM for Orita-13 [52]. The phenol functionality in Orita-13 is also found in the MIF tautomerase substrates D-dopachrome and p-hydroxyphenylpyruvate and proved to be a succesfull design motif for MIF inhibitors (Table 1).
Isoxazolines as MIF inhibitors
The most frequently used reference inhibitor for MIF tautomerase activity is ISO-1, which was discovered in 2002. This inhibitor of the isoxazoline class was reported to inhibit MIF tautomerase activity in a dose-dependent manner. It binds at the same position as the substrate p-hydroxyphenylpyruvate with an IC50 of about 7 μM [53]. A later study described a Ki of 24 μM for inhibition of MIF tautomerase activity [52]. A MIF-CD74 binding study reported a maximum of 40% inhibition at 10 μM (Table 1) [54]. Further studies showed that inhibition of MIF by ISO-1 in a mouse model significantly reduced prostate cancer [42], colon cancer [45], and blocked melanoma cell growth [55]. Another study in a mouse model showed that this MIF inhibitor blocks corticosteroid-insensitive lung inflammation [35]. In addition, ISO-1 was also reported to inhibit MIF activity in a mouse model of type 1 diabetes and to result in the delayed onset of this disease [56]. Altogether, ISO-1 is a valuable compound that is widely used as a reference inhibitor in the initial validation of small molecule MIF tautomerase inhibitors as potential therapeutics in diseases with an inflammatory component.
Another small molecule MIF tautomerase inhibitor with an isoxazoline scaffold is CPSI-1306 (Table 2). This inhibitor lacks the characteristic phenol functionality, which is advantageous for applications in vivo. Phenol functionalities are generally considered to be non-druglike because they are prone to phase II bioconjugation reactions thus resulting in quick inactivation and excretion in in vivo experiments. Oral administration of this inhibitor resulted in less severe symptoms in a mouse model for multiple sclerosis as compared to untreated mice [57]. Further small molecules with the same scaffold were synthesized and evaluated for MIF-inhibitory activity. The IC50 of the most active compound, Alam-4b (Table 1), was 7.3 μM in a MIF tautomerase assay and this compound was shown to be nontoxic in a cell viability assay [58]. Subsequently, in 2014, another small molecule inhibitor of isoxazoline class, ISO-66 (Table 1), was reported. Its IC50 in the MIF tautomerase assay was 1.5 μM and long-term administration of ISO-66 in a mouse model of colon cancer or melanoma was shown to be nontoxic and to decrease tumor burden significantly [59]. Thus, studies on MIF inhibitors with an isoxazoline scaffold demonstrate that the development and application of small molecule MIF inhibitors has potential to provide novel therapeutics.
1,2,3-triazoles as MIF inhibitors
In 2010, 1,2,3-triazole derivatives were reported as inhibitors of MIF. The most potent compounds, Jorgensen-3g and Jorgensen-3h (Table 1), showed IC50 values of about 1 μM for MIF tautomerase activity and MIF-CD74 binding [60]. Subsequently, in 2015, improvements were made by synthesis of several optimized biaryltriazoles. This provided potent compounds with a phenolic hydroxyl group that bind to the MIF tautomerase active site. Neverthless, some compounds had limited water solubility. The activity of this class of compounds was further improved by the addition of a fluorine atom adjacent to the phenolic hydroxyl group to enhance the hydrogen bond interaction with residue Asn-97 of MIF. This yielded the most potent compound, Dziedzic-3bb (Table 1), having a Ki value of 0.057 μM and a solubility that is in the normal range for orally avialable drugs [61].
The synthesis of fluorescently-labeled MIF inhibitors with a biaryltriazole scaffold was described in 2016. These inhibitors were used in a fluorescence polarization assay to assess the direct binding of inhibitors to the active site of MIF. The two most potent inhibitors, denoted Cisneros-3i and Cisneros-3j, were reported to have Ki’s of 0.057 and 0.034 μM in the tautomerase assay and Kd’s of 0.071 and 0.063 μM in the fluorescence polarization-based binding assay, respectively (Table 1) [62].
Covalent MIF inhibitors
The specific reactivity of the proline in the MIF active site provides opportunities to develop covalent inhibitors. In 2008, a compound of the phenyl-pyrimidine class, 4-IPP (Table 2), was reported to inactivate the MIF catalytic function by dehalogenation and formation of a covalent bond between C-4 of pyrimidine and the N-terminal nitrogen of Pro-1 in the MIF tautomerase active site. This compound also inferferes with the biological functions of MIF as it was reported to irreversibly inhibit lung adenocarcinoma cell migration and anchorage-independent growth [63]. Later on, it was described that 4-IPP inhibits the growth of thyroid cancer cells by inducing apoptosis and mitotic cell death [64]. In 2009, isothiocyanates were discovered as irreversible MIF tautomerase inhibitors. The isothiocyanate BITC (Table 2) was shown to covalently modify the Pro-1 residue in the MIF active site. This drastically alters the MIF tertiary structure and results in loss of its tautomerase activity and in inhibition of MIF binding to CD74 [65].
The Woodward’s reagent K is a classical heterocyclic electrophile with a specific reactivity [66][67]. Taking advantage of the specific reactivity of Woodward's Reagent K, covalent MIF inhibitors were developed. These inhibitors were shown to react with the active site Pro-1 of MIF and were applied for covalent labeling of MIF that proved to be selective. The covalent inhibitors were used as probes for labeling and imaging of MIF activities in living cells [68]. These examples demonstrate that it is possible to develop covalent active site-directed inhibitors of MIF that bind with a reasonable level of selectivity. Such inhibitors have great potential for labeling and imaging of enzyme activity in vitro and in vivo. In contrast to their application in imaging, covalent inhibitors are not preferred in pharmacotherapy due to concerns about their off-target effects.
Other type of MIF inhibitors
Apart from the isoxazolines and 1,2,3-triazoles, other types of reversible MIF inhibitors were also developed. In 2006, MIF inhibitors with a benzoxazinone scaffold were described and patented. The most potent compound, NVS-2, was reported to have an IC50 of 0.020 μM (Table 2) [69]. A later assay by Cisneros et al. reported a similar value for the Ki of 0.027 μM in the tautomerase assay and a Kd of 0.055 μM in the fluorescence polarization-based binding assay [62]. Subsequently, in 2010, substituted benzoxazol-2-ones were discovered as MIF antagonists (Table 2). One potent inhibitor from this class, MIF098 (Alissa-5), showed noncovalent inhibition in the MIF tautomerase assay, with an IC50 of around 0.010 μM. This inhibitor was further reported to attenuate MIF-dependent ERK phosphorylation in human synovial fibroblasts, which demonstrates possible use of MIF inhibition as therapy in rheumatoid arthritis [70].
In 2012, an allosteric MIF tautomerase inhibitor p425 was identified in a high-throughput screening of a library consisting of 230,000 small molecules. However, this compound is a sulfonated azo compound (also known as pontamine sky blue), which has poor druglike properties [71]. Another study, in 2016, reported inhibitor K664-1 (Table 2) with a pyrimidazole scaffold as a novel MIF inhibitor with an IC50 of 0.16 μM in the MIF tautomerase assay [72]. This inhibitor provided protection to β-cells from cytokine-triggered apoptosis in a mouse model, which demonstrates its potential for the prevention of diabetes progression [73].
In 2016, compound T-614 (also known as iguratimod) was found to selectively inhibit MIF in vitro and in vivo. The compound has synergic effects with glucocorticoids to slow disease progression in a mouse model of multiple sclerosis. The IC50 of that compound in the MIF tautomerase assay was 6.81 μM (Table 2) [72]. Recently, novel types of MIF inhibitors were discovered using substitution-oriented screening (SOS). Inspired by the known chromen-4-one inhibitor Orita-13, a focused collection of compounds with a chromene scaffold was screened for MIF binding. In this study, inhibitors 10 and 17 (denoted Kok-10 and Kok-17, Table 2) provided IC50 values in the low micromolar range (18 and 6.2 μM, respectively) in the MIF tautomerase assay. The binding proved to be reversible and the enzyme kinetics suggested no direct interaction of these compounds with the substrate binding pocket [74].
Future perspective of inhibitor development
Altogether it can be concluded that a diverse array of structures can be employed to develop MIF inhibitors that interact with MIF tautomerase activity via direct competition, via allosteric modulation of substrate binding, or via covalent binding. This provides a valuable starting points to design novel structural motifs that can be employed to interfere with MIF cytokine functions. Then, inhibitors of the enzymatic activity of MIF should also be evaluated in assays for binding to its cellular receptors such as CD74, CXCR2, CXCR4 and CXCR7 and/or in disease models.Within this context it is important to note that a study by Cisneros et al. in 2016 demonstrated that the reported IC50’s of MIF tautomerase inhibitors were often not reproducible [52]. Most inhibitors were shown to be less potent than previously reported. As pointed out, for covalent or slow-tight binding inhibitors the IC50’s are time dependent. Therefore, it is important to evaluate the reversibility of binding by recovery of enzyme activity in, for example, dilution experiments [75], which is too often neglected. Another complicating factor is the enzyme kinetics of the MIF tautomerase activity for its substrate p-hydroxyphenylpyruvate (4-HPP) that provides a sigmoidal curve, which cannot be fitted to a simple one-to-one binding model. Thus, the Michaelis-menten constant Km cannot be derived easily and one needs to resort to Khalf,app [74] or [S]0.5 [16]. This issue complicates the calculation of the equilibrium constant for inhibition (Ki) from IC50 values. Therefore, we argue to include enzyme dilution experiments and enzyme kinetic studies, or direct binding assays, if IC50 values are reported in order to provide a more complete analysis of MIF binding.
Conclusive remarks
MIF has been described to play a key role in the pathogenesis of inflammatory diseases and cancer. Small molecule inhibitors of MIF have been developed and used in studies to investigate the biological role of MIF. Inhibitor ISO-1 is widely used as a reference compound for MIF inhibition in mouse models of lung inflammation, prostate cancer, colon cancer, melanoma and diabetes. Other small molecule inhibitors also provided positive effects in various disease models. Altogether this indicates the potential of MIF inhibitors for development of novel therapeutics for diseases with an inflammatory component.
It is commonly presumed that MIF inhibitors identified in a MIF tautomerase assay have potential to interfere with MIF cytokine functions. Following this line of argumentation several classes of MIF tautomerase inhibitors have been identified. The isoxazolines and the 1,2,3-triazoles are important classes of inhibitors from which potent MIF inhibitors were identified. Also covalent inhibitors that react with the active site Pro-1 of MIF have been identified and in one case used for activity-based labeling of MIF in living cells. Over time an increasing number of MIF inhibitors has been described, thus providing more insight in structure-activity relationships for MIF binding. A complicating factor in the analysis of MIF inhibitors proved to be covalent or slow-tight binding behavior that results in overestimation of the inhibitors potency. Also the sigmoidal enzyme kinetics for MIF tautomerase activity complicates analysis of MIF binding. We argue that anticipation of these issues is needed for successful further development of the field.
Ultimately, the identification of potent MIF inhibitors with favorable properties for drug discovery programs will enable the identification of novel therapeutics that target MIF functions in diseases with an inflammatory component. Furthermore, attention should be given toward the MIF structural homolog D-DT, which has been demonstrated to have an overlapping functional spectrum of action. This suggests that the combined or separate therapeutic targeting of D-DT and MIF could have additional advantages.
Acknowledgement
We thank the Directorate General of Higher Education Indonesia (DIKTI) for giving the grant 94.18/E4.4/2014, in collaboration with the University of Surabaya (Ubaya)-Indonesia and the University of Groningen (RuG)-The Netherlands (to T.K). We acknowledge the European Research Council for providing ERC starting grant 309782 (to F.J.D.) and the NWO for providing VIDI grants 723.012.005 (to F.J.D.) and 700.56.421 (to G.J.P.).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- [1].Davidt JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966;56:72–7. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–2. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- [3].Nathan CF, Karnovsky ML, David JR. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971;133:1356–76. doi: 10.1084/Jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bloom J, Sun S, Al-Abed Y. MIF, a controversial cytokine: a review of structural features, challenges, and opportunities for drug development. Expert Opin Ther Targets. 2016;20:1463–75. doi: 10.1080/14728222.2016.1251582. [DOI] [PubMed] [Google Scholar]
- [5].O’Reilly C, Doroudian M, Mawhinney L, Donnelly SC. Targeting MIF in Cancer: Therapeutic Strategies, Current Developments, and Future Opportunities. Med Res Rev. 2016;36:440–60. doi: 10.1002/med.21385. [DOI] [PubMed] [Google Scholar]
- [6].Weiser WY, Temple PA, Witek-Giannotti JS, Remold HG, Clark SC, David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1989;86:7522–6. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Merk M, Mitchell RA, Endres S, Bucala R. D-dopachrome tautomerase (D-DT or MIF-2) doubling the MIF cytokine family. Cytokine. 2012;59:10–7. doi: 10.1016/j.cyto.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Poelarends GJ, Veetil VP, Whitman CP. The chemical versatility of the beta-alpha-beta fold: catalytic promiscuity and divergent evolution in the tautomerase superfamily. Cell Mol Life Sci. 2008;65:3606–18. doi: 10.1007/s00018-008-8285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wasiel AA, Rozeboom HJ, Hauke D, Baas BJ, Zandvoort E, Quax WJ, et al. Structural and functional characterization of a macrophage migration inhibitory factor homologue from the marine cyanobacterium Prochlorococcus marinus. Biochemistry. 2010;49:7572–81. doi: 10.1021/bi1008276. [DOI] [PubMed] [Google Scholar]
- [10].Wasiel AA, Baas B-J, Zandvoort E, Quax WJ, Poelarends GJ. Dehalogenation of an anthropogenic compound by an engineered variant of the mouse cytokine macrophage migration inhibitory factor. Chembiochem. 2012;13:1270–3. doi: 10.1002/cbic.201200153. [DOI] [PubMed] [Google Scholar]
- [11].Sparkes A, De Baetselier P, Roelants K, De Trez C, Magez S, Van Ginderachter JA, et al. The non-mammalian MIF superfamily. Immunobiology. 2017;222:473–82. doi: 10.1016/J.IMBIO.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Suzuki M, Sugimoto H, Nakagawa A, Tanaka I, Nishihira J, Sakai M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996;3:259–66. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- [13].Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rosengren E, Åman P, Thelin S, Hansson C, Ahlfors S, Björk P, et al. The macrophage migration inhibitory factor MIF is a phenylpyruvate tautomerase. FEBS Lett. 1997;417:85–8. doi: 10.1016/S0014-5793(97)01261-1. [DOI] [PubMed] [Google Scholar]
- [15].Donnelly SC, Bucala R. Macrophage migration inhibitory factor: a regulator of glucocorticoid activity with a critical role in inflammatory disease. Mol Med Today. 1997;3:502–7. doi: 10.1016/S1357-4310(97)01133-7. [DOI] [PubMed] [Google Scholar]
- [16].Lubetsky JB, Swope M, Dealwis C, Blake P, Lolis E. Pro-1 of macrophage migration inhibitory factor functions as a catalytic base in the phenylpyruvate tautomerase activity. Biochemistry. 1999;38:7346–54. doi: 10.1021/bi990306m. [DOI] [PubMed] [Google Scholar]
- [17].Cooke G, Armstrong ME, Donnelly SC. Macrophage migration inhibitory factor (MIF), enzymatic activity and the inflammatory response. Biofactors. 2009;35:165–8. doi: 10.1002/biof.27. [DOI] [PubMed] [Google Scholar]
- [18].Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF Signal Transduction Initiated by Binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pantouris G, Syed MA, Fan C, Rajasekaran D, Cho TY, Rosenberg EM, et al. An Analysis of MIF Structural Features that Control Functional Activation of CD74. Chem Biol. 2015;22:1197–205. doi: 10.1016/j.chembiol.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Su H, Na N, Zhang X, Zhao Y. The biological function and significance of CD74 in immune diseases. Inflamm Res. 2017;66:209–16. doi: 10.1007/s00011-016-0995-1. [DOI] [PubMed] [Google Scholar]
- [21].Merk M, Zierow S, Leng L, Das R, Du X, Schulte W, et al. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF) Proc Natl Acad Sci USA. 2011;108(34):E577–85. doi: 10.1073/pnas.1102941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benedek G, Meza-Romero R, Jordan K, Zhang Y, Nguyen H, Kent G, et al. MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc Natl Acad Sci USA. 2017;114:E8421. doi: 10.1073/PNAS.1712288114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O ’reilly C, Doroudian M, Mawhinney L, Donnelly SC. Targeting MIF in Cancer: Therapeutic Strategies, Current Developments, and Future Opportunities. Inc Med Res Rev. 2016;36:440–60. doi: 10.1002/med.21385. [DOI] [PubMed] [Google Scholar]
- [24].Weber C, Kraemer S, Drechsler M, Lue H, Koenen RR, Kapurniotu A, et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc Natl Acad Sci USA. 2008;105:16278–83. doi: 10.1073/pnas.0804017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- [26].Grieb G, Kim B-S, Simons D, Bernhagen J, Pallua N. MIF and CD74 - Suitability as Clinical Biomarkers. Mini-Reviews Med Chem. 2015;14:1125–31. doi: 10.2174/1389557515666150203143317. [DOI] [PubMed] [Google Scholar]
- [27].Schindler L, Dickerhof N, Hampton MB, Bernhagen J. Post-translational regulation of macrophage migration inhibitory factor: Basis for functional fine-tuning. Redox Biol. 2018;15:135–42. doi: 10.1016/j.redox.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schinagl A, Kerschbaumer RJ, Sabarth N, Douillard P, Scholz P, Voelkel D, et al. Role of the Cysteine 81 Residue of Macrophage Migration Inhibitory Factor as a Molecular Redox Switch. Biochemistry. 2018;57:1523–32. doi: 10.1021/acs.biochem.7b01156. [DOI] [PubMed] [Google Scholar]
- [29].Thiele M, Kerschbaumer RJ, Tam FWK, Völkel D, Douillard P, Schinagl A, et al. Selective Targeting of a Disease-Related Conformational Isoform of Macrophage Migration Inhibitory Factor Ameliorates Inflammatory Conditions. J Immunol. 2015;195:2343–52. doi: 10.4049/jimmunol.1500572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schinagl A, Thiele M, Douillard P, Völkel D, Kenner L, Kazemi Z, et al. Oxidized macrophage migration inhibitory factor is a potential new tissue marker and drug target in cancer. Oncotarget. 2016;7:73486–96. doi: 10.18632/oncotarget.11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Al-Abed Y, VanPatten S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3:45–63. doi: 10.4155/fmc.10.281. [DOI] [PubMed] [Google Scholar]
- [32].Bruchfeld A, Wendt M, Miller EJ. Macrophage Migration inhibitory Factor in Clinical Kidney Disease. Front Immunol. 2016;7:8. doi: 10.3389/fimmu.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Meyer-Siegler KL, Vera PL. Intraluminal antibodies to macrophage migration inhibitory factor decrease substance P induced inflammatory changes in the rat bladder and prostate. J Urol. 2004;172:1504–9. doi: 10.1097/01.ju.0000140213.54457.97. [DOI] [PubMed] [Google Scholar]
- [34].Weiser JN, Roche AM, Hergott CB, LaRose MI, Connolly T, Jorgensen WL, et al. Macrophage Migration Inhibitory Factor Is Detrimental in Pneumococcal Pneumonia and a Target for Therapeutic Immunomodulation. J Infect Dis. 2015;212:1677–82. doi: 10.1093/infdis/jiv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Russell KE, Chung KF, Clarke CJ, Durham AL, Mallia P, Footitt J, et al. The MIF antagonist ISO-1 attenuates corticosteroid-insensitive inflammation and airways hyperresponsiveness in an ozone-induced model of COPD. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tynan A, Mawhinney L, Armstrong ME, O’Reilly C, Kennedy S, Caraher E, et al. Macrophage migration inhibitory factor enhances Pseudomonas aeruginosa biofilm formation, potentially contributing to cystic fibrosis pathogenesis. FASEB J. 2017;31:5102–10. doi: 10.1096/fj.201700463R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Savva A, Brouwer MC, Roger T, Valls Serón M, Le Roy D, Ferwerda B, et al. Functional polymorphisms of macrophage migration inhibitory factor as predictors of morbidity and mortality of pneumococcal meningitis. Proc Natl Acad Sci. 2016;113:3597–602. doi: 10.1073/pnas.1520727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Matsui Y, Okamoto H, Jia N, Akino M, Uede T, Kitabatake A, et al. Blockade of macrophage migration inhibitory factor ameliorates experimental autoimmune myocarditis. J Mol Cell Cardiol. 2004;37:557–66. doi: 10.1016/J.YJMCC.2004.05.016. [DOI] [PubMed] [Google Scholar]
- [39].Mikulowska A, Metz CN, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–7. [PubMed] [Google Scholar]
- [40].Nobre CCG, de Araújo JMG, de M Fernandes TAA, Cobucci RNO, Lanza DCF, Andrade VS, et al. Macrophage Migration Inhibitory Factor (MIF): Biological Activities and Relation with Cancer. Pathol Oncol Res. 2017;23:235–44. doi: 10.1007/s12253-016-0138-6. [DOI] [PubMed] [Google Scholar]
- [41].Kindt N, Journe F, Laurent G, Saussez S. Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets. Oncol Lett. 2016;12:2247–53. doi: 10.3892/ol.2016.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Meyer-Siegler KL, Iczkowski Ka, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–9. doi: 10.4049/jimmunol.177.12.8730. doi:177/12/8730 [pii]. [DOI] [PubMed] [Google Scholar]
- [43].Hussain F, Freissmuth M, Völkel D, Thiele M, Douillard P, Antoine G, et al. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Mol Cancer Ther. 2013;12:1223–34. doi: 10.1158/1535-7163.MCT-12-0988. [DOI] [PubMed] [Google Scholar]
- [44].Choudhary S, Hegde P, Pruitt JR, Sielecki TM, Choudhary D, Scarpato K, et al. Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis. Carcinogenesis. 2013;34:2891–9. doi: 10.1093/carcin/bgt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].He X-X, Chen K, Yang J, Li X-Y, Gan H-Y, Liu C-Y, et al. Macrophage migration inhibitory factor promotes colorectal cancer. Mol Med. 2009;15:1–10. doi: 10.2119/molmed.2008.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mawhinney L, Armstrong ME, O’ Reilly C, Bucala R, Leng L, Fingerle-Rowson G, et al. Macrophage Migration Inhibitory Factor (MIF) Enzymatic Activity and Lung Cancer. Mol Med. 2014;20:729–35. doi: 10.2119/molmed.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mangano K, Mazzon E, Basile MS, Di Marco R, Bramanti P, Mammana S, et al. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget. 2018;9:17951–70. doi: 10.18632/oncotarget.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lechien JR, Nassri A, Kindt N, Brown DN, Journe F, Saussez S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: A systematic review. Head Neck. 2017;39:2573–84. doi: 10.1002/hed.24939. [DOI] [PubMed] [Google Scholar]
- [49].Wang S, Cen X, Liang X, Tang Y. Macrophage migration inhibitory factor: a potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget. 2017;8:10650–61. doi: 10.18632/oncotarget.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Phase 1 Study of Anti-Macrophage Migration Inhibitory Factor (Anti-MIF) Antibody in Solid Tumors. [accessed February 20, 2018];ClinicalTrials.gov. Full Text View - n.d https://clinicaltrials.gov/ct2/show/study/NCT01765790. [Google Scholar]
- [51].Orita M, Yamamoto S, Katayama N, Aoki M, Takayama K, Yamagiwa Y, et al. Coumarin and chromen-4-one analogues as tautomerase inhibitors of macrophage migration inhibitory factor: discovery and X-ray crystallography. J Med Chem. 2001;44:540–7. doi: 10.1021/jm000386o. [DOI] [PubMed] [Google Scholar]
- [52].Cisneros JA, Robertson MJ, Valhondo M, Jorgensen WL. Irregularities in enzyme assays: The case of macrophage migration inhibitory factor. Bioorg Med Chem Lett. 2016;26:2764–7. doi: 10.1016/j.bmcl.2016.04.074. [DOI] [PubMed] [Google Scholar]
- [53].Lubetsky JB, Dios A, Han J, Aljabari B, Ruzsicska B, Mitchell R, et al. The Tautomerase Active Site of Macrophage Migration Inhibitory Factor Is a Potential Target for Discovery of Novel Anti-inflammatory Agents. J Biol Chem. 2002;277:24976–82. doi: 10.1074/jbc.M203220200. [DOI] [PubMed] [Google Scholar]
- [54].Cournia Z, Leng L, Gandavadi S, Du X, Bucala R, Jorgensen WL. Discovery of human macrophage migration inhibitory factor (MIF)-CD74 antagonists via virtual screening. J Med Chem. 2009;52(2):416–24. doi: 10.1021/jm801100v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tanese K, Hashimoto Y, Berkova Z, Wang Y, Samaniego F, Lee JE, et al. Cell Surface CD74–MIF Interactions Drive Melanoma Survival in Response to Interferon-γ. J Invest Dermatol. 2015;135:2775–84. doi: 10.1038/jid.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Korf H, Breser L, Van Hoeck J, Godoy J, Cook DP, Stijlemans B, et al. MIF inhibition interferes with the inflammatory and T cell-stimulatory capacity of NOD macrophages and delays autoimmune diabetes onset. PLoS One. 2017;12:e0187455. doi: 10.1371/journal.pone.0187455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kithcart AP, Cox GM, Sielecki T, Short A, Pruitt J, Papenfuss T, et al. A small-molecule inhibitor of macrophage migration inhibitory factor for the treatment of inflammatory disease. FASEB J. 2010;24:4459–66. doi: 10.1096/fj.10-162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Alam A, Pal C, Goyal M, Kundu MK, Kumar R, Iqbal MS, et al. Synthesis and bio-evaluation of human macrophage migration inhibitory factor inhibitor to develop anti-inflammatory agent. Bioorg Med Chem. 2011;19:7365–73. doi: 10.1016/j.bmc.2011.10.056. [DOI] [PubMed] [Google Scholar]
- [59].Ioannou K, Cheng KF, Crichlow GV, Birmpilis AI, Lolis EJ, Tsitsilonis OE, et al. ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models. Int J Oncol. 2014;45:1457–68. doi: 10.3892/ijo.2014.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jorgensen WL, Gandavadi S, Du X, Hare AA, Trofimov A, Leng L, et al. Receptor agonists of macrophage migration inhibitory factor. Bioorg Med Chem Lett. 2010;20:7033–6. doi: 10.1016/j.bmcl.2010.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dziedzic P, Cisneros JA, Robertson MJ, Hare AA, Danford NE, Baxter RHG, et al. Design, Synthesis, and Protein Crystallography of Biaryltriazoles as Potent Tautomerase Inhibitors of Macrophage Migration Inhibitory Factor. J Am Chem Soc. 2015;137:2996–3003. doi: 10.1021/ja512112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cisneros JA, Robertson MJ, Valhondo M, Jorgensen WL. A Fluorescence Polarization Assay for Binding to Macrophage Migration Inhibitory Factor and Crystal Structures for Complexes of Two Potent Inhibitors. J Am Chem Soc. 2016;138:8630–8. doi: 10.1021/jacs.6b04910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Winner M, Meier J, Zierow S, Rendon BE, Crichlow GV, Riggs R, et al. A Novel, Macrophage Migration Inhibitory Factor Suicide Substrate Inhibits Motility and Growth of Lung Cancer Cells. Cancer Res. 2008;68:7253–7. doi: 10.1158/0008-5472.CAN-07-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Varinelli L, Caccia D, Volpi CC, Caccia C, De Bortoli M, Taverna E, et al. 4-IPP, a selective MIF inhibitor, causes mitotic catastrophe in thyroid carcinomas. Endocr Relat Cancer. 2015;22:759–75. doi: 10.1530/ERC-15-0299. [DOI] [PubMed] [Google Scholar]
- [65].Ouertatani-Sakouhi H, El-Turk F, Fauvet B, Roger T, Le Roy D, Karpinar DP, et al. A new class of isothiocyanate-based irreversible inhibitors of macrophage migration inhibitory factor. Biochemistry. 2009;48:9858–70. doi: 10.1021/bi900957e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Woodward RB, Olofson RA, Mayer H. A NEW SYNTHESIS OF PEPTIDES. J Am Chem Soc. 1961;83:1010–2. doi: 10.1021/ja01465a072. [DOI] [Google Scholar]
- [67].Woodward RB, Olofson RA. THE REACTION OF ISOXAZOLIUM SALTS WITH BASES. J Am Chem Soc. 1961;83:1007–9. doi: 10.1021/ja01465a069. [DOI] [Google Scholar]
- [68].Qian Y, Schürmann M, Janning P, Hedberg C, Waldmann H. Activity-Based Proteome Profiling Probes Based on Woodward’s Reagent K with Distinct Target Selectivity. Angew Chemie Int Ed. 2016;55:7766–71. doi: 10.1002/anie.201602666. [DOI] [PubMed] [Google Scholar]
- [69].Billich A, Lehr P, Gstach H. MIF-Inhibitors. US 20070219189A1. 2007 United States Patent Application Publication.
- [70].Hare AA, Leng L, Gandavadi S, Du X, Cournia Z, Bucala R, et al. Optimization of N-benzyl-benzoxazol-2-ones as receptor antagonists of macrophage migration inhibitory factor (MIF) Bioorg Med Chem Lett. 2010;20:5811–4. doi: 10.1016/j.bmcl.2010.07.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bai F, Asojo OA, Cirillo P, Ciustea M, Ledizet M, Aristoff PA, et al. A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF) J Biol Chem. 2012;287:30653–63. doi: 10.1074/jbc.M112.385583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bloom J, Metz C, Nalawade S, Casabar J, Cheng KF, He M, et al. Identification of Iguratimod as an Inhibitor of Macrophage Migration Inhibitory Factor (MIF) with Steroid-sparing Potential. J Biol Chem;2016;291:26502–14. doi: 10.1074/jbc.M116.743328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vujicic M, Nikolic I, Krajnovic T, Cheng KF, Vanpatten S, He M, et al. Novel inhibitors of macrophage migration inhibitory factor prevent cytokine-induced beta cell death. Eur J Pharmacol. 2014;740:683–9. doi: 10.1016/j.ejphar.2014.06.009. [DOI] [PubMed] [Google Scholar]
- [74].Kok T, Wapenaar H, Wang K, Neochoritis CG, Zarganes-Tzitzikas T, Proietti G, et al. Discovery of chromenes as inhibitors of macrophage migration inhibitory factor. Bioorg Med Chem. 2017;26(5):999–1005. doi: 10.1016/j.bmc.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Strelow J, Dewe W, Iversen PW, Brooks HB, Radding JA, McGee J, et al. Mechanism of Action Assays for Enzymes. Eli Lilly & Company and the National Center for Advancing Translational Sciences. 2004 [PubMed] [Google Scholar]