Abstract
Previous studies in children with autism spectrum disorder (ASD) have shown elevated evening cortisol; however, few studies have examined diurnal rhythm in adolescents with ASD. Adolescence is a time of significant physical and psychological change, and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis may put adolescents with ASD at increased risk for internalizing disorders, such as anxiety and depression. The extent to which cortisol levels are associated with age, puberty and symptom profile was examined in 113 youth (ages 7–17) with ASD and typical development. Salivary samples were collected over 3 days in the home, 4 times per day (waking, 30-min post-waking, afternoon, evening). Results showed youth with ASD had higher evening cortisol and a blunted diurnal slope relative to TD youth. Pubertal development and age were significant predictors of evening cortisol, and adolescents with ASD had higher evening cortisol levels compared to children with ASD. The study extends previous reports of elevated evening cortisol in children with ASD to reveal high levels in adolescence as well. Adolescents with ASD also show a significantly blunted diurnal slope, which may be associated with risk of internalizing symptoms. Findings suggest elevated evening cortisol persists across development in youth with ASD, thus emphasizing a need to identify potential negative effects of excess cortisol exposure on health in ASD individuals.
Keywords: Autism spectrum disorder, cortisol, HPA axis, age, development, puberty, adolescents
Introduction
The hypothalamic-pituiatry-adrenal (HPA) axis is a highly-regulated system that maintains homeostasis, activates in response to stress, and restores basal levels by negative feedback mechanisms. The primary human glucocorticoid, cortisol, is secreted by the adrenal cortices of the HPA axis in response to stress (Herman & Cullinan 1997). Cortisol is also released for homeostatic purposes to maintain a diurnal rhythm, with levels highest in the morning, followed by a steady decline until reaching a nadir in the evening (Smyth et al. 1997). In a majority of individuals, there is a sharp increase in cortisol 30 minutes after waking (Pruessner et al. 1997), known as the cortisol awakening response (CAR).
The transition into adolescence is a time of significant change in neuroendocrine systems, including the HPA axis (Marceau et al. 2012). The effects of age and puberty should be considered when conducting research in youth as both have been shown to influence activity of the HPA axis (Kiess et al. 1995; Walker et al. 2001; Rosmalen et al. 2005). Adolescence and the onset of puberty is also a time marked by significant biological change, especially increased gonadal hormone release (Dahl 2004; Spear 2000), which may also influence on HPA functioning (e.g. Kudielka & Kirschbaum 2005).
Children with autism spectrum disorder (ASD) have been shown to frequently experience HPA axis dysfunction, both in response to stress and in regulation of the diurnal rhythm (see (Taylor & Corbett 2014) for review). For example, many children with ASD have elevated cortisol in response to social interaction (Lopata et al. 2008; Corbett et al. 2014; Corbett et al. 2012; Corbett et al. 2010; Schupp et al. 2013). During social evaluative threat, however, both children and adolescents with ASD show a maladaptive blunted cortisol response (Edmiston et al. 2017; Lanni et al. 2012; Corbett et al. 2012; Levine et al. 2012; Jansen et al. 2000). There is substantial evidence for elevated evening cortisol (Corbett et al. 2009; Corbett et al. 2008; Tomarken et al. 2015), as well as lower morning values (Corbett et al. 2008; Corbett et al. 2009) in children with ASD. Additionally, children and adolescents with low-functioning ASD have shown higher cortisol at several points across the diurnal rhythm (Tordjman et al. 2014; Putnam et al. 2015). Alterations in evening and/or morning values may ultimately lead to a blunted diurnal slope in at least a subgroup of individuals (Corbett et al. 2008; Corbett et al. 2009; Tomarken et al. 2015; Tordjman et al. 2014), and this elevated evening cortisol and/or blunted diurnal slope may be associated with accumulation of stress throughout the day (G. E. Miller et al. 2007) or with difficulty tolerating change (Tordjman et al. 2014).
Notably, some investigations have found no differences in diurnal rhythm between TD and ASD populations (Corbett et al. 2006; Kidd et al. 2012; Brosnan et al. 2009). In studies of the CAR, those that found no group differences were in children (Corbett & Schupp 2014; Zinke et al. 2010), while the one study that did find significant differences included an older adolescent population (Brosnan et al. 2009), suggesting that physical development may be a contributing factor in these differing results. Similarly, older children with ASD have shown higher cortisol levels compared to younger children in response to a relatively benign social encounter on a playground (Corbett et al. 2010). In TD populations, cortisol response to social evaluative threat increases throughout adolescence (Gunnar et al. 2009; van den Bos et al. 2014), becoming significantly higher throughout pubertal maturation (van den Bos et al. 2016). These findings suggest that not only do age and development play an important role in cortisol rhythm, but they may also contribute to some of the mixed findings in youth with ASD.
Chronic HPA dysfunction in ASD likely has significant negative effects on physical and mental health. Chronic stress is related to cognitive deficits in ASD (Ogawa et al. 2017), and heightened stress reactivity is associated with increased prevalence of gastrointestinal symptoms (Ferguson et al. 2016). Elevated basal cortisol levels are frequently reported in individuals with depression (e.g. Burke et al. 2005), and in neurotypical adolescents, elevated evening cortisol is associated with depressive symptoms (Van den Bergh & Van Calster 2009; Dahl et al. 1991) and elevated trait anxiety (Van den Bergh et al. 2008). Furthermore, blunted diurnal slopes are associated with internalizing symptom severity in both children and adolescents (Doane et al. 2013; Shirtcliff & Essex 2008).
The transition through puberty often coincides with increased incidence of psychiatric disorders, such as depression and anxiety (Paus et al. 2008). As HPA axis dysfunction is associated with increased prevalence of internalizing symptoms (see (Stetler & G. E. Miller 2011) for review), changes in HPA functioning during pubertal development are important to consider when investigating the increased rates of psychiatric symptoms in adolescents. This issue is particularly salient for adolescents with ASD, as youth in this population already show a high prevalence of comorbid diagnoses such as anxiety and depression (e.g. Mayes et al. 2011; Simonoff et al. 2008). Therefore, investigating age and pubertal differences in diurnal rhythm, as well as associations with internalizing symptoms, will provide further insight into the unique struggles faced by adolescents with ASD in regards to increased risk of physical and/or mental health impairments which may be related to consistently elevated or altered cortisol circadian rhythm from childhood into adolescence.
The current study sought to extend previous research investigating the diurnal regulation of cortisol in TD and ASD populations by examining developmental effects on cortisol levels. It was hypothesized that there would be significant between-group differences in cortisol levels, especially in the evening. Furthermore, it was hypothesized that within the ASD group, there would be a significant elevation in cortisol levels in adolescents relative to children. Age and pubertal development were hypothesized to be significant predictors of cortisol levels in both the TD and ASD groups. Further, associations between parent-reported levels of stress and internalizing symptoms were expected for youth with ASD.
Methods and Materials
Participants
The current study consisted of youth ages 7-to-17 (N=113). The sample included 64 participants with ASD (mean age=12.02) and 49 TD participants (mean age=11.17). Participants were primarily male (n=99), with few females in each group (n=14, 7 ASD and 7 TD). There is some overlap with the current sample and the sample reported in (Tomarken et al. 2015); however, the current study includes a much larger sample of ASD and TD participants as well as an expanded sample to include adolescents with ASD and TD. Children were defined as ages 7–12 (n=70) and adolescents as ages 13–17 (n=43).
ASD diagnosis was based on Diagnostic and Statistical Manual (DSM-V) criteria (American Psychiatric Association 2013). Diagnosis was established by: (1) previous clinical diagnosis, (2) current clinical judgement (BAC), and (3) corroborated by the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000), administered by research reliable personnel. In order to be included in the study, participants in both groups were required to have an IQ ≥ 70 as measured on the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999). Children and adolescents in the TD group had to receive a score of <10 on the Social Communication Questionnaire ((Rutter et al. 2003); described below).
The study was approved by the Vanderbilt University Institutional Review Board and performed in accordance with the Helsinki Declaration for research involving human subjects. Written informed consent was obtained from parents prior to inclusion in the study, and research participants gave verbal and written assent. Recruitment was conducted using IRB approved flyers, university-wide email announcements, and several recruitment systems via clinics, subject tracking systems, and resource centers.
Diagnostic and Assessment Measures
Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000)
The ADOS is a semi-structured interview used to assess characteristics associated with the diagnosis of ASD. All ASD participants were administered the ADOS by research reliable personnel in order to corroborate diagnosis. Internal consistency for all domains and modules ranges from 0.47 to 0.94 (Lord et al. 2000).
Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999)
The WASI is a general measure of intelligence, which can be used to estimate intellectual functioning. All participants had to score an IQ ≥ 70 on the WASI to be included. Test-retest reliability for full-scale estimated IQ is 0.95 (Wechsler 1999).
Social Communication Questionnaire (SCQ) (Rutter et al. 2003)
The SCQ is a 40-item parent-report measure of impairment in social communication associated with ASD. The SCQ was also used to screen typically developing children to confirm that scores were well below the ASD threshold (≥15 is suggestive of ASD, ≥22 is suggestive of autism). Discriminative ability to differentiate ASD from non-ASD ranges from 0.74 to 0.94.
Social Responsiveness Scale, Second edition (SRS-2) (Constantino & Gruber 2012)
The SRS-2 is a parent-report questionnaire developed to identify severity of ASD symptoms across several domains, including Social Awareness, Social Motivation, Social Cognition, Social Communication, and Restricted and Repetitive Behaviors. Domain and total scores are presented as standardized T scores. The SRS shows high sensitivities (0.74 to 0.80) and specificities (0.69 to 1.00) for ASD (Bölte et al. 2011).
Pubertal Development Scale (PDS) (Petersen et al. 1988)
The PDS is a measure of pubertal development. Though originally designed as a self-report measure, many studies have since used the measure as a parent-report form. Due to concerns regarding some of the younger children’s ability to accurately self-report, the current study used the PDS as a parent-report measure. Correlation between parent and child ratings are relatively strong for females (r=0.75) and males (r=0.77), and correlations between parent and doctor are good as well (r=0.54–0.76) (C. L. Miller et al. 1988). Parents estimated the child’s development on a scale of “1” (change has not begun) to “4” (complete) on five categories, three related to general pubertal development and two related to sex-specific development. Scores were averaged to calculate a mean development score for analyses. Pubertal development was defined as the averaged total score.
Child Behavior Checklist (CBCL) (Achenbach et al. 1991)
The CBCL is a parent-report measure of problem behaviors that may be indicative of psychiatric problems in children ages 6–18 (Achenbach et al. 1991). The questionnaire identifies behaviors across several domains, with frequency of behaviors rated on a Likert scale from 0 (“Not True”) to 2 (“Very Often True”). For the purpose of the current study, the primary subscales of interest were the Anxiety, Withdrawn/Depressed, and Internalizing disorders subscales. Cronbach’s alpha for individual subscales ranges from 0.63 to 0.97.
Stress Survey Schedule (SSS) (Groden et al. 2001)
The SSS is a parent-report questionnaire to assess daily stress of individuals with ASD and other developmental disabilities. Stress is measured across eight domains, such as Changes and Threats, Social/Environmental Interactions, and more. The questionnaire rates stress on a Likert scale range from 1 (“none or mild stress”) to 5 (“Severe stress”). Higher total scores indicate higher stress levels. Cronbach’s alpha ranges from 0.57 to 0.91.
Socioeconomic Status (SES)
Parents provided highest level of education to serve as a proxy measure of socioeconomic status. The primary outcome variable for analyses was parent education level in years. Parents chose from seven categories of education level (i.e. high school graduate, graduate degree, etc.) derived from the original Hollingshead Index of Social Status (Hollingshead 1975). Selections were coded from 1–7, and following methods from previous studies (e.g. Cohen et al. 2006), codes were then converted to approximate years of education (e.g. high school graduate = 12 years, college degree = 14 years, etc.).
Cortisol Sampling Procedure
Salivary cortisol sampling was conducted at home using well-established methods (Corbett et al. 2008). Participants provided samples over three days at four time points each day, for a total of 12 cortisol samples. Three days of sampling have previously shown acceptable stability estimates, while single-day sampling may be more sensitive to variability from day-specific factors (Tomarken et al. 2015). The four sampling times were as follows: Immediately upon waking (M1), 30-minutes post waking (M2), afternoon between 1 pm and 4 pm (AFT), and in the evening before bedtime (EVE). Participants were instructed not to eat or drink 1-hour prior to collection and to refrain from brushing their teeth in the morning until after the second morning sample. Participants passively drooled into a test tube using a straw, with approximately 1 mL of saliva collected for analysis. All samples were refrigerated until being returned to the lab, where they were placed in a −80° C freezer.
Cortisol Assay
The salivary cortisol assay was performed using a Coat-A-Count® radioimmunoassay kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA) modified to accommodate lower levels of cortisol in human saliva relative to plasma. Saliva samples, which had been stored at −80°C, were thawed and centrifuged at 3460 rpm for 15 minutes to separate the aqueous component from mucins and other suspended particles. The coated tube from the kit was substituted with a glass tube into which 100 µl of saliva, 100 µl of cortisol antibody (courtesy of Wendell Nicholson, Vanderbilt University, Nashville, TN), and 100 µl of 125I-cortisol were mixed. After incubation at 4°C for 24 hours 100 µl of normal rat serum in 0.1% PO4/EDTA buffer (1:50) and precipitating reagent (PR81) were added. The mixture was centrifuged at 3460 rpm for 30 minutes, decanted, and counted. Serial dilution of samples indicated a linearity of 0.99. Interassay coefficient of variation was 6.7%. Samples were assayed in batches, with an equal mix of ASD and TD samples for each batch.
Statistical Analysis
Independent-sample t-tests were conducted to assess differences between ASD and TD groups on several demographic and dependent measures, including age, pubertal development, IQ, parent education, SCQ total, SRS total T-score, CBCL Withdrawn, Anxiety, and Internalizing Symptoms, and SSS total score. If the assumption of normality was violated, the equivalent nonparametric test was used. Equal variance was tested with Levene’s test of homogeneity, and the Welch degree of freedom approximation was used if the assumption was violated. Effect sizes for independent sample t-test were calculated as Cohen’s d or a nonparametric alternative for Mann-Whitney tests (r; (Rosenthal 1991)).
Cortisol values were averaged for each time point, and the CAR was calculated by subtracting M2 from M1 and averaged for the three days. Area under the curve (AUC) with respect to ground was calculated according to formula 2 presented by Pruessner and colleagues (Pruessner et al. 2003). The peak-to-trough slope, representing the linear decline in cortisol throughout the day, was calculated by subtracting evening cortisol from the maximum morning cortisol value. Descriptive statistics revealed two clear outliers for evening cortisol, with one ASD participant having values more than four standard deviations below the mean and another with values more than three standard deviations above the mean. Both were removed from the sample in analyses of evening cortisol. Cortisol values were positively skewed toward large values; thus, values were log transformed (base 10) to achieve approximate normality. All log-transformed values were used in statistical analyses.
Repeated measure ANOVAs were used to assess differences in cortisol across time between TD and ASD groups and between children with ASD and adolescents with ASD. Time of collection consisted of four levels (time points). In cases of violation of sphericty, degrees of freedom were Greenhouse-Geisser corrected. Univariate ANOVAs were conducted for between group differences in the CAR, AUC, and Peak-to-trough slope. Partial η2 was calculated for ANOVA analyses to measure effect size.
Hierarchal regression assessed the contributions of age, IQ, diagnosis, and puberty in predicting evening cortisol. Step one tested the unique contribution of age and IQ, while the second step assessed associations of diagnosis and pubertal development. Moderation analyses were conducted to determine whether age or puberty was a moderator of the relationship between evening cortisol and diagnosis. Partial correlations measured associations between cortisol rhythm and psychological symptoms while controlling for age. All statistical analyses were performed using IBM SPSS Version 24.0 (IBM Corp, 2016). Moderation analyses were conducted using the PROCESS application for SPSS (Hayes 2013).
Results
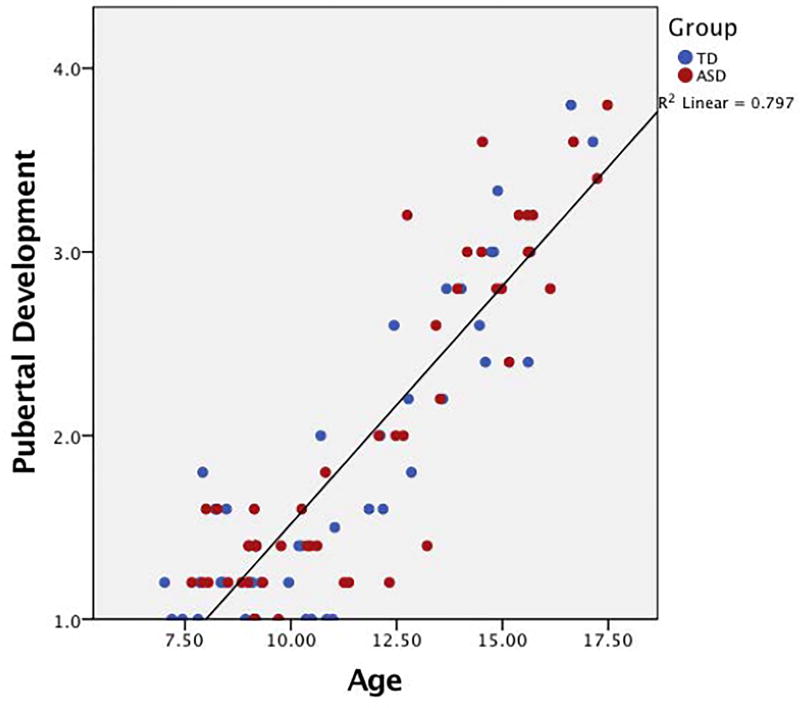
Between-group differences in demographic and diagnostic information for both groups are presented in Table 1. Tests revealed expected group differences in SCQ, SRS, SSS, CBCL, and IQ, though the ASD group did score in the average range on the WASI (see Table 1). Importantly, the two groups did not differ in age or pubertal development. Pearson product correlations revealed a strong positive correlation between age and pubertal development (r=0.89, p<0.001; Figure 1), as expected.
Table 1.
Demographic and Dependent Variables.
| Variable | ASD | Typically Developing | p |
Cohen’s d or r |
||
|---|---|---|---|---|---|---|
|
|
||||||
| M | SD | M | SD | |||
| Age | 12.02 | 2.83 | 11.17 | 2.94 | 0.12 | 0.29 |
| ADOS | 13.16 | 4.47 | --- | --- | --- | --- |
| PDS | 2.03 | 0.88 | 1.79 | 0.80 | 0.18 | 0.29 |
| IQ* | 103.92 | 21.52 | 119.31 | 13.28 | <0.001 | 0.86 |
| SES | 16.27 | 2.88 | 16.71 | 2.49 | 0.43 | 0.16 |
| SCQ* | 20.74 | 8.44 | 2.32 | 2.08 | <0.001 | 0.84 |
| SRS* | 78.41 | 10.45 | 43.02 | 5.01 | <0.001 | 0.85 |
| CBCL-Withdrawn* | 62.88 | 11.35 | 52.98 | 4.76 | <0.001 | 1.14 |
| CBCL-Anxiety* | 63.14 | 9.46 | 52.77 | 5.20 | <0.001 | 1.36 |
| CBCL-Internalizing* | 62.31 | 10.18 | 46.46 | 9.80 | <0.001 | 1.59 |
| SSS* | 115.51 | 30.22 | 65.17 | 14.71 | <0.001 | 2.12 |
ASD, Autism Spectrum Disorder; ADOS, Autism Diagnostic Observation Schedule; PDS, Pubertal Development Scale; IQ, Intelligence quotient; SES, Socioeconomic status (parent education in years); SCQ, Social Communication Questionnaire; SRS, Social Responsiveness Scale; CBCL, Child Behavior Checklist; SSS, Stress Survey Schedule
p<0.001
Figure 1. Scatterplot of association between puberty and age.
A strong positive relationship is seen between pubertal development (average score on PDS) and age for both groups.
Diurnal Cortisol: TD vs. ASD
Uncontrolled
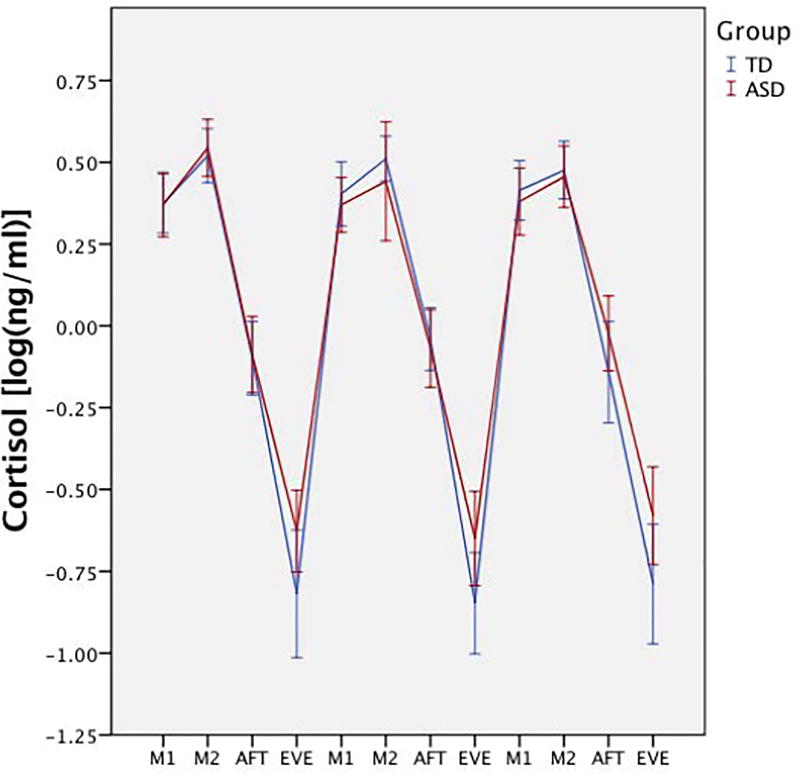
Repeated measures ANOVA, with diagnosis as a between-group variable and time as the within-group variable, showed there was no main effect for diagnosis on cortisol (F(1,103)=1.73, p=0.19, η2=0.02); however, there was a significant diagnosis × time effect (F(2.20,226.99)=4.08, p=0.02, η2=0.04), suggesting cortisol levels at different time points were dependent upon diagnosis. Comparisons showed significantly higher evening cortisol values in the ASD group (F(1,103)=6.88, p=0.01, η2=0.06; Figure 2), but other time points were non-significant (p>0.05; Table 2). Additional ANOVAs for the CAR, AUC, and peak-to-trough showed a significant effect for peak-to-trough (F(1,103)=6.70, p=0.01, η2=0.01). Descriptive statistics (Table 2) show the TD group had a significantly steeper slope compared to the ASD group.
Figure 2. Average group patterns of diurnal rhythm.
Diurnal cortisol rhythm between groups across the three home sampling days, with higher evening cortisol in the ASD group. Cortisol values are log transformed. M1 = morning sample 1; M2 = morning sample 2; AFT = afternoon; EVE= evening.
Table 2.
Descriptive statistics for cortisol
| Between Diagnostic Group (ASD vs. TD) | ||||
|
| ||||
| Time of Collection | ASD mean (SD) | TD mean (SD) | p | η2 |
|
| ||||
| Morning 1 | 0.33 (0.25) | 0.34 (0.26) | 0.82 | 0.001 |
| Morning 2 | 0.44 (0.31) | 0.48 (0.19) | 0.45 | 0.01 |
| Afternoon | −0.05 (0.32) | −0.10 (0.28) | 0.42 | 0.01 |
| Evening* | −0.60 (0.40) | −0.81 (0.41) | 0.01 | 0.06 |
| CAR (M2-M1) | 0.10 (0.32) | 0.11 (0.26) | 0.79 | 0.001 |
| AUC | 3.12 (0.19) | 3.08 (0.19) | 0.27 | 0.01 |
| Peak-to-Trough* | 1.11 (0.44) | 1.32 (0.38) | 0.01 | 0.06 |
|
| ||||
| Within ASD Group (Child with ASD vs. Adolescent ASD) | ||||
|
| ||||
| Time of Collection | ASD Child mean (SD) | ASD Adolescent mean (SD) | p | η2 |
|
| ||||
| Morning 1* | 0.39 (0.20) | 0.25 (0.29) | 0.03 | 0.08 |
| Morning 2 | 0.48 (0.29) | 0.38 (0.33) | 0.27 | 0.02 |
| Afternoon | −0.10 (0.35) | 0.02 (0.28) | 0.14 | 0.04 |
| Evening* | −0.69 (0.43) | −0.47 (0.32) | 0.04 | 0.08 |
| CAR (M2-M1) | 0.08 (0.36) | 0.12 (0.28) | 0.66 | 0.003 |
| AUC | 3.10 (0.21) | 3.13 (0.16) | 0.55 | 0.01 |
| Peak-to-Trough* | 1.25 (0.41) | 0.93 (0.43) | 0.01 | 0.13 |
Values represent log-transformed cortisol in ng/ml. ASD, Autism Spectrum Disorder; TD, Typically Developing; CAR, Cortisol Awakening Response; AUC, Area under the Curve
p<0.05
Controlling for IQ and Education
Due to group differences in total IQ, analyses were repeated with IQ was a covariate. Additionally, socioeconomic status has been shown to effect stress levels and may potentially interact with IQ differences (e.g. Bradley & Corwyn 2002), thus parent education was also included as a covariate. Notably, not all participants reported on parent education, thus analyses included a slightly reduced subset of total participants (n=93). Repeated measures analysis of covariance remained significant for a diagnosis × time interaction (F(2.12,188.77)=3.28, p=0.03, η2=0.04), with evening cortisol significantly higher in the ASD group (F(1,89)=5.54, p=0.02, η2=0.06).
Age and Puberty Associations and Interactions
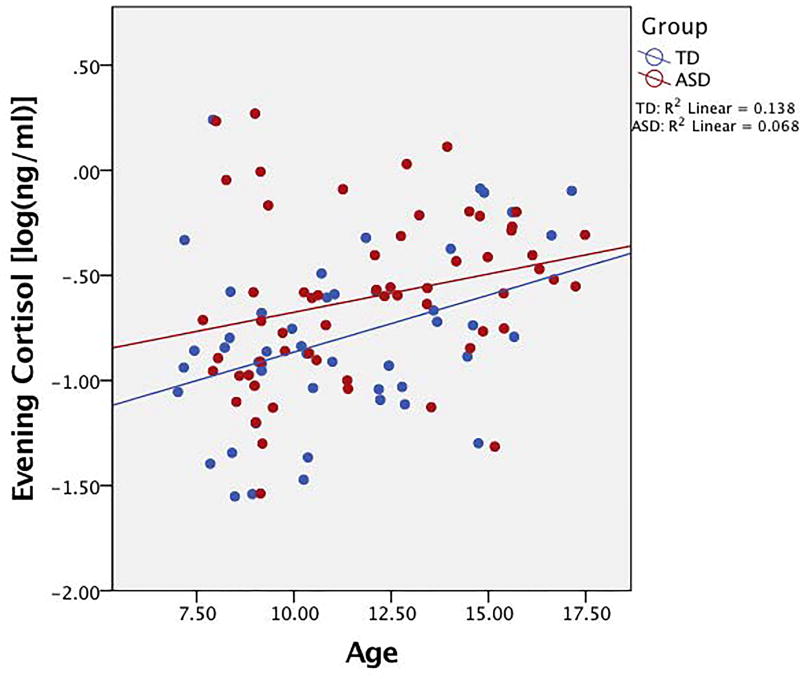
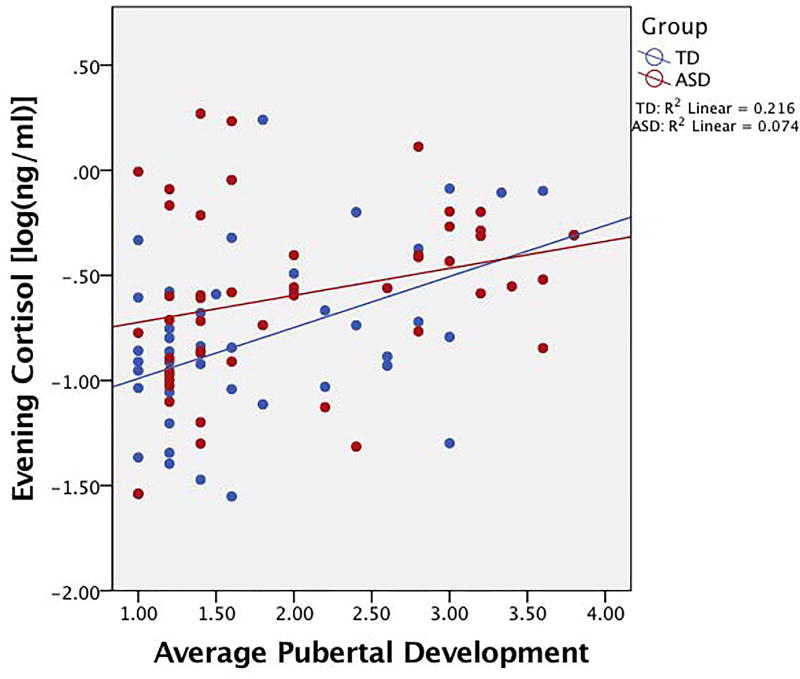
A two-step hierarchal regression with age, IQ, puberty, and diagnosis as predictors of evening cortisol was performed. The model at step one was significant (F(2,89)=5.74, p=0.01), with age and IQ accounting for 11.4% of the variation. Only age, however, was a significant contributor to the model (β=0.30, t=3.02, p=0.003). When diagnosis and pubertal development were added to the model, an additional 6.9% of variation in evening cortisol was explained, and this change in R2 was significant (F(2,87)=3.65, p=0.03). While pubertal development was a significant predictor of evening cortisol (β=0.45, t=2.11, p=0.04), diagnosis was at trend-level significance (β=0.19, t=1.73, p=0.08) after accounting for age and total IQ. Detailed results are presented in Table 3. Findings suggest significant relationships for diagnosis, age, and puberty with evening cortisol (Figures 3 and 4).
Table 3.
Regression Results for Predictors of Evening Cortisol
| Step 1 | Step 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | β | t-statistic | p | β | t-statistic | p |
| Age | 0.30 | 3.02 | 0.003 | −0.11 | −0.53 | 0.60 |
|
| ||||||
| Total IQ | −0.13 | −1.32 | 0.19 | −0.03 | −0.28 | 0.78 |
| Diagnosis | -- | -- | -- | 0.19 | 1.73 | 0.08 |
| Puberty | -- | -- | -- | 0.45 | 2.11 | 0.04 |
|
| ||||||
| Step 1 | Step 2 | |||||
|
| ||||||
| R2 | 0.114 | 0.183 | ||||
| F for change in R2 | 5.74* | 3.65* | ||||
p-value < 0.05;
IQ, Intelligence quotient
Figure 3. Plot of association with evening cortisol and age.
Positive association between evening cortisol and age in both groups. Cortisol values are log transformed.
Figure 4. Scatterplot of relationship between evening cortisol and puberty.
Positive association between evening cortisol and pubertal development (average score on PDS) for both groups. Cortisol values are log transformed.
As Figures 3 and 4 suggest possible interactions, the moderating effects of age or puberty were explored. Neither age (ΔR2=0.004, F(1,102)=0.37, p=0.54) nor puberty (ΔR2=0.012, F(1,88)=1.58, p=0.21) moderated the association between diagnosis and evening cortisol. Partial correlations between evening cortisol and pubertal development while controlling for age did show that while the TD group had a significant positive correlation between evening cortisol and PDS score (r=0.30, p=0.05), the ASD group did not (r=0.17, p=0.25).
Diurnal Cortisol: Children with ASD vs. Adolescents with ASD
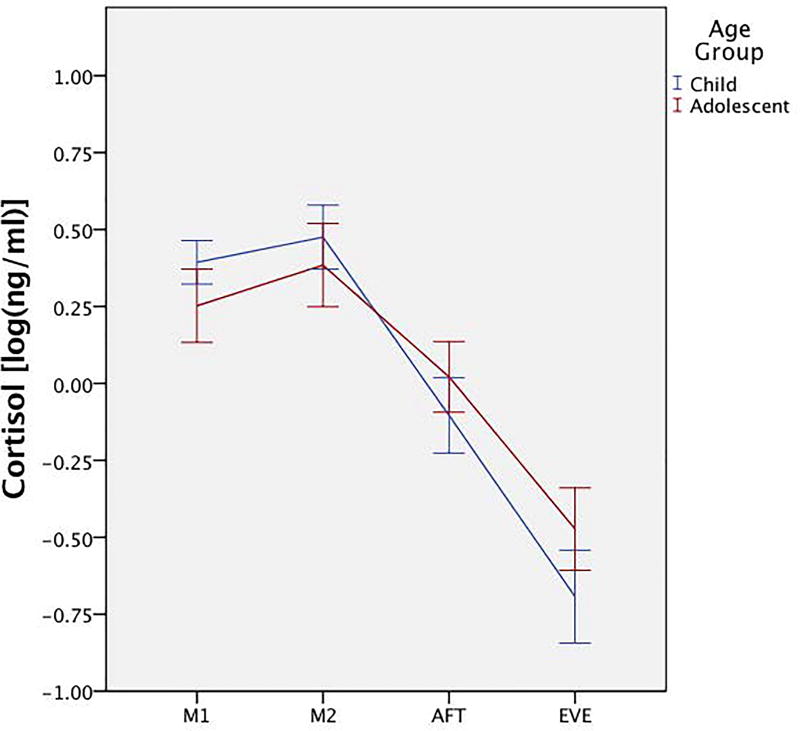
An additional repeated-measure ANOVA between children and adolescents with ASD showed no main effect for age group on average cortisol (F(1,56)=0.33, p= 0.57, η2=0.01), but there was a significant age group × time interaction (F(2.25,125.87)=4.78, p=0.01, η2=0.08). Pairwise comparisons showed differences between age groups for morning cortisol (F(1,56)=4.85, p=0.03, η2=0.08) and evening cortisol (F(1,56)=4.61, p=0.04, η2=0.08). Univariate ANOVAs for AUC, the CAR, and slope (peak-to-trough) showed a significant between-group effect for peak-to-trough (F(1,56)=8.42, p=0.01, η2=0.13), with the adolescent group having a blunted diurnal slope (Figure 5).
Figure 5. Diurnal rhythm pattern for children and adolescents with ASD.
Average diurnal rhythm between children and adolescents with ASD shows lower morning cortisol, higher evening cortisol, and flatter diurnal slope in adolescents. Cortisol values are log transformed. M1 = morning sample 1; M2 = morning sample 2; AFT = afternoon; EVE = evening.
Correlations between Cortisol and Symptom Profile in ASD
To determine whether symptoms contribute to altered diurnal cortisol rhythm in ASD, correlations were conducted between all measures shown to differ between diagnostic groups (i.e., CBCL Withdrawn, CBCL Anxiety, CBCL Internalizing, and SSS total; Table 1). Given the large age range and possible differences in internalizing symptoms with older children/adolescents (e.g. Paus et al. 2008; Zahn-Waxler et al. 2000), we chose to control for age. Analyses revealed a trend-level positive association with total area under the curve and the CBCL Withdrawn (r=0.26, p=0.07), suggesting more cortisol released throughout the day may be associated with more symptoms of withdrawal/depression when controlling for the effects of age. There were no significant associations with evening cortisol and any of the symptom variables (all p>0.05).
Discussion
The primary aim of the study was to assess the developmental effects on cortisol diurnal rhythm in TD and ASD children and adolescents. As hypothesized, youth with ASD had elevated evening cortisol compared to TD peers. Additionally, diurnal slope was significantly altered, as youth with ASD had a blunted slope relative to typically developing peers, There were no differences, however, in total cortisol output or at any other time point in the diurnal rhythm, suggesting that the HPA axis of youth with ASD may reflect the cumulative effects of stress throughout the day (Corbett et al., 2009), leading to an elevation in cortisol in the evening.
Given findings that age affects stress reactivity in both ASD and TD populations (e.g. (Schupp et al. 2013), it was hypothesized that age and pubertal development would be associated with diurnal cortisol levels in both groups. Age, puberty, and diagnosis were all significant predictors of evening cortisol values. Importantly, puberty was a unique, significant contributor to increased evening cortisol levels after controlling for the effects of age. Adolescents with ASD additionally showed significantly elevated evening cortisol levels relative to younger children with ASD. The HPA axis is known to mature throughout development, leading to a steady rise in overall cortisol levels as physical development progresses (Walker et al. 2001; Kiess et al. 1995); however, development changes of the diurnal rhythm in adolescents with ASD have previously been under-investigated. The current findings are significant as they show that like in typical development, the HPA axis matures throughout pubertal development in ASD. Diagnosis appears to be contributing to an altered diurnal rhythm, as an autism diagnosis was a significant predictor of evening cortisol levels. Interestingly, partial correlations within the ASD group did not show a correlation between evening cortisol and puberty. These findings suggest not only is evening cortisol consistently elevated throughout development in youth with ASD compared to TD youth, but this elevation cannot be solely attributable to age or puberty effects on HPA axis maturation.
Consistently elevated evening cortisol across development in youth with ASD may have significant health-related implications. Increases in evening cortisol may indicate chronic stress exposure (G. E. Miller et al. 2007) and/or an inability to disengage from daily stressors (Corbett et al. 2009; Sapolsky et al. 1986), as well as increased risk of developing psychopathology such as depression or anxiety (e.g. Burke et al. 2005; Paus et al. 2008; Kamin & Kertes 2016). Additionally, chronic exposure to stress hormones can lead to significant neuronal damage according to the neurotoxicity hypothesis (Sapolsky et al. 1986), which may result in damage to many structures implicated in cognitive and emotional regulation (e.g. Lupien et al. 2009; Paus et al. 2008; Kamin & Kertes 2016). Further, elevated arousal prior to sleep has been associated with sleep disturbance in youth with ASD (Richdale et al. 2014 Malow 2006). Clearly, if children with ASD continue to experience elevated cortisol levels throughout development, the negative effects of excess glucocorticoid exposure may pose significant physical and mental health risks.
Because previous studies have found cumulative daily stress, especially from changes throughout the day, to be strongly correlated with evening cortisol (Corbett et al. 2009), it was hypothesized that parent-reported stress would be positively associated with cortisol. However, there was no statistically significant association between stress and evening cortisol in the ASD group. Additionally, despite evidence that diurnal rhythm may be related to internalizing symptoms (e.g. Van den Bergh et al. 2008; Van den Bergh & Van Calster 2009; Dahl et al. 1991; Gunnar & Vazquez 2001), no associations were found between cortisol or diurnal slope and parent-report measures of depression and anxiety. Notably, a trend-level correlation with total cortisol output and withdrawn/depressed symptoms was seen in the ASD group, suggesting there may be a relationship between cortisol and internalizing symptoms in at least some individuals with ASD which warrants further investigation.
A few explanations may exist for why the current study did not find significant associations. First, the current study used the CBCL, a parent-report measure of internalizing symptoms, while previous reports used self-report measures (Van den Bergh & Van Calster 2009; Van den Bergh et al. 2008). Potential discrepancies in observable behaviors that may be reported by parents versus internal feelings that can be reported by the child might contribute to differences in findings. In studies of individuals with ASD, however, use of self-report can be challenging given that some individuals with ASD may have difficulty reporting on internal feelings and emotions (e.g. Losh & Capps 2006; Zamzow et al. 2016). Moreover, much of previous research has been conducted in adolescents clinicially diagnosed with an internalizing disorder (e.g. (Dahl et al. 1991), while the current study did not compare those with or without a clinical diagnosis of anxiety or depression. With a lack of strong associations between cortisol and psychological symptoms in the current study despite some evidence from previous reports (Corbett et al. 2009; Bitsika et al. 2015; Hollocks et al. 2016), it appears that relationships with diurnal cortisol and other symptoms may be related to individual variations in traits, rather than being driven by the ASD diagnosis.
Limitations and Future Directions
Strengths of the current study include utilization of a relatively large ASD sample and a comprehensive age range of children and adolescents. However, there were limitations, such as the predominantly male sample and differences in IQ between groups. Additionally, the study would have benefitted from a larger sample of adolescents and a more objective measure of pubertal development, such as clinical exam by a physician. Further, the current sample consisted only of those with high-functioning ASD (IQ ≥ 70), and thus the sample was not fully representative of the full autism spectrum. Future study across the broader range of functioning is important for identifying potential physiological differences across the broad spectrum. Future studies with a larger sample of adolescents with ASD may better determine whether diurnal cortisol is associated with significant psychopathology in these individuals. Moreover, research suggests important gender differences in the effects of HPA axis activity on mental health (Gunnar et al. 2009; Spear 2000), thus studies targeted at recruitment of female youth with ASD are warranted. Finally, the cross-sectional design did not allow for investigation of whether those with elevated evening cortisol in childhood will continue to show consistently elevated or even further increases in cortisol during development. Longitudinal studies which follow children through puberty and into adolescence may provide a better understanding of how cortisol levels across development may be related to ASD diagnosis and comorbid symptoms.
Conclusions
The current study aimed to assess diurnal profile differences throughout development in youth with ASD. Results revealed persistent evening cortisol elevation in youth with ASD, while morning and afternoon cortisol values were not different between TD and ASD groups. Additionally, adolescents with ASD show a flatter diurnal slope, due to lower morning and higher evening cortisol, relative to children with ASD. While puberty and age contribute to the elevated evening levels, there are likely influences from other symptoms in adolescents with ASD to explain why these alterations in HPA axis rhythm remain throughout early development. While there were no significant associations with internalizing symptoms in the current sample, relationships are likely complex and thus require future studies to further delineate the interactions between HPA axis dysfunction and psychopathology in adolescents with ASD. Future research will be critical in identifying what effects, if any, elevated cortisol levels may have on behavior and global functioning in autism.
Acknowledgments
Acknowledgements and Funding
The authors thank the Vanderbilt Hormone Assay and Analytical Core (supported by DK059637 and DK020593) for completion of the cortisol assays. The authors would also like to thank the families for their participation in the study.
This study was funded by NIMH R01 MH085717 awarded to BAC. No funding body had any role in the design of the study, data collection, analysis, interpretation of data, or manuscript writing. Additional support was provided by NICHD P30 HD15052 (Vanderbilt Kennedy Center) and NCATS/NIH UL1 TR000445 (Vanderbilt REDCap).
Footnotes
The authors declare that they have no conflict of interest.
References
- Achenbach TM, et al. National survey of problems and competencies among four- to sixteen-year-olds: parents' reports for normative and clinical samples. Monographs of the Society for Research in Child Development. 1991;56(3):1–131. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Bitsika V, et al. Hypothalamus-pituitary-adrenal axis daily fluctuation, anxiety and age interact to predict cortisol concentrations in boys with an autism spectrum disorder. Physiology & behavior. 2015;138:200–207. doi: 10.1016/j.physbeh.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Bölte S, et al. Autistic traits and autism spectrum disorders: the clinical validity of two measures presuming a continuum of social communication skills. Journal of Autism and Developmental Disorders. 2011;41(1):66–72. doi: 10.1007/s10803-010-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual review of psychology. 2002;53(1):371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brosnan M, et al. Absence of a normal Cortisol Awakening Response (CAR) in adolescent males with Asperger Syndrome (AS) Psychoneuroendocrinology. 2009;34(7):1095–1100. doi: 10.1016/j.psyneuen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Burke HM, et al. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Cohen S, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale- Second Edition (SRS-2) Los Angeles: Western Psychological Corporation; 2012. [Google Scholar]
- Corbett BA, Schupp CW. The cortisol awakening response (CAR) in male children with autism spectrum disorder. Hormones and behavior. 2014;65(4):345–350. doi: 10.1016/j.yhbeh.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, et al. Biobehavioral profiles of arousal and social motivation in autism spectrum disorders. Journal of child psychology and psychiatry. 2014;55(8):924–934. doi: 10.1111/jcpp.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, et al. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Research. 2009;2(1):39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, et al. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, et al. Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular autism. 2010;1(1):13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, et al. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of psychiatry & neuroscience. 2008;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Lanni KE. Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Molecular autism. 2012;3(1):13. doi: 10.1186/2040-2392-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021(1):1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE, et al. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biological psychiatry. 1991;30(1):25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Doane LD, et al. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and psychopathology. 2013;25(3):629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- Edmiston EK, Blain SD, Corbett BA. Salivary cortisol and behavioral response to social evaluative threat in adolescents with autism spectrum disorder. Autism Research. 2017;10(2):346–358. doi: 10.1002/aur.1660. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, et al. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain, behavior, and immunity. 2016;58:57–62. doi: 10.1016/j.bbi.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J, et al. The development of a stress survey schedule for persons with autism and other developmental disabilities. Journal of Autism and Developmental Disorders. 2001;31(2):207–217. doi: 10.1023/a:1010755300436. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and psychopathology. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, et al. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and psychopathology. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. New York, NY: Guilford Press; 2013. [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in neurosciences. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-Factor Index of Social Status. New Haven, CT: Unpublished manuscript; 1975. [Google Scholar]
- Hollocks MJ, et al. Dual Cognitive and Biological Correlates of Anxiety in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2016;46(10):3295–3307. doi: 10.1007/s10803-016-2878-2. [DOI] [PubMed] [Google Scholar]
- Jansen LM, et al. Unresponsiveness to psychosocial stress in a subgroup of autistic-like children, multiple complex developmental disorder. Psychoneuroendocrinology. 2000;25(8):753–764. doi: 10.1016/s0306-4530(00)00020-2. [DOI] [PubMed] [Google Scholar]
- Kamin HS, Kertes DA. Cortisol and DHEA in development and psychopathology. Hormones and behavior. 2016;89:69–85. doi: 10.1016/j.yhbeh.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Kidd SA, et al. Daytime secretion of salivary cortisol and alpha-amylase in preschool-aged children with autism and typically developing children. Journal of Autism and Developmental Disorders. 2012;42(12):2648–2658. doi: 10.1007/s10803-012-1522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess W, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatric research. 1995;37(4):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lanni KE, et al. Verbal ability, social stress, and anxiety in children with autistic disorder. Autism : the international journal of research and practice. 2012;16(2):123–138. doi: 10.1177/1362361311425916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, et al. Physiologic Arousal to Social Stress in Children with Autism Spectrum Disorders: A Pilot Study. Research in Autism Spectrum Disorders. 2012;6(1):177–183. doi: 10.1016/j.rasd.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata C, et al. Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(10):1866–1877. doi: 10.1007/s10803-008-0575-5. [DOI] [PubMed] [Google Scholar]
- Lord C, et al. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Losh M, Capps L. Understanding of emotional experience in autism: insights from the personal accounts of high-functioning children with autism. Developmental psychology. 2006;42(5):809–818. doi: 10.1037/0012-1649.42.5.809. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marceau K, Dorn LD, Susman EJ. Stress and puberty-related hormone reactivity, negative emotionality, and parent--adolescent relationships. Psychoneuroendocrinology. 2012;37(8):1286–1298. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Mayes SD, et al. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Research in Autism Spectrum Disorders. 2011;5(1):474–485. [Google Scholar]
- Miller CL, et al. Measuring pubertal development: A comparison of different scales and different sources. Biennial meeting of the Society for Research in Adolescence 1988 [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Ogawa S, et al. Associations of acute and chronic stress hormones with cognitive functions in autism spectrum disorder. Neuroscience. 2017;343:229–239. doi: 10.1016/j.neuroscience.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life sciences. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putnam SK, et al. Salivary Cortisol Levels and Diurnal Patterns in Children with Autism Spectrum Disorder. Journal of Developmental and Physical Disabilities. 2015;27(4):453–465. [Google Scholar]
- Richdale AL, et al. The role of insomnia, pre-sleep arousal and psychopathology symptoms in daytime impairment in adolescents with high-functioning autism spectrum disorder. Sleep medicine. 2014;15(9):1082–1088. doi: 10.1016/j.sleep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research. 2. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Rosmalen JGM, et al. Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30(5):483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine reviews. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schupp CW, Simon D, Corbett BA. Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. Journal of Autism and Developmental Disorders. 2013;43(10):2405–2417. doi: 10.1007/s10803-013-1790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental psychobiology. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Smyth JM, et al. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22(2):89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosomatic medicine. 2011;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology. 2014;49:207–228. doi: 10.1016/j.psyneuen.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Han GT, Corbett BA. Temporal patterns, heterogeneity, and stability of diurnal cortisol rhythms in children with autism spectrum disorder. Psychoneuroendocrinology. 2015;62:217–226. doi: 10.1016/j.psyneuen.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, et al. Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology. 2014;50:227–245. doi: 10.1016/j.psyneuen.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children's Depression Inventory. Psychoneuroendocrinology. 2009;34(5):791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, et al. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Hormones and behavior. 2008;54(2):253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- van den Bos E, et al. Adolescents' increasing stress response to social evaluation: pubertal effects on cortisol and alpha-amylase during public speaking. Child development. 2014;85(1):220–236. doi: 10.1111/cdev.12118. [DOI] [PubMed] [Google Scholar]
- van den Bos E, van Duijvenvoorde ACK, Westenberg PM. Effects of Adolescent Sociocognitive Development on the Cortisol Response to Social Evaluation. Developmental psychology. 2016 doi: 10.1037/dev0000133. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and psychopathology. 2001;13(3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and psychopathology. 2000;12(3):443–466. [PubMed] [Google Scholar]
- Zamzow RM, et al. Effects of propranolol on conversational reciprocity in autism spectrum disorder: a pilot, double-blind, single-dose psychopharmacological challenge study. Psychopharmacology. 2016;233(7):1171–1178. doi: 10.1007/s00213-015-4199-0. [DOI] [PubMed] [Google Scholar]
- Zinke K, et al. Children with high-functioning autism show a normal cortisol awakening response (CAR) Psychoneuroendocrinology. 2010;35(10):1578–1582. doi: 10.1016/j.psyneuen.2010.03.009. [DOI] [PubMed] [Google Scholar]