Abstract
Adipose-derived stem cells (ASCs) are multipotent mesenchymal progenitor cells that have functional and phenotypic overlap with pericytes lining microvessels in adipose tissue. The role of CD140b [platelet-derived growth factor receptor- β (PDGFR-β)], a constitutive marker expressed by ASCs, in the angiogenic behavior of human retinal endothelial cells (HREs) is not known. CD140b was knocked down in ASCs using targeted siRNA and lipofectamine transfection protocol. Both CD140b+ and CD140b− ASCs were tested for their proliferation (WST-1 reagent), adhesion (laminin-1 coated plates), and migration (wound-scratch assay). Angiogenic effect of CD140b+ and CD140b− ASCs on HREs was examined by co-culturing ASCs:HREs in 12:1 ratio for 6 days followed by visualization of vascular network by Isolectin B4 staining. The RayBio® Membrane-Based Antibody Array was used to assess differences in human cytokines released by CD140b+ or CD140b− ASCs. Knockdown of CD140b in ASCs resulted in a significant 50% decrease in proliferation rate, 25% decrease in adhesion ability to Laminin-1, and 50% decrease in migration rate, as compared to CD140b+ ASCs. Direct contact of ASCs expressing CD140b+ with HREs resulted in robust vascular network formation that was significantly reduced with using CD140b− ASCs. Of the 80 proteins tested, 45 proteins remained unchanged (>0.5–<1.5 fold), 6 proteins including IL-10 downregulated (<0.5 fold) and 29 proteins including IL-16 & TNF-β were upregulated (>1.5 fold) in CD140b− ASCs compared to CD140b+ ASCs. Our data demonstrate a substantial role for CD140b in the intrinsic abilities of ASCs and their angiogenic influence on HREs. Future studies are needed to fully explore the signaling of CD140b in ASCs in vivo for retinal regeneration.
Keywords: Mesenchymal stem cells, Pericyte, Endothelial, PDGF, Migration, Retina
Introduction
Adipose-derived stem cells (ASCs) are heterogenous cell population, which exists in the perivascular region of adipose tissue (1) and share functional and phenotypic markers of pericytes (2–4). ASCs are plastic adherent cell population that can be isolated from collagenase digests of adipose tissue (5). They gained big interest because they can be isolated from adipose tissue in quantities appropriate for clinical application (6). Adipose tissue is becoming widely accessible because of increased incidence of obesity and liposuction procedures in USA and worldwide (7).
Others and we have reported that ASCs have a remarkable property to self-assemble into vascular structures when in contact with a number of endothelial cell types(8–12). While it is interesting to note that ASCs take up perivascular position and the endothelial cells form angiogenic tube like structures, several studies point to the fact that ASCs enhanced vasculogenic and angiogenic potential of endothelial cells through secretion of multiple angiogenic factors (12–14). More recently, the interaction between ASCs and endothelial cells was suggested to be bi-directional, with ASCs secreting angiogenic cytokines and endothelial cells changing the expression of cell surface markers such as PECAM-1 (15).
A variety of retinal diseases involve ischemia as a predisposing factor aggravating multiple diseases including, diabetic retinopathy, age-related macular degeneration and retinopathy of prematurity (16). While majority of the studies aim at protecting against the loss of retinal neurovascular cells, much less attention is paid to regeneration of endothelial-pericyte interactions in the retina (17). Because ASCs resemble pericytes, the exogenous use of ASCs in retinal disease therapy has gained lot of attention(17). In this regard, however, the interaction between ASCs and retinal endothelial cells is not fully elucidated.
Platelet derived growth factor (PDGF) is one of the growth factors regulating cell growth and division (18), and the importance of its signaling in normal physiological development is well-documented (18). Platelet-derived growth factor receptor(s); PDGFRα (CD140a) and PDGFRβ (CD140b), are shown to be constitutively expressed in most ASCs, regardless of passage number (19, 20). Although the cell surface expression of CD140b is not a bonafide ASC marker(21), the presence of it thought to be a major cell surface marker defining pericytes(22). The impact of PDGF signaling on ASCs differentiation as well as proliferation was reported (23) (24, 25) (26). However, the specific role of CD140b signaling in ASCs in regulating angiogenic potential of retinal endothelial cells is still not completely understood. In the current study, we aim at investigating how modulation of CD140b alters behavior of ASCs and whether this exerts secondary effect on angiogenic behavior of HREs via direct or paracrine secretions.
Materials and Methods
Human ASC cultures and FACS analysis
Human ASC culture studies were approved for research per the University of Tennessee Institutional Biosafety and Institutional Review Board as exempt study. Human ASCs were obtained from commercial source (Cat#PT-5006, www.lonza.com), and used within passage 2–6. At least 3 healthy donor lots were tested in all experiments unless otherwise specified to control for any variability across donors. Cell surface marker expression in ASCs was performed by the standard procedures of FACS(27). In brief, cell samples were washed with 2 mL of staining buffer (containing 0.09% Sodium Azide). Cells were counted and Fc receptor was blocked by pre-immune IgG (1 µg of IgG per 1 × 105 cells). 100 µL of cell suspension containing 1 × 105 cells were transferred to 5 mL flow cytometry tube. 10 µL of each antibody or 10 µL of each corresponding isotype control antibody were added to the cells. The mixture was incubated for 30–45 min at room temperature (RT) in the dark. Excess antibody was removed by washing with 2 mL of staining buffer. The final cell pellet was re-suspended in 200µL of staining buffer and analyzed with FACSAria flow cytometer (BD Biosciences).
CD140b knock down in ASCs
ASCs were cultured in 6-well plates at a density of 100,000 cells per well at 37°C, 5% CO2. At 80% confluency, siRNA protocol was initiated in triplicates. In brief, cells were shifted to serum-free EBM2 media for overnight (Lonza, Cat#cc-3156) then treated with negative control siRNA (Cat#: 4390844, Ambion) or a validated siRNA against CD140b (Cat#4390826, Ambion) with the aid of Lipofectamine® RNAiMAX reagent (Invitrogen, Ref#: 13778-150). After 48 hours, transfection reagents were replaced by EGM2-MV complete media (prepared by addition of EGM2-MV SingleQuots, Lonza, Cat# CC-4147 to EBM2 media) and cells were allowed to recover for 48 hours. ASC lysates were used to assess transfection efficiency both at gene and protein levels. For gene expression, total RNA was isolated using NucleoSpin® RNA Plus kit (Macherey-Nagel GmbH, Takara Bio USA). About 50 ng of RNA samples served as a template for real time qRT-PCR suing EXPRESSS SYBR Green reagents (Life Technologies, Carlsbad, CA USA). The gene specific primers for human CD140b were GGAGAGGGCAGTAAGGAGGA (sense), ATGGTGTCCTTGCTGCTGAT (anti-sense); BMG were CGA GAT GTC TCG CTC CGT GG (sense), GTC GGA TGG ATG AAA CCC AGA CAC (anti-sense). PCR amplification was carried out using StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA USA) with cycle conditions (initial cycle: 50°C for 5 min, initial denaturation 95°C for 20 sec, 40 cycles of denaturation 95°C for 3 sec, and annealing/extension of 60°C for 30 sec). The expression levels of gene transcripts were determined using 2−DDCt and normalized to BMG. The knock down data at gene level was also confirmed at protein level by subjecting cells to FACS analysis as described above. All subsequent experiments were performed 48hrs post transient transfections.
Proliferation assay
Proliferation of CD140b+ and CD140b− ASC was assessed by Cell Proliferation Assay kit based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases (EMD Millipore) as described by us previously (8). Briefly, ASCs were cultured in flat-bottomed 96-well plate at a density of 10,000 cells per well in 100µL of EGM2-MV media (Lonza, Cat.#: cc-3156). Cells were incubated at 37°C, 5% CO2 for up to 24hrs. After incubation, 10 µL of the WST-1 dye working solution were added to the wells and plates were incubated for further 4 hours. The absorbance (A) values of each well were read at 460 nm using an automatic microplate reader (BioTek Instruments, Inc). The percentage viability was calculated using the background-corrected absorbance as follows: % viability = [(A of experimental well)/A of control well] *100.
Adhesion assay
Adhesion of CD140b+ and CD140b− ASC was assessed using Laminin as the substrate. ASCs cultured in T75 flask at 80% confluency were dislodged with 1ml TrypLE (Thermofisher). ASCs at density of 1×106/ml were taken in EBM2 medium and labeled with 5µl of cell-labeling dye DiO (Vybrant Cell labeling solutions, ThermoFisher scientific). The mixture was incubated for 20 minutes at 37°C, 5% CO2. The labeled cells were washed 3 times with PBS at 300g for 5 minutes each and resupended in the warm EGM2-MV medium. About 10,000 ASC were plated per well of a 96-well flat bottom plate coated with Laminin-1. After 2 hours of incubation, the wells were washed with 100µl PBS for 3 times and the fluorescence was measured using fluorescence plate reader (BioTek Instruments, Inc) with an excitation of 484nm and emission of 501nm. The percentage adhesion was calculated using the background-corrected fluorescence for an equal number of input.
Migration assay
Migration ability of CD140b+ and CD140b− ASC was assessed by scratch assay as described previously (28). In brief, fully confluent ASCs were scratched by means of 200 µL sterile tips, crossing in the center. Cells were incubated with or without PDGF-bb (50 ng/mL, R&D Systems, Cat#220-BB-050) as a stimulant in EBM2 media. Images were taken at 0, 6 and 24 hours at the cross-wound area of all wells by using 10× magnification with EVOS cell imaging system (ThermoFisher). The wound gap area was calculated at mentioned different time points using image-J program by comparing the area from the initial time point and cell migration was represented as percentage reduction in wound gap.
Co-culture of ASCs and human retinal endothelial cells (HREs)
Human Retinal Endothelial Cells (HREs; ACBRI 181, Cell Systems Corporation, Kirkland, WA) were co-cultured with Cd140b+ or CD140b− ASCs as described previously (8, 15). Briefly, a mixture of 5×103 HREs and 6×104 of ASC (per cm2) were re-suspended and cultured in EGM2-MV medium with 5% FBS for 6 days with media exchange every 3 days. Co-cultures were treated with PDGF-bb (50 ng/mL). At the end of the experiment, vascular networks were visualized by staining the cultures with biotinylated Ulex Europaeus Agglutinin I (Vector labs, Burlingame, CA) followed by Streptavidin DyLight® 594 (Vector labs, Burlingame, CA). 3×3 image montage of each well was captured by means of 4× objective using LIONHEARTFX automated live cell imager (BioTek Instruments, Inc.). Images were processed for total tube length by ImageJ (FIJI) software and results were expressed as tube length (pixels)/field.
Human cytokine antibody array
Human cytokines were assessed in cell supernatants of 8 healthy human donors of CD140b+ or CD140b− ASCs using a commercially available Human Cytokine Antibody Array C5 Kit according to manufacturer instructions (RayBiotech, Code: AAH-CYT-5-2). Briefly, antibody array membranes were incubated with blocking buffer for 30 minutes at RT followed by sample incubation for overnight at 4 °C. Membranes were then washed with washing buffer for a total of 3 washes (5 minutes each, at RT) followed by incubation with biotinylated antibody cocktail for 2 hours at RT. This was followed by a wash and incubation with HRP-streptavidin for 2 hours at RT. Proteins were finally detected via chemiluminescence signal using the detection buffer. Images were captured using Kodak gel imager and the data was analyzed using Image J software. Since the signal intensity for each antigen-specific antibody spot is proportional to the relative concentration of the antigen in that sample, comparison of signal intensities between array images of CD140b+ and CD140b− ASCs could be determined by the relative differences in expression levels of each analyte across the groups.
Statistical Analysis
Results are expressed as mean ± SEM. For all quantitative experiments, statistical analyses were performed with an unpaired Student T-test (Prism 4 software; GraphPad Software, La Jolla, CA, USA) and a p-value < 0.05 was considered statistically significant.
Results
Characterization of human ASCs
Human ASCs obtained from the commercial vendor (Lonza) were expected to be >90% positive for CD13, CD29, CD44, CD73, CD90, CD105 and CD166 and <5% positive for CD14, CD31 and CD45 (www.lonza.com). We used CD140b, CD105 and CD31 cell surface markers to further verify ASCs in the laboratory and in due course of passaging and culture. While the expression of CD105 is >80%, the expression of CD31 is less than 10% across all donors (Supplemental Fig. 1A&B). On the other hand, the expression of CD140b varied from 19.9% to 50.6% across three donors tested at passage 5. Our observations are in line with a previous report of about 40% positivity in cultured adipose tissue stromal vascular fraction are CD140b(29).
Knocking down CD140b alters intrinsic capabilities of ASCs
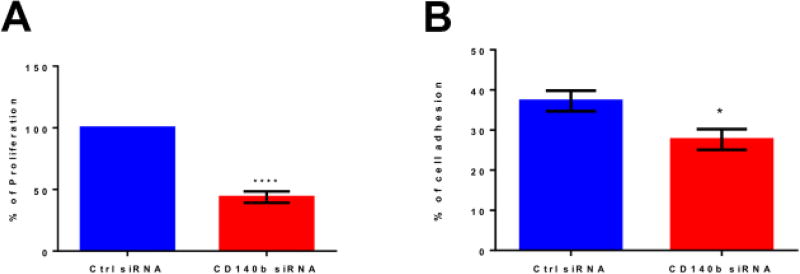
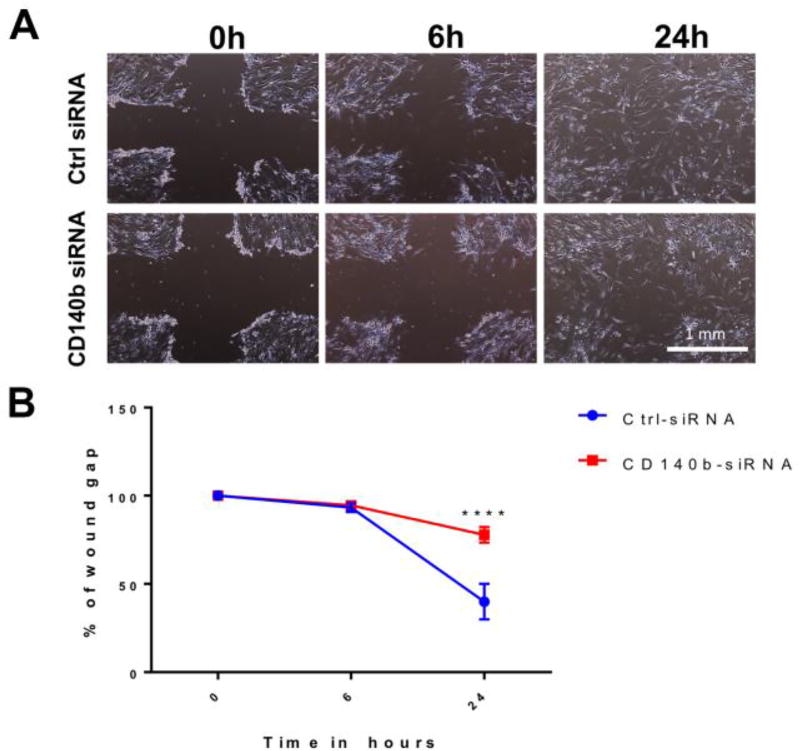
Transient transfection of ASCs with CD140b siRNA resulted in >80% knockdown (KD) of CD140b expression in cells both at gene transcript level and at protein level as compared to control siRNA treated cells (Supplemental Fig. 1C&D). Since there was no dose dependence in the KD, we used 30 picomoles of siRNA concentration in further experiments. Knocking down the expression of CD140b on the surface of ASCs resulted in significant alterations in ASCs intrinsic proliferation, adhesion and migration capabilities (Figures 1–2). As shown in Fig. 1A, the percentage proliferation of CD140b KD ASCs decreased approximately by half of those ASCs treated with control siRNA (43.8±2.68, p< 0.001; Fig. 1A). While the percentage of adhesion to extracellular matrix protein Laminin-1 by control siRNA-treated ASCs were 37.2±2.6 %, the percentage adhesion decreased in CD140b KD-ASCs to 27.6±2.6 % (p<0.05, Fig. 1B). Corroborating the proliferation and adhesion, CD140b KD ASCs demonstrated a decreased migration capacity in the scratch wound assay (Fig. 2). While the wound gap in ASCs treated with control siRNA at 6 h is 93.4±2.5%, CD140b KD ASCs were similar at 94.2±1.5%, however, the wound gap at 24h showed a remarkable decrease in migration with CD140b KD ASCs with a significantly wider wound gap of 77.8±4.3 as compared to those cells treated with control siRNA with a wound gap of 39.9±10.0% (P<0.0001; Fig. 2).
Figure 1. Genetic deletion of CD140b decreases proliferation and adhesion ability of ASCs.
Proliferation of ASCs as measured by WST-1 cell proliferation reagent (A) and adhesion to Laminin-1 (B). Data represent Mean ± SEM performed in duplicates. ****, p<0.0001; *, p<0.05; n=3–4 donors.
Figure 2. Genetic deletion of CD140b decreased migration ability of ASCs.
(A) Representative cross-wounded images of control siRNA treated ASCs and CD140b siRNA treated ASCs at the tested time points; 0, 6 and 24 hrs (4× magnification). (B) Wound area analysis expressed as % wound gap at 0, 6 and 24 hours of control siRNA treated ASCs (squares) and CD140b siRNA treated ASCs (rounds). Data represent Mean ± SEM performed in duplicates. ****, p<0.0001; n=3 donors.
Knocking down CD140b impairs vascular network formation of co-cultured human retinal endothelial cells (HREs)
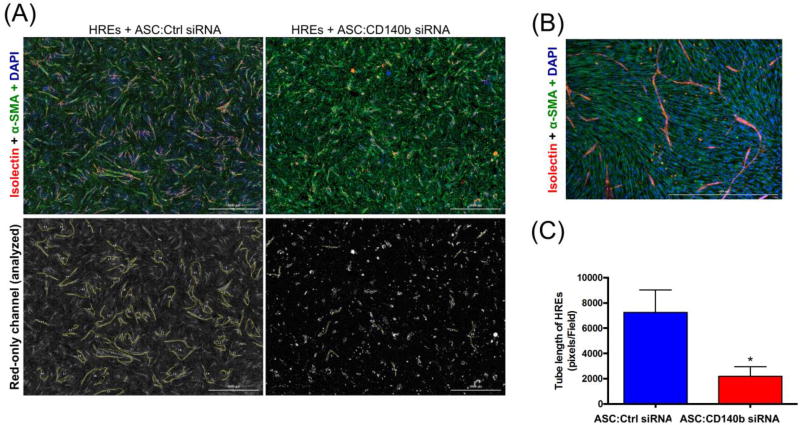
Previously we have shown that co-culturing HREs with ASCs enhanced the angiogenic tube formation ability of HREs, as compared to HREs-only cultures (8). To dissect the specific contribution of CD140b role in ASCs we knocked down the expression of CD140b in ASCs as described in materials and methods section, and co-cultured with HREs. While the HREs co-cultured with control siRNA treated ASCs showed robust angiogenic response as illustrated by isolectin-staining of tubes (Fig. 3A), the HREs co-cultured with CD140b siRNA treated ASCs demonstrated a remarkable decrease in angiogenic tubes (Fig. 3A). Image analysis of total tube length in HREs (Fig. 3B) showed significant decrease by co-culturing with CD140b− ASCs (2205±760.7 pixels/field) as compared to co-culturing with CD140b+ ASCs (7256±1782 pixels/field, p<0.05).
Figure 3. Genetic deletion of CD140b in ASCs impaired direct angiogenic effect on co-cultured HREs.
Representative images of HREs co-cultured with control siRNA treated ASCs or CD140b siRNA treated ASCs for 6 days. (A) Upper panel shows colored images of HREs, stained with Isolectin B4 (red); ASCs, stained for α-SMA (green), and co-cultures counter-stained with DAPI (blue). Lower panel shows Red-only channels representing angiogenic tubes stained with Isolectin B4 (4× magnification). (B) Representative high magnification images of ASCs and HREs co-culture. (C) Image analysis of vascular tube length calculated by image J software as pixels/field. Data represent Mean ± SEM performed in triplicates. *, p<0.05 using unpaired Student T-test; n=3 donors.
CD140b in ASCs controls expression of differential human cytokines
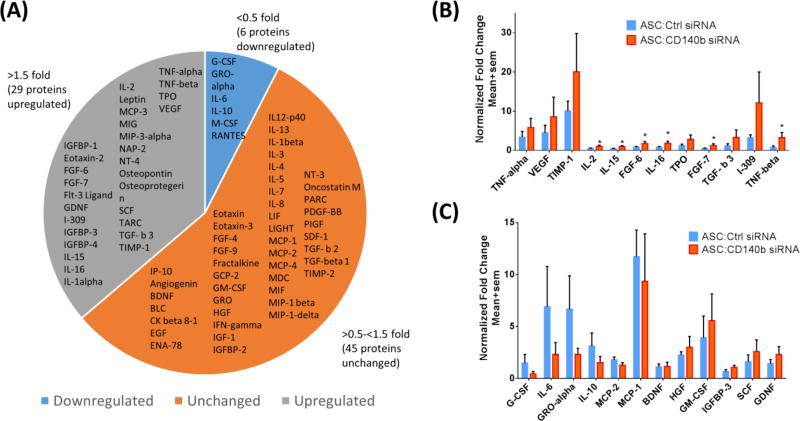
To better understand the role of CD140b expression in ASCs, we measured cytokines using a commercially available protein array for the simultaneous detection of 80 human proteins in the supernatant of the CD140b siRNA treated ASCs and compared it to those cells treated with control siRNA (Fig. 4). To better define the data, the signal intensities (arbitrary units) for each cytokine from 8 different donors were averaged to obtain a ratio of cytokine in CD140b siRNA treated cells to ctrl siRNA treated ASCs. Any expression below 0.5 fold was considered downregulated; 0.5–1.5 fold considered unchanged and any value above 1.5 fold was considered upregulated. Whereas 29 proteins including VEGF, TNF-α, glial cell-derived neurotrophic factor (GDNF), epidermal growth factor (EGF), TNF-β were found to be upregulated in CD140b siRNA treated ASCs, 6 proteins including IL-6, RANTES and IL-10 were found to be downregulated. About 45 proteins were unchanged across the two populations of cells. Among the proteins that were up regulated, significance was reached with IL-15 (p=0.03); IL-16 (p=0.047) and TNF-β (p=0.042). Complete results from all 80 proteins and the internal controls are shown in the Supplemental Figure 2.
Figure 4. Knocking-down CD140b in ASCs altered expression profile of multiple human cytokines.
(A) Collective pie-chart of all assessed human cytokines in ASCs supernatant, showing up-regulation in 29 cytokines (>1.5 fold); down-regulation in 6 cytokines (<0.5 fold); and no change in 45 cytokines (0.5–1.5 fold) by knocking-down CD140b. (B) Bar graph of representative upregulated cytokines showing significant up-regulation in IL-2, IL-15, FGF-6, IL-16, FGF-7 and TNF-β. (C) Bar graph of the representative downregulated cytokines. Data represent Mean ± SEM performed in duplicates. *, p<0.05; n=8 donors.
Discussion
In this study using multiple batches of ASCs isolated from healthy donors, we were able to show that CD140b (PDGFRβ) plays a crucial role in controlling the intrinsic proliferation; adhesion to extracellular matrix and migration abilities of ASCs in vitro. Furthermore, our data point to the fact that loss of CD140b severely impairs ASCs angiogenic potential to aid retinal endothelial cell assembly into vascular network. Finally, we show a correlation between CD140b and multiple cytokines secreted from ASCs, to control signal transduction pathways related to angiogenesis, cellular stress and apoptosis.
It is well known that PDGF/PDGFRs signaling is crucial for normal physiological development (18), proper renal, cardiovascular and circulatory functions (30–32), as well as formation of blood vessel (33) and functional microvascular pericytes (34). In the context of stem cells, PDGFR signaling was shown to control expression of differentiation markers and therapeutic capacity of mesenchymal stem cells (MSCs) (35). MSCs were shown to respond to endogenous PDGF-driven stimulation by changing the expression of PDGF receptors; PDGFR-α (CD140a) and PDGFR-β on their surface (36).
In our study we have observed that CD140b controls proliferation of ASCs which is in agreement with a previous study that demonstrated PDGF signaling significantly increased proliferation of ASCs, an effect that was attributed in part to generation of reactive oxygen species, and subsequent upregulation of miR-210 (24). Furthermore, a large body of literature indicates PDGF signaling as a major pathway responsible for MSC proliferation (37–39). While it is interesting to note that CD140 knock down in ASCs impair their intrinsic proliferation ability, it had no further effect when exogenous cellular stress is applied. Specifically, when CD140 siRNA treated cells are subjected to high glucose, oxidative stress, protein kinase inhibitor (staurosporine) and TNFα, their proliferation rate was unaffected (Supplemental Fig 3). This data is in line with our previous observation that ASCs are resistant to high glucose stress (8). Our data also point to the fact that CD140b controls migration ability of ASCs, where cells lacking CD140b failed to migrate efficiently is in agreement with Gehmert S et al. who reported similar results, where migration of ASCs were induced by breast cancer cells in a PDGF-B/PDGFR-β-dependent manner (40). Additional literature supporting PDGFRβ signaling in MSCs demonstrates CD140b as an inducer of proliferative and migratory responses of MSCs, but inhibit their osteogenic differentiation (41). In another study, PDGF-AB was shown to regulate migratory capability of MSCs through Urokinase-type plasminogen activator receptor interaction with β1 integrin that may be relevant to controlling MSCs function and tissue remodeling after injury (42). Future studies need to assess the loss or gain of CD140b in proliferation and migration abilities of ASCs in vivo.
Interaction between ASCs and endothelial cells has been investigated. Some studies suggest interaction between ASCs and endothelial cells in a cooperative manner on Matrigel resulting in network assembly with enhanced stability of endothelial networks (13). Same group reported the vascular co-operation between ASCs and human progenitor endothelial cells in vivo as well where, implantation of a construct of both ASCs and progenitor endothelial cells subcutaneously in NOD/SCID mice resulted in robust functional vascular network formation (43). In other studies using HUVEC, ASCs were shown to induce outgrowth and perivascular organization in an in vitro model of fibrin-embedded spheroids (10) and in another chorioallantoic membrane model (11). Nevertheless, the role of PDGFR-β in controlling the interaction between ASCs and endothelial cells is not known. Our data demonstrate for the first time that CD140b on the surface of ASCs is crucial to aid HREs assemble into vascular tubes. Whereas HREs co-cultured with CD140b+ ASCs formed vascular network, HREs co-cultured with CD140b siRNA treated ASCs failed to form vascular network. While CD140b siRNA influenced ASCs proliferation rates in a short-term experiment (Fig. 1), the transient transfection of CD140b siRNA did not influence the overall proliferation rates in tube formation assay (6 days time frame, data not shown) suggesting the observed difference in vascular network is a consequence of cell surface expression of CD140b. Previously it was shown that ASCs increase the expression of α-smooth muscle actin and support vascular network and its likely to play a role here (44). It is also possible that HREs may display a bidirectional downregulation of PDGF in the absence of CD140b in ASCs. More studies are warranted to better understand the signaling between CD140b in ASCs and HREs in the observed co-operative angiogenic behavior.
Although the exact mechanisms are sparse, angiogenic behavior of ASCs could be partially attributed to the paracrine secretion of different angiogenic factors in their conditioned medium (CM) (14, 45–47). ASCs secrete various bioactive molecules, including; vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and granulocyte macrophage colony-stimulating factor (GM-CSF), which promote endothelial cell survival (13) and proliferation (14). In the current study, we have investigated how ASCs secretion of different cytokines is influenced by genetic deletion of CD140b. Evaluation of 80 different human cytokines showed down-regulation in 6 proteins including IL-6 &IL-10 and up-regulation in 29 proteins including IL-2, 15, FGF, VEGF & TNF-β. The correlation between altered cytokines and angiogenesis is established elsewhere; IL-6 (48, 49); IL-10 (50); IL-2 (51); IL-15 (51); VEGF and FGF (52) and TNF (53). Recently it has been suggested that besides soluble factors, extracellular vesicles (EVs) that include exosomes and microvesicles may play a major role in the angiogenic potential of ASCs via PDGF signaling (54). Future studies need to evaluate if EVs correlate with CD140b cell surface expression.
The limitations of our study include the use of transient transfection protocol to knockdown the expression of CD140b on surface of ASCs. Alternatively, FACS sorting could have been used to separate subpopulations of ASCs based on cell surface expression of CD140b. Taking into consideration the fact that, expression of cell surface marker being very dynamic, the negative population is likely to develop some positive expression of CD140b after few passages. Future studies using lentiviral forced expression of CD140b or shRNA genetic deletion may be more useful.
In conclusion, CD140b is an important cell surface marker, controlling intrinsic capabilities of ASCs including modulation of paracrine secretions. Moreover, CD140b-enriched ASCs enhance angiogenic potential of endothelial cells. Future studies are warranted to investigate the underlying mechanism for enhanced angiogenic potential of HREs, which may include possible differentiation of ASCs to pericytes, supporting vascular networks as well as the underlying signal transduction pathway and secretion of different angiogenic markers from ASCs. A complete understanding of stem cell identity, scale up methodology and intrinsic signaling mechanisms are necessary to engineer these cells for future tissue engineering purposes.
Supplementary Material
Supplemental Figure 1. Flow cytometric characterization of cell surface proteins and transient transfection with CD140b siRNA in human ASCs. (A) Representative flow cytometric histograms of the expression of characteristic markers of CD105, CD31 and CD140b. Line shows discrimination of negative cells (isotype controls). (B) Mean expression of the surface markers from 3 representative human donors. (C) Relative gene expression of CD140b as normalized to β2-Micro globulin (BMG) internal control as measured by RT-PCR in ASCs lysates following transient transfection with siRNA against CD140b. (D) Flow cytometric analysis for surface expression of CD140b after transient transfection in ASCs. Data expressed as Mean±SEM of n=3 donors. *, p<0.05.
Supplemental Figure 2. Multiple cytokine expression profile in the cell supernatants of ASCs. Data expressed as Mean of 8 donors.
Supplemental Figure 3. Genetic deletion of CD140b does not alter proliferation of ASCs subjected to cellular stress. Proliferation of ASCs as measured by WST-1 cell proliferation reagent from cell subjected to (A) high glucose (B) oxidative stress (C) staurosporine and (D) Tumor Necrosis Factor alpha (TNF-α) cytokine. Data represent Mean ± SEM performed in duplicates. ***, p<0.001; n=3 donors.
Lay Summary.
Adipose-derived stem cells (ASCs) obtained from human fat can differentiate into multiple tissues and also exhibit features of pericytes. In this study, we addressed the role of CD140b, a surface protein in ASCs that serves as a pericyte marker in angiogenic functioning of retinal endothelial cells. Our results demonstrate that CD140b is not only required for ASC survival but also mediates the production of certain paracrine factors that positively affect the angiogenic properties of retinal endothelial cells. Our study paves the way for future studies that are needed to fully explore CD140b signaling in ASCs in vivo for retinal regeneration.
Future studies.
Long-term studies are needed to study the safety and effectiveness of CD140b positive ASCs in retinal disease models to rule out any complications of stem cell treatment, including potential rejection and need for reinjection.
Acknowledgments
Authors wish to acknowledge Jack Anderson, BS for technical support and Daniel Johnson, PhD for help with statistical analysis. This study was funded by grants from National Eye Institute (EY023427), and unrestricted funds from Research to Prevent Blindness to R.G. The funders played no role in the conduct of the study, collection of data, management of the study, analysis of data, interpretation of data, or preparation of the manuscript.
Footnotes
Author Contributions
Conceived and designed the experiments: RP, SLE, RG. Performed the experiments: RP, SLE. Analyzed the data: RP, SLE, RG. Wrote and reviewed the paper: RP, SLE, RG. Conceptualization and final approval: RG.
Competing financial interests
RG is a co-founder and hold equity in Cell Care Therapeutics Inc., whose interest is in the use of adipose derived stromal cells in visual disorders. None of the other authors declare any financial conflicts.
References
- 1.Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103(9):435–47. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- 2.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geevarghese A, Herman IM. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res. 2014;163(4):296–306. doi: 10.1016/j.trsl.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva Meirelles L, de Deus Wagatsuma VM, Malta TM, Bonini Palma PV, Araujo AG, Panepucci RA, et al. The gene expression profile of non-cultured, highly purified human adipose tissue pericytes: Transcriptomic evidence that pericytes are stem cells in human adipose tissue. Exp Cell Res. 2016;349(2):239–54. doi: 10.1016/j.yexcr.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–20. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2016;43(4):268–74. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 8.Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, et al. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014;9(1):e84671. doi: 10.1371/journal.pone.0084671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorrell JM, Baber MA, Traktuev DO, March KL, Caplan AI. The creation of an in vitro adipose tissue that contains a vascular-adipocyte complex. Biomaterials. 2011;32(36):9667–76. doi: 10.1016/j.biomaterials.2011.08.090. [DOI] [PubMed] [Google Scholar]
- 10.Verseijden F, Posthumus-van Sluijs SJ, Pavljasevic P, Hofer SO, van Osch GJ, Farrell E. Adult human bone marrow- and adipose tissue-derived stromal cells support the formation of prevascular-like structures from endothelial cells in vitro. Tissue Eng Part A. 2010;16(1):101–14. doi: 10.1089/ten.TEA.2009.0106. [DOI] [PubMed] [Google Scholar]
- 11.Strassburg S, Nienhueser H, Bjorn Stark G, Finkenzeller G, Torio-Padron N. Co-culture of adipose-derived stem cells and endothelial cells in fibrin induces angiogenesis and vasculogenesis in a chorioallantoic membrane model. J Tissue Eng Regen Med. 2016;10(6):496–506. doi: 10.1002/term.1769. [DOI] [PubMed] [Google Scholar]
- 12.Falcon BL, Swearingen M, Gough WH, Lee L, Foreman R, Uhlik M, et al. An in vitro cord formation assay identifies unique vascular phenotypes associated with angiogenic growth factors. PLoS One. 2014;9(9):e106901. doi: 10.1371/journal.pone.0106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 14.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 15.Merfeld-Clauss S, Gollahalli N, March KL, Traktuev DO. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16(9):2953–66. doi: 10.1089/ten.tea.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivera JC, Dabouz R, Noueihed B, Omri S, Tahiri H, Chemtob S. Ischemic Retinopathies: Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2017;2017:3940241. doi: 10.1155/2017/3940241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajashekhar G. Mesenchymal stem cells: new players in retinopathy therapy. Front Endocrinol (Lausanne) 2014;5:59. doi: 10.3389/fendo.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu YJ, Cho TJ, Lee DS, Choi JY, Cho J. Phenotypic characterization and in vivo localization of human adipose-derived mesenchymal stem cells. Mol Cells. 2013;35(6):557–64. doi: 10.1007/s10059-013-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43(10):596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–8. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851–60. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghavan SS, Woon CY, Kraus A, Megerle K, Pham H, Chang J. Optimization of human tendon tissue engineering: synergistic effects of growth factors for use in tendon scaffold repopulation. Plast Reconstr Surg. 2012;129(2):479–89. doi: 10.1097/PRS.0b013e31823aeb94. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Park SG, Song SY, Kim JK, Sung JH. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013;4:e588. doi: 10.1038/cddis.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hye Kim J, Gyu Park S, Kim WK, Song SU, Sung JH. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells. 2015;33(2):542–56. doi: 10.1002/stem.1865. [DOI] [PubMed] [Google Scholar]
- 26.Gehmert S, Gehmert S, Hidayat M, Sultan M, Berner A, Klein S, et al. Angiogenesis: the role of PDGF-BB on adipose-tissue derived stem cells (ASCs) Clin Hemorheol Microcirc. 2011;48(1):5–13. doi: 10.3233/CH-2011-1397. [DOI] [PubMed] [Google Scholar]
- 27.Rajashekhar G, Traktuev DO, Roell WC, Johnstone BH, Merfeld-Clauss S, Van Natta B, et al. IFATS collection: Adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells. 2008;26(10):2674–81. doi: 10.1634/stemcells.2008-0277. [DOI] [PubMed] [Google Scholar]
- 28.Elshaer SL, Abdelsaid MA, Al-Azayzih A, Kumar P, Matragoon S, Nussbaum JJ, et al. Pronerve growth factor induces angiogenesis via activation of TrkA: possible role in proliferative diabetic retinopathy. J Diabetes Res. 2013;2013:432659. doi: 10.1155/2013/432659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maumus M, Peyrafitte JA, D'Angelo R, Fournier-Wirth C, Bouloumie A, Casteilla L, et al. Native human adipose stromal cells: localization, morphology and phenotype. International journal of obesity (2005) 2011;35(9):1141–53. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, et al. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125(17):3313–22. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 31.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8(16):1888–96. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 32.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 33.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125(4):917–28. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 35.Ball SG, Shuttleworth A, Kielty CM. Inhibition of platelet-derived growth factor receptor signaling regulates Oct4 and Nanog expression, cell shape, and mesenchymal stem cell potency. Stem Cells. 2012;30(3):548–60. doi: 10.1002/stem.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan HB, Giannoudis PV, Boxall SA, McGonagle D, Jones E. The systemic influence of platelet-derived growth factors on bone marrow mesenchymal stem cells in fracture patients. BMC Med. 2015;13:6. doi: 10.1186/s12916-014-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1(11):771–82. doi: 10.5966/sctm.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pountos I, Georgouli T, Henshaw K, Bird H, Jones E, Giannoudis PV. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma. 2010;24(9):552–6. doi: 10.1097/BOT.0b013e3181efa8fe. [DOI] [PubMed] [Google Scholar]
- 39.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 40.Gehmert S, Gehmert S, Prantl L, Vykoukal J, Alt E, Song YH. Breast cancer cells attract the migration of adipose tissue-derived stem cells via the PDGF-BB/PDGFR-beta signaling pathway. Biochem Biophys Res Commun. 2010;398(3):601–5. doi: 10.1016/j.bbrc.2010.06.132. [DOI] [PubMed] [Google Scholar]
- 41.Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C, Ishizawa S, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23(9):1519–28. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 42.Chabot V, Dromard C, Rico A, Langonne A, Gaillard J, Guilloton F, et al. Urokinase-type plasminogen activator receptor interaction with beta1 integrin is required for platelet-derived growth factor-AB-induced human mesenchymal stem/stromal cell migration. Stem Cell Res Ther. 2015;6:188. doi: 10.1186/s13287-015-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104(12):1410–20. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 44.Freiman A, Shandalov Y, Rozenfeld D, Shor E, Segal S, Ben-David D, et al. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Research & Therapy. 2016;7:5. doi: 10.1186/s13287-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazo M, Cemborain A, Gavira JJ, Abizanda G, Arana M, Casado M, et al. Adipose stromal vascular fraction improves cardiac function in chronic myocardial infarction through differentiation and paracrine activity. Cell transplantation. 2012;21(5):1023–37. doi: 10.3727/096368911X623862. [DOI] [PubMed] [Google Scholar]
- 46.Cho YJ, Song HS, Bhang S, Lee S, Kang BG, Lee JC, et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res. 2012;90(9):1794–802. doi: 10.1002/jnr.23063. [DOI] [PubMed] [Google Scholar]
- 47.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2542–7. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 48.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990;87(8):3092–6. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11(4):517–27. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 50.Dace DS, Khan AA, Kelly J, Apte RS. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PloS one. 2008;3(10):e3381. doi: 10.1371/journal.pone.0003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae J, Park D, Lee YS, Jeoung D. Interleukin-2 promotes angiogenesis by activation of Akt and increase of ROS. J Microbiol Biotechnol. 2008;18(2):377–82. [PubMed] [Google Scholar]
- 52.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22(4):201–7. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 53.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111(10):4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow cytometric characterization of cell surface proteins and transient transfection with CD140b siRNA in human ASCs. (A) Representative flow cytometric histograms of the expression of characteristic markers of CD105, CD31 and CD140b. Line shows discrimination of negative cells (isotype controls). (B) Mean expression of the surface markers from 3 representative human donors. (C) Relative gene expression of CD140b as normalized to β2-Micro globulin (BMG) internal control as measured by RT-PCR in ASCs lysates following transient transfection with siRNA against CD140b. (D) Flow cytometric analysis for surface expression of CD140b after transient transfection in ASCs. Data expressed as Mean±SEM of n=3 donors. *, p<0.05.
Supplemental Figure 2. Multiple cytokine expression profile in the cell supernatants of ASCs. Data expressed as Mean of 8 donors.
Supplemental Figure 3. Genetic deletion of CD140b does not alter proliferation of ASCs subjected to cellular stress. Proliferation of ASCs as measured by WST-1 cell proliferation reagent from cell subjected to (A) high glucose (B) oxidative stress (C) staurosporine and (D) Tumor Necrosis Factor alpha (TNF-α) cytokine. Data represent Mean ± SEM performed in duplicates. ***, p<0.001; n=3 donors.