Abstract
Aluminum (Al) is the third most abundant element in the earth's crust and is omnipresent in our environment, including our food. However, with normal renal function, oral and enteral ingestion of substances contaminated with Al, such as antacids and infant formulae, do not cause problems. The intestine, skin, and respiratory tract are barriers to Al entry into the blood. However, contamination of fluids given parenterally, such as parenteral nutrition solutions, or hemodialysis, peritoneal dialysis or even oral Al-containing substances to patients with impaired renal function could result in accumulation in bone, parathyroids, liver, spleen, and kidney. The toxic effects of Al to the skeleton include fractures accompanying a painful osteomalacia, hypoparathyroidism, microcytic anemia, cholestatic hepatotoxicity, and suppression of the renal enzyme 25-hydroxyvitamin D-1 alpha hydroxylase. The sources of Al include contamination of calcium and phosphate salts, albumin and heparin. Contamination occurs either from inability to remove the naturally accumulating Al or from leeching from glass columns used in compound purification processes. Awareness of this long-standing problem should allow physicians to choose pharmaceutical products with lower quantities of Al listed on the label as long as this practice is mandated by specific national drug regulatory agencies.
Keywords: Aluminum toxicity, Bone, Parathyroid glands, Liver, Osteomalacia
1. Introduction
Aluminum (Al) toxicity to bone has been the subject of several reviews, though none recent. The majority of the work was performed in the 1970s through the 1990s and involved either Al contamination of fluid used in hemodialysis or peritoneal dialysis secondary to chronic renal disease or Al contamination of fluids used for the intravenous nutrition of patients with intestinal disease or premature infants who could not tolerate enteral feeds. In both settings it is notable that Al contaminated fluids that were administered by a parenteral route, not through the gastrointestinal tract.
To understand this setting a bit better we will first discuss Al the element in nature and then discuss its kinetics as it relates to handling by the body. Following that we will discuss the manifestations of Al toxicity and the therapeutic options to manage it.
Al is the third most abundant element in the earth's crust and is ubiquitous in nature [1]. There is no known physiological requirement for Al by the body, but it has been known to interact with iron, especially in its affinity for the chelating agent deferoxamine [2,3] and in the use of the aurin tricarboxylic acid stain to identify either Al or elemental iron in bone histology [4]. In addition, 95% of Al circulates in the blood bound to transferrin [2,5]. Al may also interact with calcium and has a strong affinity for phosphate [6].
2. How do we measure Al in fluids and in tissue?
Al in blood, urine, and tissue is still measured by electrothermal atomic absorption spectroscopy with a Zeeman background correction [7,8]. A machine with a Zeeman correction applies a magnetic field to the atomic emissions from specific elements more clearly separating background from substance emissions.
Normal values for human plasma, urine and tissues were published by LeGendre and Alfrey [9]. The analyses are not widely available or often performed and this makes for scarce data on the subject. Specimens once obtained are dried and ashed prior to analysis [7,8].
3. Al handling by the body
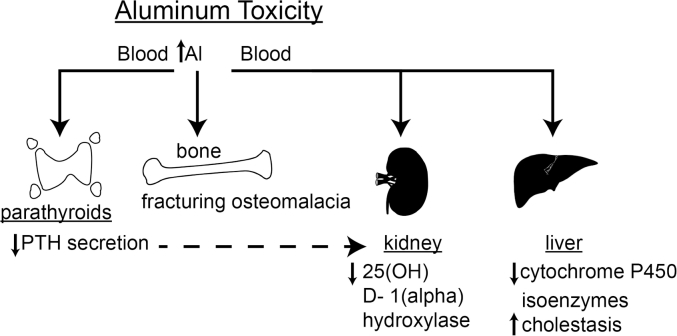
Al can be ingested in any number of foods or medications. Intestinal absorption is very low, estimated at 0.005% [10] of intake. Al injected subcutaneously does not enter into the circulation but instead accumulates within the dermal layers [11]. Thus, the intestinal mucosa as well as the skin serve as barriers to Al entry into the blood. What little Al is absorbed is excreted by the kidney, although only about 5% of Al in the circulation is ultrafilterable [5]. Al ingested orally, for example in antacids or in infant formula, will not accumulate in the body as long as renal function is intact. If the kidney cannot excrete Al, it will accumulate in the body, resulting in manifestations of Al toxicity in chronic renal disease. However, impaired renal function is not required to produce Al toxicity. Bypassing the natural barriers to Al entry into the body, for example, by providing nutrition to a patient by an intravenous route, known as total parenteral nutrition (TPN), Al can still accumulate in the body, attributable to the 95% binding of Al to circulating transferrin. The consequences of the circulation of bound Al are its establishment of equilibrium between the blood and various tissues. Among the tissues in which Al can build up are the bone, liver, spleen, parathyroid glands, and kidney. While little is known of the consequences of Al accumulation in the spleen, a great deal has been learned about the consequences of Al accumulation in bone and parathyroid glands, and some information is available about the effects of Al in liver and kidney (Fig. 1).
Fig. 1.
Illustration of the various toxic roles of aluminum (Al) in different body organs. PTH, parathyroid hormone.
4. Al accumulation in bone
Al has been demonstrated in the bones of patients with chronic renal disease undergoing either hemodialysis [12] or peritoneal dialysis [12]. It can also accumulate in bone with impaired renal function just by the oral ingestion of Al-containing antacids [13]. Al also accumulates in the bones of patients receiving long-term treatment with TPN to manage an intestinal disorder that impairs the patient's ability to feed enterally [14]. The upper limits of normal Al content in bone is below 10 μg/g dry weight [5,14]. Al content of bone in either renal disease or TPN treatment has reached over 100 μg/g dry weight, although the amount in bone samples of premature infants obtained at autopsy have been somewhat lower [15]. In either condition Al accumulates at the mineralization front of the bone surface, where osteoblasts have just laid down new type I collagen. Instead of the accumulation of calcium at that spot, a phenomenon that occurs normally, Al preferentially occupies the unmineralized type I collagen, impairing calcification and resulting in osteomalacia. In addition to bone, Al is also taken up by the parathyroid glands [16] and impairs parathyroid hormone (PTH) secretion [16]. Whether Al serves to up-regulate the parathyroid calcium-sensing receptor (CaSR) is unclear. No effect of Al on the CaSR was discernible in rat studies [17]. However, the end result was still a functional hypoparathyroidism [16] with consequent hypercalciuria [5,14,15]. The end result of these changes is Al displacement of calcium on the bone surface, leading to osteomalacia, hypercalcemia, and hypercalciuria. The apparent contradiction of the coexistence of hypoparathyroidism and hypercalcemia can be explained in parenteral nutrition patients by the constant infusion of calcium-containing solutions while the Al is blocking skeletal uptake of the infused calcium. This leads to an iatrogenic hypercalcemia, which is partially ameliorated by the hypercalciuria associated with the functional hypoparathyroidism. Moreover, the mild hypercalcemia may also contribute to the secondary suppression of PTH secretion. This is the scenario in patients receiving Al-contaminated parenteral nutrition solutions. In patients who suffer from chronic renal disease, inability to excrete phosphate leads to secondary and sometimes tertiary hyperparathyroidism. Thus Al accumulation in the parathyroids leads to a partial amelioration of the secondary hyperparathyroidism rather than to frank hypoparathyroidism [17]. A potential consequence of this Al displacement of calcium at the bone surface is the occurrence of hungry bone syndrome. This phenomenon occurs when Al is chelated out of bone by use of deferoxamine [3]. When the Al is freed from the bone by the chelating agent the bone takes up excessive calcium from the circulation leaving the patient with hypocalcemia and an inappropriately low serum concentration of PTH [3]. This situation is analogous to the hungry bone syndrome that occurs with severe secondary hyperparathyroidism and partial parathyroidectomy which occurs with chronic renal disease. Once the PTH stimulus to bone resorption has been removed by surgery the bone, which had been releasing calcium in response to the hyperparathyroidism sucks the calcium back into the bone from the blood at least temporarily overriding the effect of circulating PTH.
Another result of the Al accumulation in bone and parathyroid is the inhibition of the renal enzyme 25 hydroxyvitamin D-1 alpha hydroxylase (25(OH)D-1-alpha hydroxylase), which converts 25(OH)D to 1 alpha, 25 dihydroxyvitamin D [18]. Whether it is the relatively low PTH that causes the reduction in conversion of 25(OH)D to its metabolically active form [19], the renal accumulation of Al that poisons this enzymatic conversion as lead accumulation similarly does, or, especially in the case of chronic renal disease, the accumulation of fibroblast growth factor 23, which suppresses the enzymatic conversion of 25(OH)D to 1,25(OH)2D [20] is presently unclear. One or more of these factors may be involved.
With regard to physical manifestations of Al accumulation in bone, severe bone pain has been manifested in patients with dialysis osteomalacia [21] as well as in those with TPN-associated osteomalacia [22,23]. When Al is removed from the parenterally administered solutions, either dialysis or parenteral nutrition solutions, bone pain resolves, PTH and 1,25-dihydroxyvitamin D concentrations return to normal in the blood [23]. Exactly what is responsible for the bone pain is unknown, but it is linked in manifestation to the abnormalities in calcium metabolism presented here.
Al toxicity is also associated with anemia [24,25], and Al has been identified in macrophages of the bone marrow and may affect the erythrocyte cell line. It is thought to interfere with heme synthesis, possibly by preventing iron incorporation, or it may interfere with the erythrocyte enzyme delta amino levulinic acid dehydratase, the last step in heme synthesis. Al-induced anemia is not necessarily common but may be associated with the generalized uptake of Al by bone and marrow and has only been observed in patients with chronic renal disease, not in those receiving TPN.
5. Al uptake by liver
Al is also taken up by the liver. This has been documented in patients receiving long-term TPN treatment [5]. The amount of Al taken up by the liver on a dry weight basis seems to be less than what has been measured in bone [5]. However, the manifestations of Al toxicity in liver have still been described. They are accumulation of bile acids in serum in rats [26] and piglets [27], a change in predominance of amino acids conjugating the bile acids from glycine to taurine [28] increased transferrin excretion in bile [29], and, in rats given parenteral Al, reduction of certain cytochrome P450 isoenzymes, NADPH cytochrome c reductase, and a fourfold increase in glucuronyl transferase activity, indicating increased conjugating activity [30]. Al appears to be preferentially taken up by hepatocyte lysosomes by use of X-ray microanalysis [27]. Also, in this same study there was a small but significant decrease in serum 25(OH)D, suggesting that liver 25-hydroxylation of the vitamin might also be affected by Al [27]. The implications of these changes for bone, although the precise pathogenesis of these manifestations is not known, could include a reduction in intestinal calcium and/or vitamin D absorption as well as alteration of drug metabolism by means of changes in cytochrome P450 isoenzymes.
6. Sources of Al contamination
In chronic renal disease, the documented sources of Al contamination have been the water in the fluid used in hemodialysis [12] and peritoneal dialysis [12] as well as oral Al-containing phosphate binders [6]. In patients receiving TPN, the contaminating sources were not as obvious. Our initial investigations revealed that casein hydrolysate, used as the protein source at the time, was the principal source of Al [14]. When an essential amino acid solution was substituted for the casein hydrolysate in adult TPN patients suffering from Al toxicity bone pain resolved, PTH and 1,25-dihydroxyvitamin D concentrations in serum rose to normal [23].
However, that was not the end of the problem. Studies in premature infants, whose renal function is developmentally reduced, revealed that Al in smaller quantities still contaminating TPN solutions were capable of contributing to the incidence of a rachitic like presentation seen in these patients [15]. The remaining mystery was the source of the Al contamination.
Detailed analysis of the TPN solution components and intravenously administered medications revealed Al contamination of many of the components. A full list, when last determined by our group, is given in Table 1 below [15,31], but the main sources were calcium and phosphate salts, albumin and heparin. On careful examination of the contaminated solutions shown in Table 1 the wide variability of Al content is evidence supporting the lack of quality control during the manufacturing process.
Table 1.
Aluminum content of common components of intravenous nutrition solutions.
| Solution | Concentration | Aluminum content (μg/L) | No. of lots tested |
|---|---|---|---|
| Salt | |||
| Calcium gluconate | 10% | 5056 ± 335 | 5 |
| Sodium phosphate | 3000 mmol/L | 5977 | 1 |
| Potassium phosphate | 3000 mmol/L | 16,598 ± 1801 | 3 |
| Serum | |||
| Albumin | 25% | 1822 ± 2503 | 4 |
| Heparin | 1000 units/mL | 684 ± 761 | 3 |
| Heparin | 5000 units/mL | 359 | 1 |
| Heparin | 10,000 units/mL | 468 | |
| Lipid emulsion | 195 | 1 | |
| Dextrose | 5% | 72 ± 1 | 2 |
| Sodium chloride | 4000 mmol/L | 6 ± 4 | 3 |
| Potassium chloride | 3000 mmol/L | 6 | 1 |
Values are presented as mean ± standard deviation.
Data derived from Sedman et al. N Engl J Med 1985; 312:1337-43 [15].
7. How could this happen?
As mentioned at the beginning of this piece, there is no known nutritional requirement for Al, so how did this element end up in TPN solutions, or dialysis fluid for that matter? In the case of dialysis fluid, Al contaminated the water used in dialysis. This problem is now corrected. However, the problem of Al contamination of a variety of salts and other medications is still with us. The U.S. Food and Drug Administration (FDA) has identified that in the case of albumin, a product of human blood, with a normal Al content of less than 10 μg/L, it is the purification process, including the use of glass columns that produces the contamination [32]. Glass, which contains silicates, also contains Al, and in the purification process the Al is leached from the glass into the product, which has been reported to contain as much as 5 times the Al content of normal serum [15,31]. In the case of the calcium and phosphate salts, the problem is a bit more complex.
In 1985, one of my colleagues (Ravi Pottathil, PhD, City of Hope National Medical Center, Duarte CA) showed me a copy of the Merck Manual, which lists chemical reagents and their degree of purity. He noted that several designated as ultrapure still contained small amounts of contaminants, such as lead. However, the designation ultrapure was reserved for those reagents that contained contaminants <1 part per million (ppm). When communicating with the quality control officers of the companies that manufactured these reagents 2 things became clear. The first was that the method for measuring Al was complicated and expensive and was not performed routinely in any industrial setting. Therefore, Al was not listed as a contaminant in any of the reagents in the Merck Manual. The second realization was that no one thought that biological effects, especially toxic effects, could be present in quantities less than 1 ppm (1 mg/L). What was clear from our work and from the work of others, however, is that Al exerted biological effects in the parts per billion (ppb) range, in other words, in micrograms/L. Thus, the likely explanation for the contamination of the calcium and phosphate salts with Al is that Al was never measured in the raw material used to make the hospital-grade salts and was not thought to cause toxicity.
The FDA has now partially addressed the problem by publishing a rule regarding the allowable Al content of components of TPN solutions. The amounts of Al at expiry of the solution components must be measured by methods reviewed and acceptable to FDA and must be placed on the label of the solution component along with a boxed warning about the toxicity of Al [33]. While this rule raises awareness of the problem, in practical terms, it is not clear that there is a uniform way to address this problem. Poole et al. [34] have demonstrated that while the rule can reduce the amount of Al delivered to preterm infants by selecting the TPN solution components lowest in Al, the FDA designated ‘safe’ limit of 5 μg/kg/day cannot be met. This ‘safe’ limit was calculated from the limited published data indicating which types of TPN solutions did not produce Al toxicity. There have to date been no Al dose-response studies to provide a more reliable threshold of toxicity. Currently, the only therapeutic option in managing the Al load received is that advocated by Poole et al. [34] and implied by the current FDA rule.
While this problem has at least been formally addressed in the United States and in parts of Europe, the degree to which Al contamination and toxicity is a problem in Asia and elsewhere in the world has not been ascertained, especially among patients with renal disease requiring dialysis and premature infants and others requiring TPN.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The author acknowledges collaborative help from the late Jack W Coburn MD of the UCLA David Geffen School of Medicine, Los Angeles, Allen C Alfrey MD and Nancy L Miller MS of the VA Hospital and the University of Colorado Medical Center, Denver, and Donald J Sherrard MD and Susan M Ott MD of the VA Medical Center, Harborview Hospital, and the University of Washington School of Medicine, Seattle. ORCID. Gordon L. Klein: 0000-0002-3011-4186.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Flaten T.P., Alfrey A.C., Birchall J.D., Savory J., Yokel R.A. Status and future concerns of clinical and environmental aluminum toxicology. J Toxicol Environ Health. 1996;48:527–541. doi: 10.1080/009841096161050. [DOI] [PubMed] [Google Scholar]
- 2.Mujika J.I., Lopez X., Rezabal E., Castillo R., Marti S., Moliner V. A QM/MM study of the complexes formed by aluminum and iron with serum transferrin at neutral and acidic pH. J Inorg Biochem. 2011;105:1446–1456. doi: 10.1016/j.jinorgbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Klein G.L., Snodgrass W.R., Griffin M.P., Miller N.L., Alfrey A.C. Hypocalcemia complicating deferoxamine therapy in an infant with parenteral nutrition-associated aluminum overload: evidence for a role of aluminum in the bone disease of infants. J Pediatr Gastroenterol Nutr. 1989;9:400–403. doi: 10.1097/00005176-198910000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Ott S.M., Maloney N.A., Klein G.L., Alfrey A.C., Ament M.E., Coburn J.W. Aluminum is associated with low bone formation in patients receiving chronic parenteral nutrition. Ann Intern Med. 1983;98:910–914. doi: 10.7326/0003-4819-98-6-910. [DOI] [PubMed] [Google Scholar]
- 5.Klein G.L., Ott S.M., Alfrey A.C., Sherrard D.J., Hazlet T.K., Miller N.L. Aluminum as a factor in the bone disease of long-term parenteral nutrition. Trans Assoc Am Phys. 1982;95:155–164. [PubMed] [Google Scholar]
- 6.Pepper R., Campbell N., Yaqoob M.M., Roberts N.B., Fan S.L. Do oral aluminium phosphate binders cause accumulation of aluminium to toxic levels? BMC Nephrol. 2011;12:55. doi: 10.1186/1471-2369-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillard O., Tiphaneau K., Reiss D., Piriou A. Improved determination of aluminum in serum by electrothermal atomic absorption spectroscopy together with a graphite furnace and Zeeman background correction. Anal Lett. 1984;17:1593–1605. [Google Scholar]
- 8.D'Haese P.C., Van de Vyver F.L., de Wolff F.A., De Broe M.E. Measurement of aluminum in serum, blood, urine, and tissues of chronic hemodialyzed patients by use of electrothermal atomic absorption spectrometry. Clin Chem. 1985;31:24–29. [PubMed] [Google Scholar]
- 9.LeGendre G.R., Alfrey A.C. Measuring picogram amounts of aluminum in biological tissue by flameless atomic absorption analysis of a chelate. Clin Chem. 1976;22:53–56. [PubMed] [Google Scholar]
- 10.Haram E.M., Weberg R., Berstad A. Urinary excretion of aluminium after ingestion of sucralfate and an aluminium-containing antacid in man. Scand J Gastroenterol. 1987;22:615–618. doi: 10.3109/00365528708991908. [DOI] [PubMed] [Google Scholar]
- 11.Webb C.L., Schoen F.J., Flowers W.E., Alfrey A.C., Horton C., Levy R.J. Inhibition of mineralization of glutaraldehyde-pretreated bovine pericardium by AlCl3. Mechanisms and comparisons with FeCl3, LaCl3, and Ga(NO3)3 in rat subdermal model studies. Am J Pathol. 1991;138:971–981. [PMC free article] [PubMed] [Google Scholar]
- 12.Mion C. Aluminium in continuous ambulatory peritoneal dialysis and post dilutional hemofiltration. Clin Nephrol. 1985;24(Suppl 1):S88–S93. [PubMed] [Google Scholar]
- 13.Chappard D., Bizot P., Mabilleau G., Hubert L. Aluminum and bone: review of new clinical circumstances associated with Al(3+) deposition in the calcified matrix of bone. Morphologie. 2016;100:95–105. doi: 10.1016/j.morpho.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Klein G.L., Alfrey A.C., Miller N.L., Sherrard D.J., Hazlet T.K., Ament M.E. Aluminum loading during total parenteral nutrition. Am J Clin Nutr. 1982;35:1425–1429. doi: 10.1093/ajcn/35.6.1425. [DOI] [PubMed] [Google Scholar]
- 15.Sedman A.B., Klein G.L., Merritt R.J., Miller N.L., Weber K.O., Gill W.L. Evidence of aluminum loading in infants receiving intravenous therapy. N Engl J Med. 1985;312:1337–1343. doi: 10.1056/NEJM198505233122101. [DOI] [PubMed] [Google Scholar]
- 16.Morrissey J., Rothstein M., Mayor G., Slatopolsky E. Suppression of parathyroid hormone secretion by aluminum. Kidney Int. 1983;23:699–704. doi: 10.1038/ki.1983.81. [DOI] [PubMed] [Google Scholar]
- 17.González-Suárez I., Naves M., Díaz-Corte C., Fernández-Martín J.L., Menéndez-Rodríguez P., Cannata-Andía J.B. Effect of aluminium on calcium-sensing receptor expression, proliferation, and apoptosis of parathyroid glands from rats with chronic renal failure. Kidney Int Suppl. 2003;(85):S39–S43. doi: 10.1046/j.1523-1755.63.s85.10.x. [DOI] [PubMed] [Google Scholar]
- 18.Henry H.L., Norman A.W. Interactions between aluminum and the actions and metabolism of vitamin D3 in the chick. Calcif Tissue Int. 1985;37:484–490. doi: 10.1007/BF02557831. [DOI] [PubMed] [Google Scholar]
- 19.Klein G.L., Horst R.L., Norman A.W., Ament M.E., Slatopolsky E., Coburn J.W. Reduced serum levels of 1 alpha,25-dihydroxyvitamin D during long-term total parenteral nutrition. Ann Intern Med. 1981;94:638–643. doi: 10.7326/0003-4819-94-5-638. [DOI] [PubMed] [Google Scholar]
- 20.Felsenfeld A.J., Levine B.S., Rodriguez M. Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial. 2015;28:564–577. doi: 10.1111/sdi.12411. [DOI] [PubMed] [Google Scholar]
- 21.Drüeke T., Cournot-Witmer G. Dialysis osteomalacia: clinical aspects and physiopathological mechanisms. Clin Nephrol. 1985;24(Suppl 1):S26–S29. [PubMed] [Google Scholar]
- 22.Klein G.L., Targoff C.M., Ament M.E., Sherrard D.J., Bluestone R., Young J.H. Bone disease associated with total parenteral nutrition. Lancet. 1980;2:1041–1044. doi: 10.1016/s0140-6736(80)92271-0. [DOI] [PubMed] [Google Scholar]
- 23.Vargas J.H., Klein G.L., Ament M.E., Ott S.M., Sherrard D.J., Horst R.L. Metabolic bone disease of total parenteral nutrition: course after changing from casein to amino acids in parenteral solutions with reduced aluminum content. Am J Clin Nutr. 1988;48:1070–1078. doi: 10.1093/ajcn/48.4.1070. [DOI] [PubMed] [Google Scholar]
- 24.Hewitt C.D., Innes D.J., Herman M.M., Savory J., Wills M.R. Hematological changes after long-term aluminum administration to normal adult rabbits. Ann Clin Lab Sci. 1992;22:85–94. [PubMed] [Google Scholar]
- 25.Alfrey A.C., LeGendre G.R., Kaehny W.D. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294:184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- 26.Klein G.L., Heyman M.B., Lee T.C., Miller N.L., Marathe G., Gourley W.K. Aluminum-associated hepatobiliary dysfunction in rats: relationships to dosage and duration of exposure. Pediatr Res. 1988;23:275–278. doi: 10.1203/00006450-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Klein G.L., Sedman A.B., Heyman M.B., Marathe G., Battifora H.A., Worrall J.L. Hepatic abnormalities associated with aluminum loading in piglets. JPEN - J Parenter Enter Nutr. 1987;11:293–297. doi: 10.1177/0148607187011003293. [DOI] [PubMed] [Google Scholar]
- 28.Klein G.L., Lee T.C., Heyman M.B., Rassin D.K. Altered glycine and taurine conjugation of bile acids following aluminum administration to rats. J Pediatr Gastroenterol Nutr. 1989;9:361–364. doi: 10.1097/00005176-198910000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Klein G.L., Goldblum R.M., Moslen M.T., Pyron D.L., Mann P.A., Lee T.C. Increased biliary transferrin excretion following parenteral aluminium administration to rats. Pharmacol Toxicol. 1993;72:373–376. doi: 10.1111/j.1600-0773.1993.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 30.Bidlack W.R., Brown R.C., Meskin M.S., Lee T.C., Klein G.L. Effect of aluminum on the hepatic mixed function oxidase and drug metabolism. Drug-Nutr Interact. 1987;5:33–42. [PubMed] [Google Scholar]
- 31.Klein G.L. Nutritional aspects of aluminium toxicity. Nutr Res Rev. 1990;3:117–141. doi: 10.1079/NRR19900009. [DOI] [PubMed] [Google Scholar]
- 32.Klein G.L. Aluminum in parenteral products: medical perspective on large and small volume parenterals. J Parenter Sci Technol. 1989;43:120–124. [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration. Code of federal regulations (CFR) Title 21 Vol 4. 21CFR201.323 [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; [updated 2019 Sep 4; cited 2018 Nov 19}. Available form: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=201.323.
- 34.Poole R.L., Pieroni K.P., Gaskari S., Dixon T., Kerner J.A. Aluminum exposure in neonatal patients using the least contaminated parenteral nutrition solution products. Nutrients. 2012;4:1566–1574. doi: 10.3390/nu4111566. [DOI] [PMC free article] [PubMed] [Google Scholar]