Abstract
Introduction
Cystatin C has been suggested as a sensitive marker of renal function. A high level of cystatin C is related to cardiovascular disease and stroke in elderly patients. We investigated the relationship between levels of cystatin C and early neurological deterioration with acute ischaemic stroke in elderly patients without chronic kidney disease.
Patients and methods
We evaluated a total of 771 elderly patients (mean age, 72.2; male, 59.0%) without chronic kidney disease who were admitted following acute ischaemic stroke between March 2010 and January 2015. The patients were divided into four groups based on the quartiles of serum cystatin C values. Early neurological deterioration was defined as an increase of ≥2 points from the baseline National Institutes of Health Stroke Scale score during the 7 days following onset. We compared the clinical characteristics and cystatin C concentrations between patients with and without early neurological deterioration.
Results
Eighty-six patients (11.2%) experienced early neurological deterioration. The percentage values of the higher (third and fourth) quartiles were significantly higher in the early neurological deterioration group (30.2% vs. 24.4% and 34.9% vs. 23.8%, P = 0.002). After adjustment for covariates, higher cystatin C levels were independently associated with a higher risk of early neurological deterioration: odds ratio (95% confidence interval) for second quartile 1.59 (0.70–3.58), third quartile 2.75 (1.25–6.04), fourth quartile 3.12 (1.36–7.16); P for trend 0.026.
Discussion and conclusions
This study demonstrated that cystatin C concentrations in elderly patients without chronic kidney disease were associated with early neurological deterioration following acute stroke. This suggests that cystatin C level could be a useful predictor for early neurological deterioration following acute stroke.
Keywords: Cystatin C, early neurological deterioration, normal renal function, elderly patients
Introduction
Cystatin C is a cysteine protease inhibitor produced in all nucleated cells at a constant rate.1 Cystatin C has been suggested as a sensitive marker of renal function than the creatinine-based equation, because its serum concentrations are independent of muscle mass and do not seem to be affected by age.1–4 It might be also associated with declining kidney function, systemic inflammation and metabolic syndrome.3–6 A high level of cystatin C has been reported to be positively associated with the increased risk of stroke, cardiovascular morbidity and mortality, with renal dysfunction appearing to be the most plausible mechanism related to inflammation, atherosclerosis and vascular risk factors.4–6 Moreover, and paradoxically, a few studies reported that higher cystatin C concentration is more strongly associated with cardiovascular disease, stroke and mortality among elderly persons with normal renal function.4,5,7,8
Early neurological deterioration (END) is classified as substantial neurological deterioration that occurs following an acute ischaemic stroke. END occurs in approximately 13–38% of patients with acute ischaemic stroke.9,10 Although previous studies have reported radiological, biological and clinical factors (including age) that can predict END, many of these factors are not useful because of the difficulties inherent in identifying reliable predictors and their underlying mechanisms.9–11
However, the predictive value of cystatin C for END following ischaemic stroke among elderly patients with normal renal function has not been elucidated. We hypothesized that cystatin C could be an important predictor for the risk of END in elderly patients with normal renal function.
Methods
Study population
We initially screened 1537 patients with acute ischaemic stroke and transient ischaemic attack (TIA), who were admitted within the first 7 days following the onset of stroke. They were registered into our prospective stroke registry system within 7 days of the onset of symptoms at Seoul National University Hospital between March 2010 and January 2015. We excluded patients based on the following criteria: chronic kidney disease (CKD; n = 259), lack of cystatin C data (n = 91), lack of clinical data (n = 42), age younger than 60 years (n = 304) and TIA (n = 70). Eventually, 771 patients (age range, 60–97 years) with acute ischaemic stroke were enrolled. The patients were divided into four groups based on the quartiles of serum cystatin C level. The study was approved by the Institutional Review Board of Seoul National University Hospital (IRB NO H-1009-062-332).
Baseline and clinical assessment
Baseline characteristics were recorded, including demographic data (age and sex), and conventional vascular risk factors (hypertension, diabetes mellitus, hyperlipidaemia, a history of smoking (current or past regular smoking), body mass index (BMI), initial body temperature and a history of stroke/TIA). Several laboratory findings, such as white blood cell (WBC) count, initial fasting blood sugar (FBS), haemoglobin (Hb), triglycerides, total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, fibrinogen, C-reactive protein and microalbuminuria, were also evaluated using routine laboratory methods. Blood samples were drawn after overnight fasting. Serum cystatin C was measured from blood samples using a BN II nephelometer (Siemens Healthcare Diagnostics, Inc.) with a particle-enhanced immunonephelometric assay (N Latex cystatin C, Siemens Healthcare Diagnostics, Inc.). The estimated glomerular filtration rate (eGFR; expressed in mL/min per 1.73 m2) was estimated using the four-variable Modification of Diet in Renal Disease (MDRD) formula, as follows: eGFR = 175 × (serum creatinine−1.154) × (age−0.203) × (1.212 if black) × (0.742 if female).12
Hypertension was defined as a history of antihypertensive treatment, a systolic blood pressure ≥140 mmHg, or a diastolic blood pressure ≥90 mmHg.13 Hyperlipidaemia was defined as a history of lipid-lowering medication, a serum TC level >240 mg/dL or a serum level of low-density lipoprotein (LDL) cholesterol >160 mg/dL.14 Diabetes mellitus was defined as HbA1c ≥6.5%, fasting blood glucose ≥7.0 mmol/L, non-fasting blood glucose ≥11.1 mmol/L, or the use of insulin or oral hypoglycaemic drugs.15
Ischaemic stroke was categorized as large-artery atherosclerosis, small-vessel occlusion, cardioembolism, other determined and undetermined, based on the Trial of Org 10172 in Acute Stroke Treatment criteria.16,17 The ischaemic lesion locations were divided into the anterior circulation (anterior cerebral artery and/or middle cerebral artery territories), posterior circulation (posterior cerebral artery and/or vertebrobasilar artery territories) and multiple artery territory. Moreover, we collected data regarding infections such as pneumonia, urinary tract infection, etc., occurring in the first week after stroke onset. On admission, we investigated the stroke onset time for all patients, and all the patients were initially graded for stroke severity based on the National Institutes of Health Stroke Scale (NIHSS). NIHSS and neurological evaluation were determined every 4 h in the stroke unit and at least 3 times daily in the general ward by specialized nurses or stroke neurologists. Whenever END was suspected by the patients, caregivers, or nursing staff, NIHSS was evaluated in detail by neurologists. The timing of END was identified by a review of the medical records. Considering the registration requirement of our stroke registry system, END was defined as an increase in NIHSS score by ≥2 points from baseline during the first 7 days following symptom onset.18
Imaging information
The patients underwent brain MRI on a 1.5 T or 3.0 T superconducting magnet systems within 24 h after admission. We evaluated significant stenosis, which was defined as ≥50% stenosis or occlusion of the corresponding artery.19 In addition, to evaluate the change in the infarct lesion, follow-up brain MRI or brain CT studies were performed after END (n = 71, 82.6%). We evaluated the follow-up images by dividing the results into five categories: no interval change, enlarged lesion, new lesion in new vascular territory, oedematous change (a swelled initial lesion or presenting oedematous change on brain CT or FLAIR image) and haemorrhagic transformation.20 Two trained neurologists (T.J.K. and S.J.A.), blinded to the clinical information, assessed the degree of stenosis and the change in the infarct lesion. Disagreement was resolved by discussion and reassessment.
Statistical analysis
Continuous variables and proportions of categorical variables were compared using Student’s t-tests, Pearson’s χ2 tests, or Fisher’s exact test, as appropriate. The association between END and the quartiles of cystatin C was studied using logistic regression analyses. Covariates with statistically significant differences (P < 0.05) were investigated using univariate analysis, and those with clinically important factors, even though it was not statistically significant, were adjusted for multivariate analysis. We analysed the relationship between the level of cystatin C and END using the restricted cubic spline function. For all the analyses, a two-tailed P-value <0.05 was considered statistically significant. Statistical analyses were performed using the SPSS program (Version 21.0, IBM Statistics) and R (version 3.2.2, R Project).
Results
Baseline characteristics
A total of 771 patients with acute ischaemic stroke were enrolled in this study. The included patients were mostly male (59.0%) and mean age of total patients was 72.2 years (Table 1). Compared with the patients in the lowest quartile of cystatin C, the patients from the group corresponding to the highest levels of cystatin C were significantly older, more hypertensive and more likely to have a history of smoking (Table 1). In addition, eGFR, FBS and HDL levels were significantly lower in patients with higher levels of cystatin C compared with the lowest levels of cystatin C group (Table 1). The proportion of male was significantly greater in the highest quartile of cystatin C group (P = 0.036). Eighty-six patients (11.2%) experienced early neurological worsening after stroke onset. The mean time to the END event was 22.9 h (±32 h) after admission. The patients with END tended to be older (P < 0.001), and more likely to have coronary heart disease (P = 0.022) (Table 2). In addition, a higher percentage of patients with END had multiple territory lesion (P = 0.005) (Table 2). Stroke severity and stroke subtype did not differ between patients with and without END. Moreover, the prevalence of significant stenosis in the parent artery was 39.4% (n = 304); however, there was no significant difference between the two groups (END group, 46.5% vs. non-END group 38.5%, P = 0.154). The END group was more likely to have infections in the first week of stroke onset compared with the non-END group (Table 2). Laboratory data on admission revealed significant differences: patients with END had higher levels of FBS (P = 0.019) and WBC (P = 0.029). However, eGFR and microalbuminuria were similar between the two groups.
Table 1.
Baseline characteristics by quartiles of cystatin C.
| Total (n = 771) | Quartile 1 (0.080–0.734) (n = 192) | Quartile 2 (0.737–0.821) (n = 193) | Quartile 3 (0.822–0.935) (n = 193) | Quartile 4 (0.936–2.060) (n = 193) | P-value | |
|---|---|---|---|---|---|---|
| Age (mean ± SD), years | 72.2 ± 7.2 | 70.2 ± 7.1 | 71.0 ± 6.5 | 72.4 ± 7.3 | 75.4 ± 6.8 | <0.001 |
| Male, n (%) | 455 (59.0) | 100 (52.1) | 119 (61.7) | 109 (56.5) | 127 (65.8) | 0.036 |
| BMI (mean ± SD), kg/m2 | 23.1 ± 3.1 | 23.2 ± 3.3 | 23.0 ± 2.9 | 23.4 ± 2.9 | 22.6 ± 3.2 | 0.096 |
| Hypertension, n (%) | 337 (43.7) | 62 (32.3) | 83 (43.0) | 77 (39.9) | 115 (59.6) | <0.001 |
| Diabetes mellitus, n (%) | 152 (19.7) | 38 (19.8) | 28 (18.1) | 29 (15.0) | 50 (25.9) | 0.234 |
| Hyperlipidaemia, n (%) | 122 (15.8) | 27 (14.1) | 29 (15.0) | 27 (14.0) | 39 (20.2) | 0.139 |
| Smoking, n (%) | 41 (5.3) | 3 (1.6) | 8 (4.1) | 12 (6.2) | 18 (9.3) | <0.001 |
| Previous stroke/TIA, n (%) | 76 (9.9) | 14 (7.3) | 15 (7.8) | 21 (10.9) | 26 (13.5) | 0.024 |
| Coronary heart disease, n (%) | 21 (2.7) | 4 (2.1) | 4 (2.1) | 6 (3.1) | 7 (3.6) | 0.280 |
| Atrial fibrillation, n (%) | 73 (9.5) | 14 (7.3) | 15 (7.8) | 20 (10.4) | 24 (12.4) | 0.056 |
| Initial NIHSS (median (IQR)) | 3 (1–6) | 4 (1.25–8) | 3 (1–6) | 3 (1–5) | 3 (0.5–6.5) | 0.051 |
| Stroke mechanism, n (%) | 0.877 | |||||
| LAA | 248 (32.2) | 62 (32.3) | 67 (34.7) | 56 (29.0) | 63 (32.6) | |
| SVO | 175 (22.7) | 43 (22.4) | 45 (23.3) | 48 (24.9) | 39 (20.2) | |
| CE | 195 (25.3) | 47 (24.5) | 43 (22.3) | 52 (26.9) | 53 (27.5) | |
| Other determined | 42 (5.4) | 7 (3.6) | 12 (6.2) | 10 (5.2) | 13 (6.7) | |
| Undetermined | 111 (14.4) | 33 (17.2) | 26 (13.5) | 27 (14.0) | 25 (13.0) | |
| END, n (%) | 86 (11.2) | 13 (15.1) | 17 (19.8) | 26 (30.2) | 30 (34.9) | 0.022 |
| Infection, n (%) | 57 (7.8) | 15 (7.8) | 12 (6.2) | 15 (7.8) | 15 (7.8) | 0.915 |
| Initial BT (℃) | 36.40 ± 0.40 | 36.41 ± 0.39 | 36.40 ± 0.41 | 36.37 ± 0.37 | 36.41 ± 0.43 | 0.790 |
| Laboratory information (mean ± SD) | ||||||
| Cystatin C (mg/L) | 0.844 ± 0.178 | 0.658 ± 0.081 | 0.780 ± 0.026 | 0.871 ± 0.031 | 1.067 ± 0.171 | <0.001 |
| eGFR (mL/min/1.73 m2) | 86.6 ± 19.9 | 92.7 ± 23.2 | 90.0 ± 19.9 | 85.2 ± 16.5 | 78.3 ± 16.3 | <0.001 |
| White blood cell count (×103/µL) | 7.58 ± 2.49 | 7.56 ± 2.62 | 7.60 ± 2.55 | 7.53 ± 2.35 | 7.63 ± 2.44 | 0.981 |
| Haemoglobin (g/dL) | 13.48 ± 1.78 | 13.52 ± 1.90 | 13.59 ± 1.72 | 13.65 ± 1.66 | 13.15 ± 1.80 | 0.029 |
| Fasting blood sugar (mg/dL) | 108.55 ± 35.08 | 116.06 ± 35.46 | 108.14 ± 38.97 | 104.97 ± 30.85 | 105.09 ± 33.65 | 0.005 |
| Total cholesterol (mg/dL) | 171.28 ± 40.49 | 172.13 ± 43.89 | 172.16 ± 37.62 | 175.43 ± 38.15 | 165.44 ± 41.65 | 0.102 |
| Triglyceride (mg/dL) | 109.87 ± 56.58 | 106.27 ± 51.43 | 99.61 ± 41.47 | 116.40 ± 57.89 | 116.97 ± 69.94 | 0.005 |
| HDL cholesterol (mg/dL) | 47.22 ± 13.63 | 49.60 ± 14.23 | 48.83 ± 13.40 | 45.09 ± 12.50 | 45.38 ± 13.85 | 0.001 |
| C-reactive protein (mg/dL) | 0.65 ± 1.58 | 0.66 ± 1.69 | 0.55 ± 1.35 | 0.51 ± 1.27 | 0.88 ± 1.93 | 0.099 |
| Microalbumin (mg/dL) | 5.61 ± 13.04 | 4.78 ± 11.71 | 4.70 ± 10.31 | 5.51 ± 13.20 | 7.46 ± 16.19 | 0.164 |
BMI: body mass index; SD: standard deviation; TIA: transient ischaemic stack; LAA: large artery atherosclerosis; SVO: small-vessel occlusion; CE: cardioembolism; END: early neurological deterioration; eGFR: estimated glomerular filtration rate; HDL: high density lipid.
Table 2.
Patients profile according to early neurological deterioration.
| END group (n = 86, 11.2%) | Non-END group (n = 685, 88.8%) | P-value | |
|---|---|---|---|
| Age (mean ± SD), years | 74.8 ± 7.9 | 71.9 ± 7.0 | <0.001 |
| Male, n (%) | 55 (64.0) | 400 (58.4) | 0.323 |
| BMI (mean ± SD), kg/m2 | 23.0 ± 3.9 | 23.1 ± 3.0 | 0.840 |
| Hypertension, n (%) | 41 (47.7) | 296 (43.2) | 0.432 |
| Diabetes mellitus, n (%) | 14 (16.3) | 138 (20.1) | 0.396 |
| Hyperlipidaemia, n (%) | 14 (16.3) | 108 (15.8) | 0.902 |
| Smoking, n (%) | 3 (7.3) | 38 (5.5) | 0.610 |
| Previous stroke/TIA, n (%) | 11 (12.8) | 65 (9.5) | 0.333 |
| Coronary heart disease, n (%) | 6 (7.0) | 15 (2.2) | 0.022 |
| Atrial fibrillation, n (%) | 10 (13.7) | 63 (9.2) | 0.468 |
| Initial NIHSS (median (IQR)) | 4 (0–9.25) | 3 (1–6) | 0.844 |
| Stroke mechanism, n (%) | 0.222 | ||
| LAA | 26 (30.2) | 222 (32.4) | |
| SVO | 13 (15.1) | 162 (23.6) | |
| CE | 29 (33.7) | 166 (24.2) | |
| Other determined | 6 (7.0) | 36 (5.3) | |
| Undetermined | 12 (14.0) | 99 (14.5) | |
| Lesion location, n (%) | 0.005 | ||
| Anterior circulation | 50 (58.1) | 409 (59.7) | |
| Posterior circulation | 17 (19.9) | 202 (29.5) | |
| Multiple territory | 19 (22.1) | 74 (10.8) | |
| Significant stenosis on relevant artery, n (%) | 40 (46.5) | 264 (38.5) | 0.154 |
| Infection, n (%) | 12 (14.0) | 45 (6.6) | 0.014 |
| Initial BT (℃) | 36.46 ± 0.39 | 36.39 ± 0.40 | 0.156 |
| eGFR (mean ± SD), mL/min/1.73 m2 | 89.1 ± 23.6 | 86.3 ± 19.4 | 0.220 |
| Quartiles of Cystatin C | 0.002 | ||
| Q1 (0.080–0.734) | 13 (15.1) | 179 (26.1) | |
| Q2 (0.737–0.821) | 17 (19.8) | 176 (25.7) | |
| Q3 (0.822–0.935) | 26 (30.2) | 167 (24.4) | |
| Q4 (0.936–2.060) | 30 (34.9) | 163 (23.8) | |
| Laboratory information (mean ± SD) | |||
| White blood cell count (×103/µL) | 8.13 ± 2.56 | 7.51 ± 2.47 | 0.029 |
| Fasting blood sugar (mg/dL) | 116.88 ± 38.71 | 107.49 ± 34.48 | 0.019 |
| Cystatin C (mg/L) | 0.892 ± 0.190 | 0.838 ± 0.176 | 0.009 |
| C-reactive protein (mg/dL) | 1.03 ± 2.05 | 0.60 ± 1.51 | 0.064 |
END: early neurological deterioration; BMI: body mass index; TIA: transient ischaemic stack; LAA: large artery atherosclerosis; SVO: small-vessel occlusion; CE: cardioembolism; BT: body temperature; eGFR: estimated glomerular filtration rate.
Among the 86 patients with END, 71 (82.6%) patients underwent follow-up brain CT or brain MRI to identify the possible causes. About one third of patients (35.2%, data not shown) with END had a ‘no interval change’ pattern, while the rate of new lesion occurrence in a new vascular territory was 2.8%. Oedematous change and haemorrhagic transformation were observed in 28.2% of the patients.
The correlation between END and cystatin C
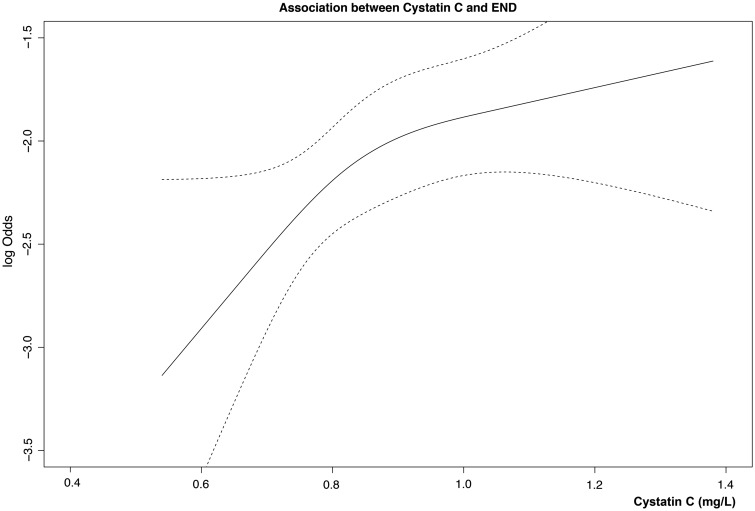
The proportion of subjects with an elevated cystatin C level was significantly higher in the END group than in the non-END group (P = 0.009) (Table 2). The risk of END increased with the serum level of cystatin C (Figure 1). An increased level of cystatin C was associated with an increased risk of END (odds ratio 1.33, 2.14 and 2.53 in the second, third and fourth quartiles, respectively; P for trend, 0.026) (Table 3). As compared with the lowest quartile, the third and fourth quartiles of cystatin C were significantly associated with END (adjusted odds ratio, third quartile 2.75, P = 0.012; fourth quartile 3.12, P = 0.007) after adjusting for confounding variables (Table 3 and full regression analysis in Supplementary Table 1). We also performed a sensitivity multivariate analysis for the relationship between cystatin C and END using a more traditional definition for END as ≥2 points of NIHSS within 72 h of admission (n = 80, Supplementary Table 3). Cystatin C was a consistently related to END using a usual definition.
Figure 1.
Association between levels of cystatin C and the risk of early neurological deterioration (END). The risk of END is increased when the serum level of cystatin C increases. Log odds (solid black line) and 95% confidence intervals (dashed outer bands).
Table 3.
Multivariate analysis according to early neurological deterioration.
| Unadjusted OR (95% CI) | P value | Model 1 |
P value | Model 2 |
P value | |
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | |||||
| Cystatin C | 0.026 | 0.026 | 0.037 | |||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Q2 | 1.33 (0.63–2.82) | 0.457 | 1.59 (0.70–3.58) | 0.267 | 1.33 (0.61–2.90) | 0.474 |
| Q3 | 2.14 (1.07–4.31) | 0.032 | 2.75 (1.25–6.04) | 0.012 | 2.34 (1.11–4.93) | 0.025 |
| Q4 | 2.53 (1.28–5.03) | 0.008 | 3.12 (1.36–7.16) | 0.007 | 2.76 (1.25–6.11) | 0.012 |
| Age | 1.06 (1.03–1.09) | <0.001 | 1.04 (1.00–1.08) | 0.037 | 1.04 (1.00–1.08) | 0.031 |
| Infection | 2.31 (1.17–4.56) | 0.016 | 1.63 (0.74–3.59) | 0.230 | 1.61 (0.73–3.56) | 0.240 |
| WBC (10/µL) | 6.65 (1.27–34.89) | 0.025 | 4.90 (0.83–28.98) | 0.080 | 4.26 (0.74–24.61) | 0.106 |
| FBS | 1.01 (1.00–1.01) | 0.022 | 1.01 (1.00–1.01) | 0.068 | 1.01 (1.00–1.01) | 0.033 |
CHD: coronary heart disease; GFR: glomerular filtration rate; WBC: white blood cell; FBS: fasting blood sugar.
Model 1: Adjusting for cystatin C, age, sex, hypertension, smoking, CHD, previous stroke/TIA, lesion location, GFR, FBS, WBC, haemoglobin, HDL, TG, infections, significant stenosis. Model 2: Adjusting for cystatin C, age, sex, hypertension, smoking, CHD, previous stroke/TIA, lesion location, FBS, WBC, haemoglobin, HDL, TG, infection, significant stenosis.
Discussion
In this study, we found that a higher level of cystatin C was an independently significant predictor for END in elderly patients with normal renal function, and that an increased cystatin C level was associated with an approximately 3 times higher risk for END. However, we found that eGFR was not associated with END.
Several factors influence the development of END, such as the severity of the initial stroke, large vessel occlusion, blood glucose, seizure, inflammation, medical complications such as infection, age and low eGFR.9–11,21–23 In line with these observations, our study showed that patients with END tended to be older and higher levels of WBC, and more frequent infection events, and the FBS level was positively correlated with END. However, after adjustment for confounding variables, END had an independent correlation only with age. Moreover, the initial NIHSS score, eGFR and microalbuminuria were similar in the patients with and without END. Higher quartile cystatin C (third and fourth quartiles) accounted for 30.2% and 34.9% of the patients with END compared with 24.4% and 23.8% of the patients with non-END, respectively. These results suggested that a higher level of cystatin C in elderly patients was a possible predictor for the development of END, even if it was a normal level.
Cystatin C is known to be expressed constantly by all nucleated cells. Within the normal range of kidney function, cystatin C has been proposed as a more reliable marker of renal function than serum creatinine and as an indicative marker of preclinical kidney disease.1,4,24,25 A higher level of serum cystatin C, which is a cysteine protease inhibitor, might reflect an imbalance in elastolytic activity. This imbalance between proteases and inhibitors affects the cardiovascular system.7,24,26 Thus, a higher cystatin C concentration is related to inflammation and endothelial dysfunction, and is involved in the pathogenesis of atherosclerosis.27–29 These factors might explain the link between microcirculation disorders and impaired vasodilation, which leads to END. Moreover, a high concentration of cystatin C has also been associated with a hypermetabolic status, and may reflect the duration and severity of other risk factors, such as hypertension and preclinical renal dysfunction.3,6,24,30 Likewise, our analysis showed that patients from groups corresponding to higher quartiles of cystatin C were older and more likely to evidence hypertension, while they had lower eGFR. These mechanisms could affect the process of vascular wall remodelling, directly or indirectly, and could influence the relation between the risk of stroke and other cardiovascular events.4,5,24 In addition, the inflammatory response promotes a local procoagulant state and a direct toxic effect that contribute to clinical neurological deterioration.28,29,31
There are some limitations to our study. First, we did not evaluate medical conditions, such as thyroid dysfunction and the use of glucocorticoid that could have induced an unexpectedly high cystatin C concentration. Second, we did not assess the volume of the infarction. However, the initial NIHSS was similar for the two groups; we therefore assumed that the volume of infarction was not different significantly.32 Third, we included only elderly patients in our study; therefore, a selection bias was possible and our subject pool might not permit the generalization of our findings. However, cystatin C was not related to a risk of END in the excluded younger patients in our study (P = 0.162, Supplementary Table 2) in consistent with previous studies.4,5,7,8 Fourth, we used a single measurement of serum creatinine and cystatin C at admission; therefore, possible effects depending on individual differences or temporal variations were not evaluated. Fifth, we selected an unusual definition of END, which may have resulted in a possible bias in the evaluation of END. However, cystatin C was a consistently related to END in a multivariate analysis using a more traditional definition of END (Supplementary Table 3). Despite these limitations, our study is meaningful in presenting an upper normal or slightly elevated cystatin C level as a possible confounder for END in elderly patients with preserved renal function.
In conclusion, our study found that a higher cystatin C level is associated with END in elderly patients without CKD. In this context, our data may suggest that the cystatin C level could be a useful biomarker for the prediction of END in elderly patients with preserved renal function. Further, large and well-designed prospective studies are required to confirm the true relationship between cystatin C and END using a more accurate method.
Supplementary Material
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Korea Health Technology R&D project (HI10C2020) and by the Ministry of Health and Welfare, Republic of Korea (HI16C1078).
Ethical approval
The study was approved by the Institutional Review Board of Seoul National University Hospital (IRB NO H-1009-062-332).
Informed consent
Written informed consent was obtained from the enrolled patients.
Guarantor
B-WY
Contributorship
TJ Kim had full access to all data, performed statistical analyses, and took responsibility for data integrity and accuracy. S-B Ko and B-W Yoon conceptualized and designed the study. CK Kim, Y Kim, MK Kang, H-G Jeong, K-W Nam, H Mo and SJ An participated in data acquisition. B-W Yoon made intellectual contributions. TJ Kim and B-W Yoon interpreted results and wrote the manuscript.
References
- 1.Shlipak MG, Matsushita K, Ärnlöv J, et al. Cystatin c versus creatinine in determining risk based on kidney function. New Engl J Med 2013; 369: 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menon V, Shlipak MG, Wang X, et al. Cystatin c as a risk factor for outcomes in chronic kidney disease. Ann Int Med 2007; 147: 19–27. [DOI] [PubMed] [Google Scholar]
- 3.Ledoux D, Monchi M, Chapelle J-P, et al. Cystatin c blood level as a risk factor for death after heart surgery. Eur Heart J 2007; 28: 1848–1853. [DOI] [PubMed] [Google Scholar]
- 4.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin c and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Int Med 2006; 145: 237–246. [DOI] [PubMed] [Google Scholar]
- 5.Wu C-K, Lin J-W, Caffrey JL, et al. Cystatin c and long-term mortality among subjects with normal creatinine-based estimated glomerular filtration rates: Nhanes iii (third national health and nutrition examination survey). J Am Coll Cardiol 2010; 56: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 6.Ni L, Lü J, Hou LB, et al. Cystatin c, associated with hemorrhagic and ischemic stroke, is a strong predictor of the risk of cardiovascular events and death in chinese. Stroke 2007; 38: 3287–3288. [DOI] [PubMed] [Google Scholar]
- 7.Sai E, Shimada K, Miyauchi K, et al. Increased cystatin c levels as a risk factor of cardiovascular events in patients with preserved estimated glomerular filtration rate after elective percutaneous coronary intervention with drug-eluting stents. Heart Vessels 2016; 31: 694–701. [DOI] [PubMed] [Google Scholar]
- 8.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin c and the risk of death and cardiovascular events among elderly persons. New Engl J Med 2005; 352: 2049–2060. [DOI] [PubMed] [Google Scholar]
- 9.Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: Predictors, mechanisms and management. Postgrad Med J 2008; 84: 412–417. [DOI] [PubMed] [Google Scholar]
- 10.Kwan J, Hand P. Early neurological deterioration in acute stroke: Clinical characteristics and impact on outcome. QJM 2006; 99: 625–633. [DOI] [PubMed] [Google Scholar]
- 11.Vila N, Castillo J, Dávalos A, et al. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000; 31: 2325–2329. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Int Med 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA 2003; 289: 2560–2571. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection E. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 15.Vijan S. Type 2 diabetes. Ann Int Med 2010; 152: ITC3-1. [DOI] [PubMed]
- 16.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 17.Kim BJ, Kim JS. Ischemic stroke subtype classification: An asian viewpoint. J Stroke 2014; 16: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto N, Kimura K, Yokota C, et al. Early neurological deterioration represents recurrent attack in acute small non-lacunar stroke. J Neurol Sci 2004; 217: 151–155. [DOI] [PubMed] [Google Scholar]
- 19.Tei H, Uchiyama S, Ohara K, et al. Deteriorating ischemic stroke in 4 clinical categories classified by the oxfordshire community stroke project. Stroke 2000; 31: 2049–2054. [DOI] [PubMed] [Google Scholar]
- 20.Kang DW, Latour LL, Chalela JA, et al. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol 2003; 54: 66–74. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Kim J-K, Oh MS, et al. A low baseline glomerular filtration rate predicts poor clinical outcome at 3 months after acute ischemic stroke. J Clin Neurol 2015; 11: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellanos M, Castillo J, García MM, et al. Inflammation-mediated damage in progressing lacunar infarctions a potential therapeutic target. Stroke 2002; 33: 982–987. [DOI] [PubMed] [Google Scholar]
- 23.Birschel P, Ellul J, Barer D. Progressing stroke: Towards an internationally agreed definition. Cerebrovasc Dis 2003; 17: 242–252. [DOI] [PubMed] [Google Scholar]
- 24.Taglieri N, Koenig W, Kaski JC. Cystatin c and cardiovascular risk. Clin Chem 2009; 55: 1932–1943. [DOI] [PubMed] [Google Scholar]
- 25.Filler G, Bökenkamp A, Hofmann W, et al. Cystatin c as a marker of gfr—history, indications, and future research. Clin Biochem 2005; 38: 1–8. [DOI] [PubMed] [Google Scholar]
- 26.Oh M-Y, Lee H, Kim JS, et al. Cystatin c, a novel indicator of renal function, reflects severity of cerebral microbleeds. BMC Neurol 2014; 14: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnusson M, Hedblad B, Engström G, et al. High levels of cystatin c predict the metabolic syndrome: The prospective malmö diet and cancer study. J Int Med 2013; 274: 192–199. [DOI] [PubMed] [Google Scholar]
-
28.Leung-Tack J, Tavera C, Gensac M, et al.
Modulation of phagocytosis-associated respiratory burst by human
cystatin c: Role of the n-terminal tetrapeptide lys
 pro
pro pro
pro arg.
Exp Cell Res
1990; 188:
16–22. [DOI] [PubMed] [Google Scholar]
arg.
Exp Cell Res
1990; 188:
16–22. [DOI] [PubMed] [Google Scholar] - 29.Balta S, Demirkol S, Ay S, et al. Serum cystatin-c levels correlate with endothelial dysfunction in patients with the metabolic syndrome. J Int Med 2013; 274: 200–201. [DOI] [PubMed] [Google Scholar]
- 30.Salgado JV, França AK, Cabral NA, et al. Cystatin c, kidney function, and cardiovascular risk factors in primary hypertension. Revista da Associação Médica Brasileira 2013; 59: 21–27. [DOI] [PubMed] [Google Scholar]
- 31.Levy E, Jaskolski M, Grubb A. The role of cystatin c in cerebral amyloid angiopathy and stroke: Cell biology and animal models. Brain Pathol 2006; 16: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong D, Yenari M, Albers G, et al. Correlation of perfusion-and diffusion-weighted mri with nihss score in acute (<6.5 hour) ischemic stroke. Neurology 1998; 50: 864–869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.