Abstract
Purpose
Intra-arterial mechanical thrombectomy combined with appropriate patient selection (image-based selection of acute ischaemic stroke patients with large artery occlusion) yields improved clinical outcomes. We conducted a systematic review and meta-analysis, with trial sequential analysis to understand the benefits, risks and impact of new trials reporting in 2016 on the magnitude/certainty of the estimates for clinical effectiveness and safety of mechanical thrombectomy.
Method
Random effects’ models were conducted of randomised clinical trials comparing mechanical thrombectomy (stent retriever or aspiration devices) with/without adjuvant intravenous thrombolysis with intravenous thrombolysis and other forms of best medical/supportive care in the treatment of acute ischaemic stroke. Study inclusion and risk of bias were assessed independently by two reviewers. Functional independence (modified Rankin Scale 0–2) and mortality at 90 days, including symptomatic intracranial haemorrhage rate were extracted. Trial sequential analysis established the strength of the evidence derived from the meta-analyses.
Findings
Eight trials of mechanical thrombectomy with a total sample size of 1841 (916 patients treated with mechanical thrombectomy and 925 treated without mechanical thrombectomy) fulfilled review inclusion criteria. The three most recent trials more precisely defined the effectiveness of mechanical thrombectomy (modified Rankin Scale 0 to 2; OR = 2.07, 95% CI = 1.70 to 2.51 based on data from eight trials versus OR = 2.39, 95% CI = 1.88 to 3.04 based on data from five trials). Meta-analyses showed no effect on mortality (OR = 0.81, 95% CI = 0.61 to 1.07) or symptomatic intracranial haemorrhage (OR = 1.22, 95% CI = 0.80 to 1.85) as found in analysis of first five trials. Trial sequential analysis indicated that the information size requirement was fulfilled to conclude the evidence for mechanical thrombectomy is robust.
Discussion
The impact of three recent trials on effectiveness and safety of mechanical thrombectomy was a more precise pooled effect size for functional independence. Trial sequential analysis demonstrated sufficient evidence for effectiveness and safety of mechanical thrombectomy.
Conclusion
No further trials of mechanical thrombectomy versus no mechanical thrombectomy are indicated to establish clinical effectiveness. Uncertainty remains as to whether mechanical thrombectomy reduces mortality or increases risk of symptomatic intracranial haemorrhage.
Keywords: Acute ischaemic stroke, intra-arterial thrombolysis, intravenous thrombolysis, meta-analysis, sequential trial analysis, stent retriever, systematic review, thrombectomy
Introduction
The benefits of intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (IV rt-PA) for acute ischaemic stroke are well-known, time dependent (with earlier treatment within the 4.5 h treatment window associated with better functional outcomes) and encapsulated by the aphorism ‘Time is Brain’.1–3
Despite the efficacy of IVT in reducing post-stroke disability, recanalisation (restoration of blood flow through a blocked artery) occurs in only ∼10–45% of patients with large artery occlusion (LAO) depending on site/length of occlusion.4,5 A number of approaches are currently being explored to increase IVT recanalisation rates, including use of more fibrin selective thrombolytic drugs, ultrasound and adjunctive anticoagulant therapy. None are yet proven.
There is overwhelming evidence that mechanical thrombectomy (MT) achieves significantly higher recanalisation rates than IV rt-PA for LAO6 and better clinical outcomes with a 13% to 31% absolute increase in patients recovering from acute stroke to be independent in activities of daily living. MT is not associated with an increased risk of symptomatic intracranial haemorrhage (SICH) or mortality.7,8
Meta-analyses of randomised controlled trials (RCTs) have since been published,9–11 each of which has taken a slightly different approach to inclusion criteria; all of which find that MT is an effective treatment with reduced disability rates. The most robust of these is likely to be the individual patient meta-analysis, based on data from 1287 patients (634 MT and 653 standard care). The results suggest that MT led to significantly reduced disability at 90 days compared with controls (adjusted OR = 2.49, 95% confidence interval (CI) = 1.76 to 3.53).11
The evidence base to define the safety and effectiveness of MT for selected acute ischaemic stroke patients has grown recently, with The Randomized, Concurrent Controlled Trial to Assess the Penumbra System's Safety and Effectiveness in the Treatment of Acute Stroke THERAPY,12 Thrombectomy after Intravenous Alteplase versus Alteplase Alone after Stroke (THRACE)13 and Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE)14 RCTs all reporting in 2016. An updated evidence synthesis is therefore warranted to understand the impact of these new trials on our understanding of the clinical effectiveness and safety of MT; in particular with stent retrievers and aspiration devices.
Meta-analyses of RCTs increase the power and precision of the estimated intervention effects. However, RCTs of MT over time have evaluated a range of devices and the population considered eligible for treatment has changed. There is therefore a need to ensure that only those RCTs that reflect the current practice to be considered. Trial sequential analysis (TSA) corresponds to group sequential analysis of a single trial and can be applied to meta-analysis to evaluate the robustness of the evidence. TSA necessitates the use of an information size to evaluate the strength of the evidence. There is a need to inform/estimate that pre-specified intervention effect, in the same manner as a power calculation in a clinical trial. TSA can help to quantify whether meta-analyses are presenting the best available and/or sufficient evidence.
Therefore we conducted a systematic review with meta-analysis alongside TSA that aimed to update the evidence-base for MT, and evaluate the benefits, risks and impact of three recent trials on the magnitude/uncertainty of the estimate for clinical effectiveness and safety of MT in the treatment of acute ischaemic stroke.
Methods
The review adhered to a published protocol15 and the reporting guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.16
Randomised clinical trials that included a minimum of 10 adult patients (aged ≥ 18) presenting with acute ischaemic stroke receiving MT (stent retrievers and aspiration devices) with or without adjuvant IVT or standard medical care were eligible for inclusion in the review. Where applicable, data on comparator interventions (IVT and other forms of best medical or supportive care) of studies evaluating MT were extracted.
Eligible studies had to include at least one of the following outcomes assessed at ≥90 days follow-up: modified Rankin Scale (mRS),17 Oxford Handicap Scale (OHS),18 National Institute of Health Stroke Scale (NIHSS)19 or Barthel ADL Index.20 Data on secondary outcomes were extracted from eligible studies: length of stay/time in acute care; recanalisation (Treatment in Cerebral Infarction (TICI) score21 as a reference measure that can be mapped onto analogous measures such as the Thrombolysis in Myocardial Ischaemia (TIMI) score22) and the EuroQol Five Dimensions Questionnaire EQ-5D23 (or analogous measures of health-related quality of life). Safety of MT was summarised as a function of 90-day mortality, and SICH within seven-days (as per the SITS-MOST definition ‘NIHSS scores worsening ≥ 4 within 24 h and an intracerebral haemorrhage type PH2’24).
Search strategy
A search strategy was designed with assistance from an experienced information scientist (SR) using a combination of MeSH/thesaurus terms and keywords. The following bibliographic databases were searched up to mid-February 2015: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, SCOPUS, and Web of Science. Additionally several trials registries were searched: ClinicalTrials.gov, International Standard Randomised Controlled Trial Number Register and the Chinese Clinical Trial Registry. Where published protocols for ongoing randomised trials were identified by the search strategy (to mid-Feb 2015), these were included if published by end of 2016 to ensure that the evidence presented in the review is complete and up-to-date.
An example search strategy for MEDLINE can be found in Supplementary material 1. As our focus was on MT with stent retriever devices and current generation aspirational devices, studies published prior to January 2009 were excluded. No restrictions were placed on country of origin. Included studies had to involve humans, and had to have a title and abstract in English. Where available, a search filter for controlled trials25 was used or adapted as appropriate. Hand searching of reference lists and citation searching of studies that fulfilled the eligibility criteria were undertaken. Reference lists of directly relevant reviews identified by the search strategy were also hand-searched.
Study selection
In stage 1, two reviewers (RF and EGA) independently assessed the titles and abstracts retrieved via the search strategy for eligibility. In stage 2, studies retained at stage 1 were independently assessed for eligibility by PW, RF and AC using a study selection form (Supplementary material 2). Disagreements were resolved via discussion or via a third reviewer (PW or AC) adjudicating on inclusion of a study.
Data extraction and risk of bias assessment
A structured data extraction form, with selected items from the template for intervention description and replication (TIDIER) checklist26 was used to capture information on the study population, intervention(s), comparator(s) and outcomes (Supplementary material 3). Data extraction was undertaken by one reviewer (PW) with fidelity of data extraction checked by a second reviewer (RF and KH) with disagreements resolved via discussion. The methodological quality assessment framework for RCTs developed by the Cochrane Collaboration27 was used independently by two reviewers (RF and DF) to assess the risk of bias within studies (low, medium and high). Where applicable, corresponding study authors were contacted to request missing data.
Data synthesis
Data on clinical outcomes were synthesised using meta-analytic techniques where sufficient data for calculation of effect sizes existed for each outcome of interest (unadjusted odds ratios and corresponding 95% CIs). To allow for differences between/within studies, random effects models were utilised. Risk of small study bias across studies was established with funnel plots. Seven out of eight trials were stopped early (truncated, two of them only modestly so) due to a pre-specified efficacy stopping point being reached (3 trials), loss of equipoise (3 trials) and in one trial due to efficacy (although a pre-specified stopping point was not reached). There has been some debate in the literature around the inclusion of truncated and non-truncated trials in a meta-analysis. Historically, the standard approach has been to incorporate truncated RCTs without any special consideration, however fears that early stopping may be an important source of bias has led to further investigation. A comprehensive investigation of the issues has concluded that early stopping of clinical trials is not a substantive source of bias in meta-analyses and recommend that all studies (truncated and non-truncated) be included.28
Trial sequential analysis
A TSA was used to establish the optimal size within our meta-analysis (maintaining Type I error of 0.05 / 5%) after accounting for heterogeneity (diversity) between trials. The TSA was conducted using TSA software version 0.9.5.5 Beta.29 An estimated optimal information size requirement was calculated using conventional parameters (power = 0.80, Type II error = 0.20; Type I error = 0.05). Based on a previous TSA of thrombectomy trials30 the following assumptions were made in the current TSA: a threshold of 30% relative risk increase for functional independence (mRS 0 to 2); 30% relative risk reduction for both all-cause mortality and SICH; and control event rates of pooled control arm rates from the eight trials (30.4%, 17.5% and 4.7%) for functional independence (mRS 0 to 2), mortality and SICH, respectively. Trial data were entered into the TSA in order of publication date.
TSA enables the estimation of information size with adjusted threshold for statistical significance – sequential monitoring boundaries.30 If the cumulative z-statistical curve crosses the sequential monitoring boundaries, then it can be inferred that future trials would not alter the conclusions about the outcome, and a sufficient level of evidence has been accumulated.30 When the z-curve crosses over into the futility area, it can be inferred that any differences between the comparators would be unlikely to change in future trials of MT.30
Findings
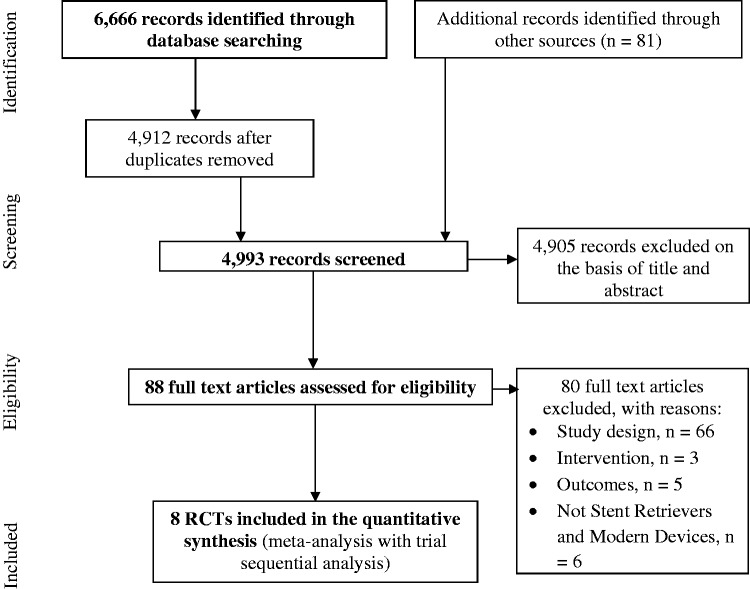
Out of 4993 records identified by the search strategy, eight randomised clinical trials were assessed to be eligible for inclusion in the meta-analyses (Figure 1). The eight trials had a combined sample size of 1841 (916 patients treated with MT and 925 treated without MT). However, the N in the treatment group across the trials for the different outcomes was variable. We also identified discrepancies in numbers of cases reported in individual published trials compared with the numbers of cases reported in previous meta-analyses (Supplementary material 4).
Figure 1.
PRISMA diagram of the process used to identify studies. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses.
The countries of origin of the eight trials were: Australia and New Zealand (EXTEND-IA31); Canada, Ireland, South Korea, UK and USA (ESCAPE32); Spain (REVASCAT33); Austria, Denmark, France, Germany, Spain, Switzerland, USA (SWIFT PRIME34); The Netherlands (MR CLEAN8); Germany and USA (THERAPY12); France (THRACE13); and UK (PISTE14).
The device types, imaging modalities and recanalisation rates for patients treated with MT in the eight trials are shown in Table 1. All eight trials were assessed to have a low risk of bias (Table 2).
Table 1.
Summary of device type, imaging modality and recanalisation in the eight trials.
| Primary author; study name | Device type | Advanced imaginga | Recanalisation % |
|---|---|---|---|
| Berkhemer et al.: MR CLEAN8 | Trevo retrievable stents and others | No | MT treatment group = 115/196 (59) |
| Campbell et al.: EXTEND:IA31 | Solitaire FR retrievable stent | CT perfusion imaging | IV rt-PA plus MT = 25/35 (86) |
| Goyal et al.: ESCAPE32 | Retrievable stents or aspiration | Yes | IV rt-PA plus MT = 113/156 (72) |
| Jovin et al.: REVASCAT33 | Solitaire FR | Y (in defined subgroups) | IV rt-PA within 4.5 h plus MT = 67/103 (65) |
| Saver et al.: SWIFT PRIME34 | Solitaire FR or Solitaire 2 | Y (in a majority) | IV-tPA plus MT = 73/83 (88) |
| Mocco et al.: THERAPY12 | Penumbra, Solitaire or Trevo | No | IV rt-PA plus MT = 30/43 (70) |
| Bracard et al.: THRACE13 | Merci, Penumbra, Catch, Solitaire | Y (MRI in a majority) | IV rt-PA plus MT = 95/138 (69) |
| Muir et al.: PISTE14 | Any CE-marked device approved for MT (stentrievers or aspiration) | No | IV rt-PA plus MT = 26/30 (87) |
CT: computed tomography; IV: intravenous; MRI: magnetic resonance imaging; MT: mechanical thrombectomy; rt-PA: recombinant tissue plasminogen activator.
Advanced imaging is taken as use of MRI techniques, perfusion CT or a systematic combination of CTA collateral scoring and ASPECTS on CT brain (ESCAPE trial).
Table 2.
Methodological quality and risk of bias assessment.
| Primary author; study name | Power calculation | Sample size achieved (reason for stopping early) | Attrition (n/%) | Adequate sequence generation | Allocation concealment | Blinding of participants/ personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Free of other problems | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berkhemer et al.: MR CLEAN8 | Yes | Yes | 2/0.4% | Low risk | Low risk | High riska | Low risk | Low risk | Low risk | Low risk | Low |
| Campbell et al.: EXTEND:IA31 | Yes | No (efficacy) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
| Goyal et al.: ESCAPE32 | Yes | No (efficacy) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
| Jovin et al.: REVASCAT33 | Yes | No (efficacy) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
| Saver et al.: SWIFT PRIME34 | Yes | No (efficacy) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
| Mocco et al.: THERAPY12 | Yes | No (loss of equipoise) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
| Bracard et al.: THRACE13 | Yes | No (efficacy) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
| Muir et al.: PISTE14 | Yes | No (loss of equipoise) | N/A | Low risk | Low risk | High riska | Low risk | N/A | Low risk | Low risk | Low |
Not feasible to blind interventionists and was unlikely to have biased outcome in these trials.
Synthesis of results
Functional independence
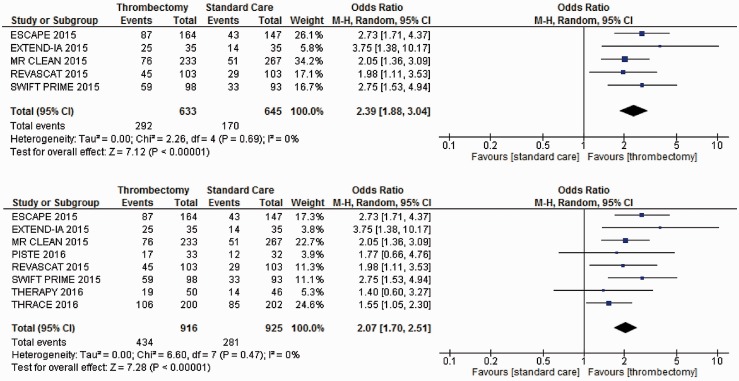
Patients treated with MT compared with those receiving IVT and other forms of best medical or supportive care were significantly more likely to be functional independent (mRS 0 to 2) at 90-days follow-up (OR = 2.39, 95% CI = 1.88 to 3.04) based on data from five trials (Figure 2). The additional impact of the three recent trials was a slightly decreased pooled effect size, but with increased certainty of the mid-point estimate (OR = 2.07, 95% CI = 1.70 to 2.51).
Figure 2.
Meta-analyses of all trial data for mRS 0 to 2 at 90 days. mRS: modified Rankin Scale.
Mortality
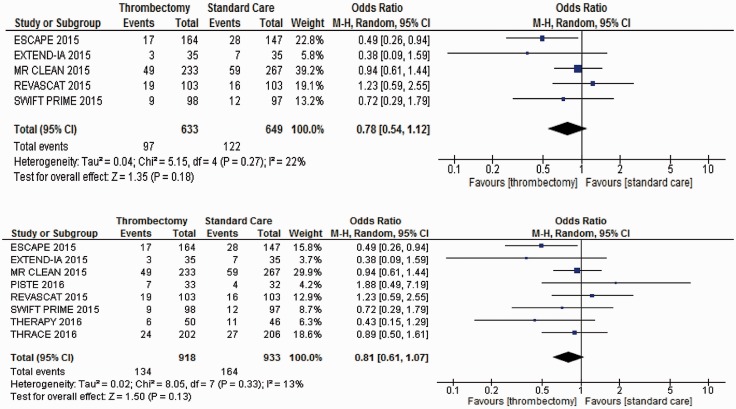
Patients treated with MT compared with those receiving IVT and other forms of best medical or supportive care did not show any effect on mortality at 90-days follow-up (Figure 3). The addition of the three most recent trials did not impact on mortality, but there was increased certainty of the mid-point estimate with a continuing trend towards reduced mortality.
Figure 3.
Meta-analyses of all trial data for mortality at 90 days.
Symptomatic intracranial haemorrhage
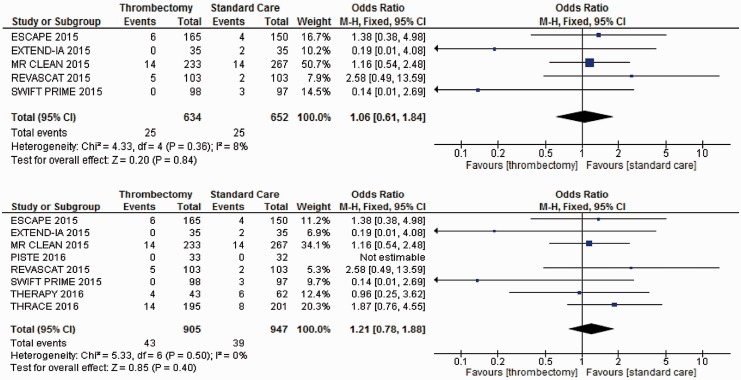
Patients treated with MT compared with those receiving IVT and other forms of best medical or supportive care (Figure 4) did not show any statistically significant increased likelihood of SICH within seven days based on data from five trials. Data from the PISTE trial were not estimable in this meta-analysis, as there were no events recorded of SICH within seven days for either the treatment or the control group. Inclusion of the remaining two recent trials did not impact on probability of SICH.
Figure 4.
Meta-analysis of all trial data for SICH within seven days (N = 7). SICH: symptomatic intracranial haemorrhage.
Findings of the trial sequential analysis
A series of TSA were undertaken using data from eight randomised clinical trials.
Functional independence TSA
The adjusted 95% CI for the TSA was 1.55 to 2.76 (heterogeneity = 0%). The adjusted information size estimate was N = 724. Figure S1 (Supplementary material 5) shows that the cumulative z-statistic curve crossed the sequential monitoring boundary for benefit of MT. The TSA demonstrates robust evidence for a 30% relative benefit for MT compared with IVT for mRS 0 to 2.
Mortality TSA
The TSA analysis (Figure S2, Supplementary material 5) shows that the cumulative z-statistic curve failed to cross the traditional boundaries for statistical significance; despite surpassing the diversity adjusted information size requirement (N = 1803), suggesting a lack of robust evidence to demonstrate a 30% relative risk reduction for MT over IVT. The TSA results suggest that future trials of MT are unlikely to demonstrate a significant effect on mortality as the adjusted 95% CI was 0.57 to 1.13.
Symptomatic intracranial haemorrhage TSA
The TSA-adjusted 95% CI was 0.53 to 2.78 (diversity = 0). The diversity adjusted information size was estimated to be N = 6057; this number was not reached, suggesting that the meta-analysis is underpowered for the SICH outcome. Figure S3 (Supplementary material 5) shows that the cumulative z-statistic curve failed to cross the traditional statistical significance boundary, nor does it cross the boundary for futility, which indicates that future trials may show differences for SICH between MT and IVT.
Discussion
Data from the five MT randomised clinical trials reporting in 2015 yielded significantly increased likelihood of functional independence (mRS 0 to 2) at 90-days follow-up (OR = 2.39, 95% CI = 1.88 to 3.04). The impact of the increased evidence base for MT (THERAPY,12 THRACE,13 PISTE14) was a marginally decreased effect size, but with increased certainty as shown by CIs with a narrower range (mRS 0 to 2; OR = 2.07, 95% CI = 1.70 to 2.51). These findings further confirm the effectiveness of MT, in particular with stent retrievers and aspiration devices. Compared with other meta-analyses of MT in the treatment of acute ischaemic stroke, including the recent Hermes meta-analysis,9–11 our effect size for functional independence is smaller in magnitude.
Our pooled effect size for functional independence derived from the five RCTs published in 2015 differed to previous meta-analyses, including meta-analysis conducted by the Hermes collaboration.11 This can be explained by discrepancies in primary dichotomous study data (Supplementary material 4) and calculating unadjusted (as opposed to adjusted) odds ratios. RCTs often adjust their analyses for prognostic factors which are thought to influence outcomes (e.g. age, severity). Trials published in 2015 used both unadjusted and adjusted pooled effect sizes, and it is worth noting that the latter are unlikely to have been adjusted using the same variables. There is no consensus about whether, or how to pool adjusted and unadjusted findings, although it is regarded best practice to avoid this approach. The simplest option to avoid heterogeneity due to the differences in adjustment with each RCT is to report unadjusted pooled effects, as we have done here.
Consistent with previous meta-analyses of MT in the treatment of stroke,11 we identified no impact on previous estimates of safety (i.e. no increased risk of mortality and SICH at 90 days and 7-days respectively). Although our meta-analyses showed a trend that MT may lead to a decreased risk of mortality this effect was not statistically significant. In the case of SICH, the divergent definitions and low event rates across the eight trials may have confounded the overall effect for this outcome. A TSA confirmed that the meta-analyses fulfilled the information size requirement to satisfy the criterion for ‘sufficient evidence’ on the effectiveness, but not all safety outcomes for MT. The information requirement was met for mortality at 90 days; however uncertainty remains as to whether MT is associated with increased risk of SICH. The robustness of efficacy data for MT would likely prohibit further randomised clinical trials of MT versus no MT. Further data on mortality and SICH could reliably be obtained from on-going or planned trials of MT versus MT plus IVT.
Questions remain around how best to image/triage emergent LAO stroke and optimal MT device types, including technical questions such as use of stentrievers or aspiration devices, including issues around use of different modes of anaesthesia35 which are currently being addressed in on-going trials.36,37 Efficacy of either MT or IVT for wake up stroke (wUS) / stroke of unknown time onset (SUTO) is also unclear.
Our findings make a strong case that no further trials to evaluate the effectiveness of MT versus no MT are warranted. Our meta-analyses included patients from different healthcare systems, patient characteristics and a range of devices which shows the generalisability of effectiveness and safety of MT (with uncertainty around SICH). This assertion is strengthened by our use of full systematic review methodology (including considerations of non-English language literature) and a supplementary TSA to establish the robustness of the effect sizes with reference to a specified information size. We also included substantially more patient numbers treated with modern MT compared with previous meta-analyses.
There is uncertainty regarding generalisability of published trials to populations of non-European ancestry (and countries where there may be marked differences in concomitant healthcare systems); however, given the compelling evidence for the efficacy of MT, it is unlikely that further randomised clinical trials of MT versus no MT will be undertaken. Therefore, registry data in other populations will be important to confirm that outcomes and safety in different populations and healthcare systems are consistent with existing trial data.
Subgroups in many categories are still small especially if the query is broken down by any categorisation of trials, e.g. advanced imaging selection/all IVT control or timing of MT since onset from symptoms. Furthermore, we did not have access to individual patient data (IPD). However, the IPD meta-analysis undertaken by the Hermes collaboration11 has already reported on patient-level evidence favouring treatment with MT (patients aged ≥80 years, patients randomised >300 min after symptom onset, and patients not eligible for IVT). An updated meta-analysis of individual patient is level data planned,38 and this will add further evidence in terms of subgroups with differential effectiveness and safety of MT.
Given the robustness and generalisability of the evidence base for MT, there is a pressing need to invest in acute stroke care services to support delivery of this complex high technology service to all eligible patients. In the UK, few centres provide 24/7 MT and there is large variability between services in MT pathways and delivery.39 A recent study has also shown that 10% of all stroke admissions in the UK could benefit from MT.40 Economic analyses of MT indicate substantial gains in quality adjusted life years and cost-savings,41,42 with one analysis reporting larger gains for younger patients.42 Costs or cost effectiveness of MT to inform service re-design is currently the subject of much research in the UK and elsewhere.43
Conclusions
The expanded evidence base for MT yielded a more precise assessment of effectiveness in terms of functional independence (mRS 0 to 2), with no increased risk of mortality or SICH. Uncertainty remains as to whether MT reduces mortality or is associated with increased risk of SICH.
Supplementary Material
Acknowledgements
We would like to thank Liam Dale and Deborah Jones for their excellent administrative support.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PW is co-PI for two randomised trials (PISTE & STABILISE) investigating different aspects of thrombectomy in acute stroke. Start-up phase of PISTE was mainly funded by Stroke Association but was also part funded by unrestricted institutional educational grants from Covidien & Codman who both manufacture devices used for stroke thrombectomy. STABILISE is part funded by Microvention. PW has also undertaken educational consultancy work within last three years for Codman & Microvention who both manufacture devices used for stroke thrombectomy. GAF is co-PI for STABILISE. GAF’s previous institution has received research grants from Boehringer Ingelheim (manufacturer of Alteplase), and honoraria from Lundbeck for stroke-related activities. GAF has also received personal remuneration for educational and advisory work from Boehringer Ingelheim and Lundbeck. GAF is supported by an NIHR Senior Investigator award. DF, GAF and PM have been involved in marketing activity for a Computerised Decision Aid for Stroke Thrombolysis (COMPASS), which may be made available for a cost payable to purchase the decision aid to cover the costs of technical maintenance and updating of the predictive equations and user interface, in accordance with user feedback and availability of new data on the effectiveness of thrombolysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grant for Applied Research Programme (RP-PG-1211-20012). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Informed consent
N/A.
Ethical approval
N/A.
Guarantor
PW.
Contributorship
PW, GAF and DF conceived the review. DF, RF, GAF, PM and PW were involved in the design of the review. SR designed, conducted and collated the search for studies. RF and EGA with assistance from PW assessed studies for inclusion. DF and RF assessed the risk of bias within included studies. KH conducted the meta-analyses. DC conducted the trial sequential analyses. RF, AC and PW extracted data on study characteristics. DF wrote the first draft of the manuscript. All authors were involved in interpretation of data and reviewed the manuscript critically for intellectual content, and approved the final manuscript.
References
- 1.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379: 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 3.Lees KR, Bluhmki E, Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010; 41: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 5.Christou I, Felberg RA, Demchuk AM, et al. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging 2002; 12: 119–123. [DOI] [PubMed] [Google Scholar]
- 6.Almekhlafi MA, Menon BK, Freiheit EA, et al. A meta-analysis of observational intra-arterial stroke therapy studies using the Merci device, penumbra system, and retrievable stents. Am J Neuroradiol 2013; 34: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 8.Berkhemer OA, Fransen PSS, Beumer D, et al. Randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 9.Balami JS, Sutherland BA, Edmunds LD, et al. A systematic review and meta-analysis of randomized controlled trials of endovascular thrombectomy compared with best medical treatment for acute ischemic stroke. Int J Stroke 2015; 10: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015; 314: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 12.Mocco J, Zaidat OO, von Kummer R, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke 2016; 47: 2331–2338. [DOI] [PubMed] [Google Scholar]
- 13.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 14.Muir KW, Ford GA, Messow CM, et al. Endovascular Therapy for Acute Ischemic Stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) Randomised, Controlled Trial. J Neurol Neurosurg Psychiatry 2017; 88: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn D, Francis R, McMeekin P, et al. Intra-arterial mechanical thrombectomy in the treatment of acute ischaemic stroke: a systematic review and meta-analysis. PROSPERO. 2015, CRD42015016649 Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015016649 (accessed 21 March 2016).
- 16.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke 1988; 19: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 18.Bamford JL, Sandercock PAG, Warlow CP, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989; 20: 828. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Neurological Disorders and Stroke / National Institutes of Health: National Institutes of Health Stroke Scale. Available at: http://www.ninds.nih.gov/disorders/stroke/strokescales.htm (accessed 30 September 2016).
- 20.Mahoney F, Barthel D. Functional evaluation: the Barthel Index. Md Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 21.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 22.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings. N Engl J Med 1985; 312: 932–936. [DOI] [PubMed] [Google Scholar]
- 23.EuroQol Research Foundation 2016. EQ-5D. Available at: http://www.euroqol.org/ (accessed 19 September 2016).
- 24.Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke 2008; 39: 3316–3322. [DOI] [PubMed] [Google Scholar]
- 25.University of Texas, School for Public Health. Search filters for Medline. Available at: http://libguides.sph.uth.tmc.edu/ovid_medline_filters (accessed 10 February 2016).
- 26.Hoffmann T, Glasziou P, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT and Green S (eds) Cochrane handbook for systematic reviews of interventions version 5.0.2, The Cochrane Collaboration, 2009. Available at: www.cochrane-handbook.org (accessed 3 March 2011).
- 28.Schou IM, Marschner IC. Meta-analysis of clinical trials with early stopping: an investigation of potential bias. Stat Med 2013; 32: 4859–4874. [DOI] [PubMed] [Google Scholar]
- 29.Thorlund K, Engstrøm J, Wetterslev J, et al. User manual for trial sequential analysis (TSA). Copenhagen: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011, pp.1–115.
- 30.Phan K, Zhao DF, Phan S, et al. Endovascular therapy including thrombectomy for acute ischemic stroke: a systematic review and meta-analysis with trial sequential analysis. J Clin Neurosci 2016; 29: 38–45. [DOI] [PubMed] [Google Scholar]
- 31.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 32.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 33.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 34.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 35.Brinjikji W, Murad MH, Rabinstein AA, et al. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. Am J Neuroradiol 2015; 36: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.University of Aarhus. “GOLIATH” – General Or Local Anaestesia in Intra Arterial THerapy. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US), 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02317237?term=stroke+conscious+sedation+general+anesthesia&rank=1 (NLM Identifier: NCT02317237). (accessed 25 September 2016).
- 37.Sahlgrenska University Hospital, Sweden. Sedation Versus General Anesthesia for Endovascular Therapy in Acute Stroke – Impact on Neurological Outcome (ANSTROKE). In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US), 2016. Available at: https://www.clinicaltrials.gov/ct2/show/NCT01872884?term=NCT01872884&rank=1 (NLM Identifier: NCT01872884). (accessed 25 September 2016).
- 38.MacIsaac RL, Khatri P, Bendszus M, et al. A collaborative sequential meta-analysis of individual patient data from randomized trials of endovascular therapy and tPA vs. tPA alone for acute ischemic stroke: ThRombEctomy And tPA (TREAT) analysis: statistical analysis plan for a sequential meta-analysis performed within the VISTA-Endovascular collaboration. Int J Stroke 2015; 10: 136–144. [DOI] [PubMed] [Google Scholar]
- 39.Flynn D, Ford GA, McMeekin PJ, et al. Characteristics of intra-arterial thrombectomy service provision in England. Int J Stroke 2016; 11: NP83–NP85. [DOI] [PubMed] [Google Scholar]
- 40.McMeekin P, White P, James M, et al. Estimating the numbers of stroke patients in the UK that are eligible for treatment with mechanical thrombectomy. Eur Stroke J. Under review.
- 41.Shireman TI, Wang K, Saver JL, et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke. Stroke 2017; 48: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steen Carlsson K, Andsberg G, Petersson J, et al. Long-term cost-effectiveness of thrombectomy for acute ischaemic stroke in real life: an analysis based on data from the Swedish Stroke Register (Riksstroke). Int J Stroke. Epub ahead of print 4 April 2017. DOI: 10.1177/1747493017701154. [DOI] [PubMed]
- 43.Ford G, Price C, White P, et al. Promoting effective and rapid stroke care (PEARS). NIHR Programme Grant for Applied Research (RP-PG-1211-20012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.