Abstract
Introduction
In randomised clinical trials (RCTs), endovascular thrombectomy (ET) was combined with intravenous thrombolysis (IVT) in the vast majority of patients. We aimed to analyse how German stroke centres manage IVT in patients receiving ET in daily routine.
Patients and Methods
We performed an online survey among neurologists and neurointerventionalists that included all German University hospitals and a selection of German community hospitals known to perform ET. The survey consisted of 20 questions and was open for reply from 20 December 2016 to 9 January 2017.
Findings
Overall, there were 110 replies, 76% (84/110) from neurologists and 20% (22/110) from neurointerventionalists. The majority of participants (75/99, 76%) reported to continue IVT after start of ET. Nine participants (9%) reported to stop IVT as a standard of care before ET and another 15 (15%) reported to stop IVT on a case-by-case basis. Thrombolysis is given intra-arterially in individual cases in 39% (37/99) and as a standard of care in 3% (3/99). Intra-arterial Heparin is given additionally as a standard procedure in 25% (24/96) and in individual cases in 11% (11/96). IVT is omitted even without contraindications before ET in 5% (5/95) as standard procedure and in 14% (13/95) in individual cases.
Discussion
We observed a wide heterogeneity with respect to the management of IVT in the context of ET. Evidence from RCTs is not implemented in a large number of cases.
Conclusion
These findings emphasise a requirement for further education and implementation of standards for the management of intravenous thrombolysis in endovascular treated stroke patients.
Keywords: Intravenous thrombolysis, endovascular treatment, mechanical thrombectomy, acute stroke, real-life data, survey
Introduction
Intravenous thrombolysis (IVT) is the standard of care in hyperacute ischemic stroke and up to 40% of acute ischemic stroke patients receive IVT in Germany.1,2 In 2015, five randomised controlled trials (RCTs) demonstrated superiority for endovascular thrombectomy (ET) plus IVT in comparison to IVT alone in patients with large vessel occlusion of the anterior circulation.3–7 In two of the five trials, only patients treated with IVT were considered for enrollment, and in the other three trials the vast majority of patients received IVT prior to ET (68–87%).3–7 Altogether, 85% of patients received IVT in addition to ET.8 Current European and German recommendations as well as the guidelines from the American Heart Association recommend that ET should not prevent IVT and IVT should not delay ET in acute stroke if both therapies are indicated.9–11 We aimed to determine, how the management of IVT together with ET is handled in clinical practice in Germany.
Patients and methods
We performed a literature search via PubMed between 2013 and 2016 and did not find any data regarding the question how the management of IVT together with ET is handled in clinical practice. Therefore we invited neurologists and neurointerventionalists from all German University hospitals, all participants of the German Stroke Registry – Endovascular Treatment (GSR), and a selected list of German Hospitals known to perform ET to participate in an online survey. Overall, we sent out 246 invitations to 115 different centres. Centres included all German University-Hospitals (n = 37) and other centres known to perform ET (n = 78). The overall response rate was 110/246 (45%). Invitations were sent to each identifiable stroke neurologist of the local centres (n = 196) and to all available neuroradiologists (n = 46). Four invitations were sent to other specialists known to perform ET. The survey was performed using an internet based questionnaire provided by a commercially available survey system (https://www.surveymonkey.de). It consisted of 20 questions with the possibility to be answered anonymously or with open identity and it was open for reply between 20 December 2016 and 9 January 2017. Statistics were calculated by Excel 2010 (Microsoft corporation®, Redmond, USA). Figures were drawn using Adobe Illustrator CC (Adobe Systems®, San José, USA).
Results
Overall, we received 110 replies, 76% (84/110) from neurologists and 20% (22/110) from neurointerventionalists, 68 of 110 (62%) answers were received anonymised. About 65% (65/100) of participants in our cohort stated to perform more than 50 ET per year, 18% (18/100) stated to perform between 20 and 50 and only 17% (17/100) mentioned to perform less than 20 annual procedures. Please see Table 1 for further detailed baseline data.
Table 1.
Baseline data.
| Specialty (n = 110 replies) | ||
| Neurologist | n = 84 | 76% |
| Neurointerventionalist | n = 22 | 20% |
| Other specialities | n = 4 | 4% |
| Type of stroke unit (n = 107 replies) | ||
| Local stroke unit | n = 27 | 25% |
| Comprehensive stroke centre | n = 79 | 74% |
| No stroke unit | N = 1 | 1% |
| Stroke unit certified by German Stroke Society (n = 108 replies) | ||
| Yes | N = 100 | 93% |
| No | N = 7 | 6% |
| Unknown | N = 1 | 1% |
| IVT/year (n = 102 replies) | ||
| <50 | N = 7 | 7% |
| 50–100 | N = 25 | 25% |
| 101–200 | N = 39 | 38% |
| >200 | N = 31 | 30% |
| ET / year (n = 100 replies) | ||
| <20 | N = 17 | 17% |
| 21–50 | N = 18 | 18% |
| 51–100 | N = 25 | 25% |
| 101–200 | N = 36 | 36% |
| >200 | N = 4 | 4% |
| Speciality performing ET (n = 98 replies) | ||
| ET exclusively performed by neurointerventionalists | N = 86 | 88% |
| ET performed by neurointerventionalists and other specialists | N = 8 | 8% |
| ET solely performed by other specialists | N = 4 | 4% |
| Availability of ET (n = 95 replies) | ||
| 24 h/7d | N = 87 | 92% |
| At working hours | N = 6 | 6% |
| Only in individual case | N = 2 | 2% |
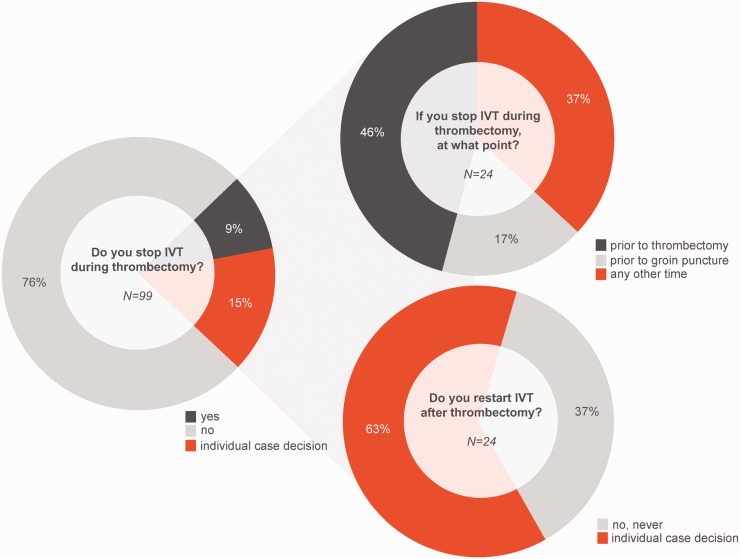
The majority of participants (75/99, 76%) reported to continue IVT after ET was started. Nine participants (9/99, 9%) reported to stop IVT as a standard of care before ET and another 15/99 (15%) to stop IVT as an individual case decision (see Figure 1). If stopping IVT, the time of stopping is before groin puncture in 17% (4/24), during ET (immediately prior thrombus retrieval) in 46% (11/24) or at any other time in 37% (9/24). Nine participants (9/24, 37%) do not re-continue with IVT after ET. Fifteen participants (15/24, 63%) proceed with IVT after ET on the basis of an individual case decision e.g. depending on thrombolysis in cerebral infarction (TICI) grade or dislocation of thrombus to distal arteries.
Figure 1.
Received answers to selected questions from the survey.
IVT: intravenous thrombolysis.
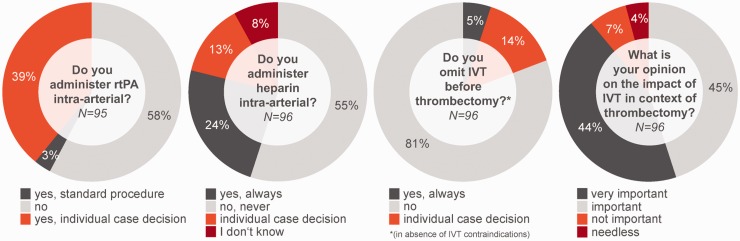
Recombinant tissue plasminogen Activator (rtPA) is given intra-arterially in individual cases in 39% (37/99) and as a standard of care in 3% (3/99) (see Figure 2). Intra-arterial Heparin is given additionally as a standard of care in 25% (24/96) and in 11% (11/96) in individual cases. IVT is omitted without contraindications before ET in total in 19% (18/96) thereof in 5% (5/96) as a standard of care and in 14% (13/96) in individual cases.
Figure 2.
Received answers to selected questions from the survey.
IVT: intravenous thrombolysis; rtPA: recombinant tissue plasminogen activator.
The importance of IVT in drip-and-ship patients was considered as very important (53/95, 56%), important (33/95, 35%), not important (8/95, 9%), needless (1/95, 1%) and harmful (0/95, 0%) and in patients treated directly in a comprehensive stroke centre (“mothership”) as very important (42/96, 44%), important (43/96, 45%), not important (7/96, 7%), needless (4/96, 4%) and harmful (0/96, 0%).
Discussion
This national survey showed a wide heterogeneity with respect to management of IVT in the setting of ET. Almost one quarter of participants reported to stop IVT before ET at least in individual cases, mostly before start of the thrombectomy manoeuvre. Moreover, nearly 20% of participants stated to withhold IVT before planned ET even in the absence of contraindications against IVT. This is noteworthy, as neither current guideline recommendations, nor the results from the large clinical trials of thrombectomy support such an approach. Furthermore some centres (re-) start IVT after ET in certain constellations (e.g. depending on TICI grade) while others generally do not proceed with IVT after ET or generally stop IVT after ET irrespective of success of recanalisation.
Besides, this survey reveals a high variability with regard to dosing and type of antithrombotic medication. This is highlighted by the fact that about one-third give variable parts of the rtPA dose intra-arterially and about one-third administer additional Heparin in different dose regimes. The majority of patients within RCTs received the full dose of IVT for that reason alone that time between IVT and groin puncture exceeded 60 min (median time from initiation of IVT to groin puncture 74–175 min).3–7 Nevertheless, without the procedure of randomisation and an increased speed of clinical routine the time interval from IVT to groin puncture may shorten to less than 60 min in a substantial number of patients. In this situation evidence for most of the above mentioned variations in ET is obviously hard to find.
In other situations, evidence may even stand in contradiction to personal experiences of the treating physicians. Besides, it should be noted that an IVT stop immediately prior to ET or groin puncture appears of limited effect due to pathophysiological considerations. Although there is a fast clearance of rtPA from the bloodstream with a half-life of 4–5 min it must be noted, that the rtPA half-life for deeper tissue compartments is 40 min and the decrease in fibrinogen levels reaches its maximum after 6 h.12 This consideration is in line with the fact that intracerebral bleeding complications do not occur within minutes but mostly within the first 24–36 h after IVT.1,13 However, the benefit of IVT in combination with ET is not only a matter of debate in daily German stroke care but also in recent literature: retrospective analyses suggested a limited effect of IVT when combined with ET although up to now there are no clinical trials showing efficacy of ET without IVT.14–16
Although this study is unique and the vast majority of participants in this survey can be considered as representatives from high volume centres, our study has several limitations. The results cannot be considered as representative for German acute stroke care in general. Nevertheless, this would not affect our main conclusion that a wide heterogeneity in IVT management is present. We further had no strict inclusion or exclusion criterion for centres to participate; however, the vast majority of participants stated a large experience with IVT and ET. Due to the possibility of anonymous participation, we could not control for incorrect or double answers. However, the results remained stable, when looking only at participants with open identity (data not shown). Some questions regarding stop and restart of thrombolysis might have been hypothetical in centres where IVT is generally fully administered due to long procedural time intervals.
Conclusion
For the first time, this national survey addresses important questions of ET management in daily stroke care. The large heterogeneity on how to proceed with IVT in the context of ET demonstrates urgent need for implementation of rational medical standards from recent trials. Where evidence is not available, further clinical trials are warranted. Until then, we propose to comply with the current guidelines that are based on recent trials and pathophysiological considerations and recommend both IVT and ET not prevent each other.
Acknowledgements
The authors thank the GSR-Steering-Committee for their help with this study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval / Informed consent
This manuscript contains results of a survey among physicians and does not contain any patient data, thus neither ethical approval nor in informed consent could be obtained.
Guarantor
LK.
Contributorship
LK and FAW developed the idea for this study together, collected and analysed the data, performed the statistical analysis, and contributed equally to writing the manuscript. GT, CHN, JF, PAR edited the manuscript for important intellectual content. FD supervised data acquisition, and edited the manuscript for important intellectual content.
GSR-Steering-Committee: Anna Alegiani, Martin Dichgans, Christian Gerloff, Peter Kraft, Gabor Petzold, Waltraud Pfeilschifter, Mirko Pham, Eberhard Siebert, Sarah Zweynert.
References
- 1.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gumbinger C, Reuter B, Hacke W, et al. Restriction of therapy mainly explains lower thrombolysis rates in reduced stroke service levels. Neurology 2016; 86: 1975–1983. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 7.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 9.http://www.dgn.org/leitlinien/3198-030-140-rekanalisierende-therapie-ergaenzung-akuttherapie-schlaganfall.
- 10.Wahlgren N, Moreira T, Michel P, et al. Mechanical thrombectomy in acute ischemic stroke: Consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke 2016; 11: 134–147. [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke 2015; 46: 3543–3546. [DOI] [PubMed] [Google Scholar]
- 13.Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011; 77: 341–348. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho JM, Liebeskind DS, Slater LA, et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: A pooled analysis of the SWIFT and STAR studies. JAMA Neurol 2017; 74: 268–274. [DOI] [PubMed] [Google Scholar]
- 15.Abilleira S, Ribera A, Cardona P, et al. Outcomes after direct thrombectomy or combined intravenous and endovascular treatment are not different. Stroke 2017; 48: 375–378. [DOI] [PubMed] [Google Scholar]
- 16.Weber R, Nordmeyer H, Hadisurya J, et al. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg 2017; 9: 229–233. [DOI] [PubMed] [Google Scholar]