Short abstract
Introduction
There are limited data on cerebrovascular events in patients with stable coronary artery disease. To study the risk of cerebrovascular event, the relative proportion of ischaemic stroke and intracranial haemorrhage, and their prognostic factors in stable coronary artery disease are investigated.
Patients and methods
The CORONOR registry prospectively recruited, between February 2010 and April 2011, 4184 unselected stable coronary artery disease outpatients. All events occurring during a five-year follow-up were adjudicated.
Results
Ninety-six patients had an ischaemic stroke and 34 had an intracranial haemorrhage, reaching a cumulative incidence after five years of 3.2 (2.7–3.8)%. During the same period, 677 deaths and 170 myocardial infarctions (ST-elevation MI, n = 55; non-ST-elevation MI, n = 115) occurred. In elderly individuals, the number of cerebrovascular events was higher than that of myocardial infarctions and largely exceeded that of ST-elevation myocardial infarctions. Predictors of ischaemic stroke were: previous history of stroke (subhazard ratio (SHR)=3.16(1.95–5.14)), absence of statin therapy at inclusion (SHR = 2.45(1.47–4.10), increasing age (SHR = 1.45(1.16–1.82) per 10-year increase) and diabetes mellitus (SHR = 1.65(1.10–2.49)). Predictors of intracranial haemorrhage were: combination of vitamin K antagonists with an antiplatelet agent at inclusion (SHR = 5.41(2.49–11.75), single antiplatelet therapy as reference), and increasing age (SHR = 1.47(1.12–1.93) per 10-year increase).
Discussion
In stable coronary artery disease patients, the brain deserves attention. In patients at high risk of ischaemic stroke, secondary prevention could be intensified. Our results raise awareness of the hazard of the association of antiplatelet drugs with oral anticoagulants in stable coronary artery disease patients.
Conclusion
While improving the prevention of future vaso-occlusive events should be our ultimate goal in coronary artery disease patients, the net clinical benefit of our treatments should carefully be studied.
Keywords: Ischaemic stroke, intracranial haemorrhage, oral anticoagulation, antiplatelet therapy, aging, myocardial infarction
Introduction
There has been significant progress over the last decades in the management of patients with acute manifestations of coronary artery disease (CAD). Stable CAD (i.e. at least 6–12 months after an acute event) is thus a highly prevalent situation that deserves an optimal secondary prevention for minimising clinical events.1,2 Although the intuitively feared recurrent event in a stable CAD population is myocardial infarction (MI),3 previous studies have shown that the risk of cerebrovascular event (CVE) should not be underestimated.4–8 In addition, the recent intensification of antithrombotic strategies for the treatment of CAD9–11 may impact the risk of CVE with a decrease in ischaemic events at the expense of an increase in bleeding complications.
There are limited recent data on the risk of CVE and on the proportion of ischaemic/bleeding events in stable CAD patients receiving ‘modern’ secondary prevention. Moreover, the determinants of ischaemic and bleeding CVE in this population remain largely unknown. The CORONOR registry includes a large contemporary (inclusion period 2010–2011) population of unselected stable CAD outpatients in which all events have been adjudicated during follow-up.12 This study presents an opportunity to better comprehend the residual risk of incident CVE in stable CAD outpatients. The present analysis aimed to assess the incidence of CVE, the relative proportion of ischaemic stroke (IS) and intracranial haemorrhage (ICrH), and the factors associated with both types of CVE in the five-year CORONOR registry.
Materials and methods
Participants
The CORONOR (suivi d’une cohorte de patients COROnariens stables en région NORd-pas-de-Calais) study is a prospective multicentre registry that included 4184 consecutive outpatients with stable CAD. The patients were included by 50 cardiologists from the region Nord Pas-de-Calais in France between February 2010 and April 2011 during outpatient visits. Patients were considered eligible if they had evidence of CAD defined by at least one of the following: previous MI (>one year ago), previous coronary revascularisation (>one year ago) and/or obstruction of ≥50% of the luminal diameter of at least one native coronary vessel on coronary angiography. The sole exclusion criterion was hospitalisation for MI or coronary revascularisation within the previous year.
Ethics approval
This study was approved by the French medical data protection committee (CCTIRS) and authorised by the Commission Nationale de l’Informatique et des Libertés (CNIL) for the treatment of personal health data. All patients consent to the study after being informed through a written document of the objectives of the study and on the treatment of data, as well as on their rights to object, of access and of rectification. The study population has been previously reported in details.11,12
Study design and definitions
At inclusion, the investigators (i.e. the cardiologist) prospectively completed a case record form, which contained information regarding demographic and clinical details of the patients including age, gender, history of hypertension, history of diabetes mellitus, smoking, prior MI, prior stroke (ischaemic or haemorrhagic), multivessel CAD, prior coronary stent implantation, prior coronary bypass, left ventricular ejection fraction and major cardiovascular treatments. History of hypertension was defined as a patient receiving ≥ 1 antihypertensive treatment; history of diabetes mellitus was defined as a patient treated with oral antidiabetic drugs or insulin, or with a previous history of elevated (>126 mg/dL) fasting blood glucose on at least two separate occasions in conjunction with ongoing dietary measures; prior MI included ST-elevation MI and non-ST-elevation MI; multivessel CAD was defined as ≥ 2 coronary arteries with ≥ 50% stenosis; left ventricular ejection fraction was the most recent echocardiographic assessment. The patients were then followed-up by their treating cardiologists. The number of outpatient visits was at the discretion of the treating cardiologists. Protocol-specified follow-up was performed at two years and at five years, using a standardised case record form to report CVEs. In case of missing information, general practitioners and/or patients themselves were contacted by a research technician. The identification of patients with events for adjudication was based on interviewing patients/relatives during outpatients’ visits, on discharge summaries for hospitalisation during follow-up that were sent to treating cardiologists and on information obtained by the research technician. When CVE was reported, all related documents (discharge summaries, brain imaging reports) were collected. All clinical events were adjudicated by two investigators blinded to each other. A third investigator joined the adjudication in case of disagreement according to pre-specified definitions. A consensus was then reached. All cases of CVE were reviewed by a neurologist specialised in stroke medicine (CC) and a cardiologist (CB). IS was defined as a sudden onset of focal neurological symptoms with the presence of cerebral infarction in the appropriate territory on brain imaging (CT or MRI), regardless of the duration of symptoms (less than or more than 24 h). The IS subtype was adjudicated centrally by the neurologist using the TOAST criteria.13 ICrH was defined as a sudden onset of focal neurological symptoms with the presence of an acute bleeding in the appropriate territory (including brain parenchyma, subdural, extradural, subarachnoid, intraventricular compartments) on brain imaging (CT or MRI), regardless of the duration of symptoms and regardless of the cause of the haemorrhage (spontaneous or secondary to trauma, vascular causes or other causes). Sudden deaths without brain imaging were not considered as CVEs.
Statistical analysis
Continuous variables were described as the mean ± standard deviation (SD). Categorical variables were presented as absolute numbers and percentages. The proportion of missing data was low (1.4% for left ventricular ejection fraction, 1.0% for multivessel CAD, and <0.1% for other variables), so missing variables were not imputed. Cumulative incidence functions are shown. The comparison of baseline variables according to IS and ICrH was performed using competitive risk regression with death as the competing event according to the method of Fine and Gray.14 Variables associated with a p value <0.05 in previous analyses were implemented into forward stepwise Fine–Gray models. Co-linearity was excluded by means of a correlation matrix between candidate predictors. The proportional subhazards assumption for each potential prognostic factor was assessed and satisfied by including interaction time-dependent terms in the regression analysis. We derived from Fine–Gray models, subhazard ratios (SHRs) as effect size measures with their 95% confidence intervals (CIs). All statistical analyses were performed using the STATA 14.1 software (STATA Corporation, College Station, Texas, USA). Statistical significance was assumed at a p value <0.05. The sample size of the cohort was calculated for the analysis of baseline variables associated with death or MI during follow-up; the study of incident CVEs was specified as a secondary analysis.
Results
The baseline characteristics of the 4184 patients included in the CORONOR study have previously been described.12 A five-year clinical follow-up was completed in 4094 (98%) patients (Supplemental Figure 1). As shown in Table 1, this was a predominantly male cohort (78%), with a mean age of 66.9 ± 11.5 years. A history of MI was documented in 62.4% of cases with 85.9% of the patients having had at least one prior coronary revascularisation procedure. Our cohort received a broad range of secondary prevention drugs (antiplatelets in 96.4%, 2 or more antihypertensive drugs in 81%, and statins in 92.2%).
Table 1.
Baseline characteristics at inclusion into the registry in the overall study population and according to the occurrence of ischaemic stroke (IS) or intracranial haemorrhage (ICrH) during follow-up.
| All patients(n = 4094) | No IS(n = 3998) | IS(n = 96) | No ICrH(n = 4060) | ICrH(n = 34) | |
|---|---|---|---|---|---|
| Age, yearsa | 66.9 ± 11.5 | 66.8±11.5 | 72.2 ± 11.6b | 66.9±11.5 | 72.3±9.4c |
| Gender (male) | 78 | 78.2 | 68.8b | 78 | 79.4 |
| History of hypertension | 60.1 | 59.8 | 71.9b | 60.1 | 58.8 |
| History of diabetes mellitus | 31 | 30.7 | 43.8b | 31 | 29.4 |
| Current smoker | 11.3 | 11.2 | 14.6 | 11.3 | 5.9 |
| Prior MI | 62.4 | 62.2 | 69.8 | 62.5 | 52.9 |
| Prior stroke | 7.6 | 7.2 | 24b | 7.5 | 20.6c |
| Multivessel CAD | 57.8 | 57.8 | 56.8 | 57.8 | 52.9 |
| Prior stent implantation | 68.8 | 69 | 61.5 | 68.9 | 58.8 |
| Prior coronary bypass | 21.4 | 21.3 | 22.9 | 21.3 | 23.5 |
| Atrial fibrillation at inclusion | 7.3 | 7.1 | 13.5b | 7.2 | 20.6c |
| LVEFa,% | 57.5 ± 10.8 | 57.6±10.8 | 55.2 ± 11.3b | 57.5±10.8 | 57.4±10.2 |
| Ongoing treatment at inclusion: | |||||
| SAPT | 67.4 | 67.5 | 66.7 | 67.6 | 44.1c |
| DAPT | 20.7 | 20.8 | 15.6 | 20.7 | 17.7 |
| VKA alone | 2.9 | 2.8 | 6.3 | 2.9 | 5.9 |
| VKA+APT | 8.3 | 8.3 | 10.4 | 8.1 | 32.4c |
| ≥2 antihypertensive drugsd | 81 | 80.8 | 86.5 | 81 | 82.4 |
| Statins | 92.2 | 92.5 | 79.2b | 92.1 | 97.1 |
MI: myocardial infarction; CAD: coronary artery disease; LVEF: left ventricular ejection fraction; SAPT: single-antiplatelet therapy; DAPT: dual-antiplatelet therapy; VKA: vitamin K antagonist; VKA + APT: vitamin K antagonist and antiplatelet therapy.
Data are percentages or amean ± SD.
bp < 0.05 vs. no IS.
cp < 0.05 vs. no ICrH.
dAngiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, aldosterone antagonists, beta-blockers, calcium antagonists, diuretics.
Incidence of IS and ICrH
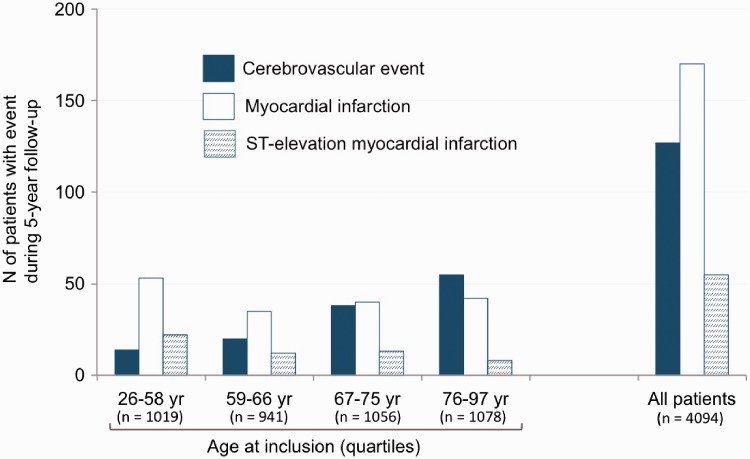
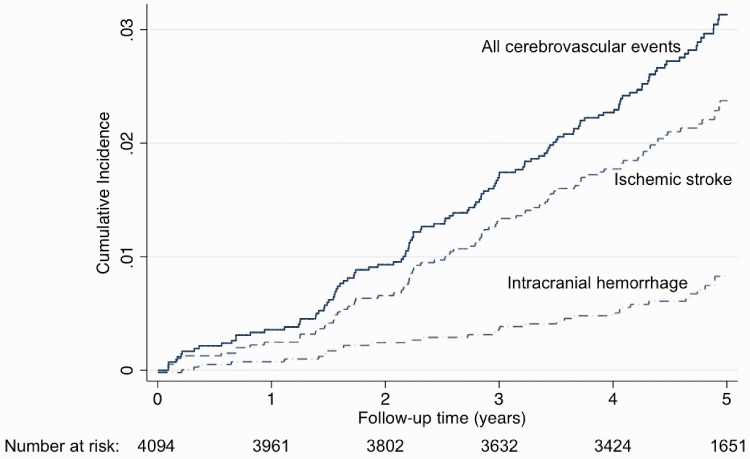
Ninety-six patients had an IS and 34 had an ICrH during a five-year follow-up (Supplemental Figure 1). Three patients had both types of CVE during follow-up, thus 127 patients in total presented a CVE. During the same period, there were 677 deaths (353 non cardiovascular deaths, 269 cardiovascular deaths (including 99 deaths from heart failure and 91 sudden deaths), and 55 deaths from unknown causes) and 170 MIs (ST-elevation MI, n = 55; non-ST-elevation MI, n = 115). Figure 1 illustrates the risks of CVE and MI according to age; in elderly individuals, the number of CVEs was higher than that of MIs and largely exceeded that of ST-elevation MIs. Most of the IS were of cardioembolic origin (n = 43/96); atrial fibrillation (AF) was reported in three-quarters (32/43) of the patients with cardioembolic strokes including 23 patients with known AF prior to stroke hospitalisation and 9 patients with AF discovered during stroke hospitalisation. The 11 other causes of cardioembolic strokes were: left ventricular systolic dysfunction (LVEF <30%) (n = 7), left ventricular aneurysm (n = 2), mechanical aortic valve (n = 1) and infective endocarditis (n = 1). Among ICrH, half were intracerebral (n = 16/34) and 14 were subdural haematoma (of which 12 were related to a trauma). At five years, the cumulative incidence was 2.4% (95% CI 1.9 to 2.9) for IS (0.5%/year) and 0.9% (95% CI 0.6 to 1.2) for ICrH (0.2%/year). It was 3.2% (95% CI 2.7 to 3.8) for all types of CVE (0.6%/year). Event curves for IS, ICrH and all types of CVE are shown in Figure 2.
Figure 1.
Clinical events during follow-up.
Figure 2.
Cumulative incidence of cerebrovascular events during the five-year follow-up.
Impact on outcome
At the end of the follow-up period, 36 patients out of the 96 with IS as the first CVE (37.5%) and 22 patients out of the 31 with ICrH as the first CVE (71%) had died. CVE-related mortality (occurring within 30 days of CVE onset) was of 15.6% (95% CI 9.0 to 24.5) (n = 15) in IS and of 45.2% (95% CI 27.3 to 64.0) (n = 14) in ICrH.
Predictors of IS and ICrH
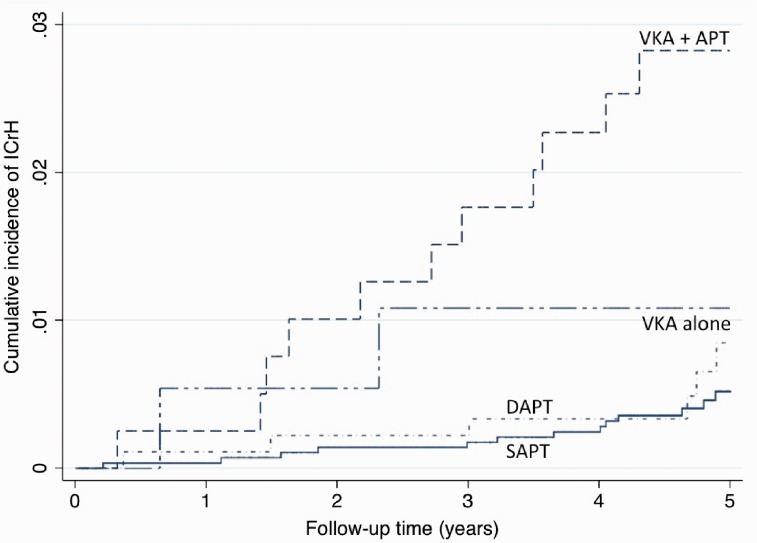
Univariate and multivariable assessments of baseline variables associated with IS and ICrH are shown in Tables 1 and 2. By multivariable analysis, predictors of IS during follow-up were: a previous history of stroke (subhazard ratio (SHR)=3.16; 95% CI 1.95 to 5.14), the absence of statin therapy at inclusion (SHR = 2.45; 95% CI 1.47 to 4.10), an increasing age (SHR = 1.45 per 10-year increase; 95% CI 1.16 to 1.82), and the presence of diabetes mellitus (SHR = 1.65; 95% CI 1.10 to 2.49). Predictors of ICrH were the combination of VKA with an antiplatelet agent at inclusion (SHR = 5.41; 95% CI 2.49 to 11.75; patients with single antiplatelet therapy as reference), and an increasing age (SHR = 1.47 per 10-year increase; 95% CI 1.12 to 1.93). Figure 3 shows cumulative incidence of ICrH according to antithrombotic regimen at inclusion.
Table 2.
Baseline variables associated with ischaemic stroke (IS) and intracranial haemorrhage (ICrH) during follow-up: Multivariable analysis.
| SHR | 95%CI | |
|---|---|---|
| IS | ||
| Previous stroke | 3.16 | 1.95–5.14 |
| No statin at inclusion | 2.45 | 1.47–4.10 |
| Age (per 10 years increase) | 1.45 | 1.16–1.82 |
| Diabetes mellitus | 1.65 | 1.10–2.49 |
| ICrH | ||
| Antithrombotic treatment at inclusion | ||
| SAPT | Reference | |
| DAPT | 1.44 | 0.56–3.65 |
| VKA alone | 2.39 | 0.54–10.50 |
| VKA + APT | 5.41 | 2.49–11.75 |
| Age (per 10 years increase) | 1.47 | 1.12–1.93 |
SHR: subhazard ratios for IS and ICrH by competitive risk regression; CI: confidence interval; SAPT: single antiplatelet therapy (either aspirin or clopidogrel alone); DAPT: dual antiplatelet therapy (aspirin and clopidogrel). VKA + APT: vitamin K antagonist and antiplatelet therapy.
Note: Candidate variables for inclusion into the IS model were age, gender, history of hypertension, history of diabetes mellitus, prior stroke, atrial fibrillation, left ventricular ejection fraction and statins. Candidate variables for inclusion into the ICrH model were age, prior stroke, atrial fibrillation and antithrombotic treatment.
Figure 3.
Cumulative incidence of intracranial haemorrhage in stable coronary artery disease patients according to antithrombotic regimen at inclusion.
SAPT: single-antiplatelet therapy; DAPT: dual-antiplatelet therapy; VKA: vitamin K antagonist; VKA + APT: vitamin K antagonist and antiplatelet therapy.
Discussion
In a real-life cohort of 4094 patients with stable CAD, the incidence of CVE reached 3.2% at five years. In stable CAD patients not only the heart is at risk, but the brain also deserves attention. Interestingly, while predictors of CVE were radically different between IS and ICrH, our data suggest that – for both types of CVE – there might be improvement in the prevention of CVEs among stable CAD patients. We identified a group of patients at high risk of IS (elderly individuals, previous history of stroke, diabetes, less statins being prescribed) in whom secondary prevention could be intensified. Regarding ICrH, the most devastating type of CVE, patients who received both oral anticoagulants and antiplatelet drugs were at very high risk (SHR = 5.78; 95% CI 2.55 to 13.11) and this association should be avoided for CAD patients in a stable setting, i.e. at a chronological distance from any acute coronary syndrome or coronary revascularisation.
Our study has several strengths. We focused on two distinct types of CVEs and used a prospective and rigorous design. All cases of CVEs were adjudicated centrally by a stroke expert and detailed clinical phenotypes were used to qualify the causes of the events. Finally, in stroke cohorts, death occurring during follow-up may be a serious competing risk. Therefore, we chose to use competing risk models rather than survival models. There are some limitations to our study. Since the inclusion was made by cardiologists, the data may not be generalisable to the overall population in the community because of selection bias. This bias is likely to overestimate the extent to which these patients are managed in relation to guidelines and the reality of management as well as the outcome may be worse; on the other hand, the inclusion of all consecutive stable CAD patients irrespective of comorbidities is one strength of the study. In addition, potential predictors of CVE were assessed at inclusion and it is acknowledged that changes may have occurred during follow-up, especially for patient medications. Major changes are however unlikely given that all patients had been stable for at least one year at inclusion, with their treating cardiologists confirming the ongoing medications. As an illustration, in the 34 patients who experienced an ICrH during follow-up, the proportion treated by the combination of an antiplatelet drug with anticoagulation was 32.4% (11/34) when evaluated at inclusion and 35.3% (12/34) when evaluated at time of admission for ICrH. Finally, our data have been obtained from a limited geographical area. However, the overall profile of our patients (including the male preponderance and a high rate of hypertension) as well as the overall incidence of CVEs is in agreement with recently published data4–8; this is reassuring regarding the external validity of our study.
The goal of any physician is to protect stable CAD patients from clinical events by achieving a high level of secondary prevention.1,2 In addition to recurrent coronary events, CVEs are also relatively frequent in this population. In the present study, we reported an overall incidence of CVEs of 0.6%/year which is globally in line with the incidence of stroke in stable CAD outpatients reported in randomised controlled trials (0.4 to 0.6%/year)4–6,9,10 and in registries performed in other geographical settings such as REACH (1%/year)7 or CLARIFY (0.5%/year).8 An important information for general practitioners and cardiologists is that the number of stable CAD patients who experienced a CVE in our cohort was similar to that of patients who had an MI and much greater than that of patients who suffered from an ST-elevation MI, the most severe form of MI. Moreover, the impact of aging on the relative risk of CVE versus MI (Figure 1) should also be emphasised.
Contrary to previous published registries, central adjudication was performed for all cases of CVE and we were able to robustly distinguish IS from ICrH. This enabled us to identify specific predictors of each type of CVE that may have an impact on the management of CAD patients. In three quarter of the cases, the CVE was an IS. Of note, for a population restricted to patients with coronary atherosclerosis, cardioembolic strokes were much more frequent than strokes related to large artery atherosclerosis. This result is important for clinical practice. In a stroke patient with a history of CAD, clinicians often jump on the possible responsibility of atheroma. Our results suggest that they should screen carefully for a cardioembolic source even though the patient is considered as atheromatous. Indeed, an underlying cardiopathy may increase the risk of cardioembolic event in an elderly CAD patient. Alternatively, thanks to the tremendous progress in the management of atheroma during the last decades, aging may be the sole driver of this finding in an elderly population of CAD survivors. Our data show that elderly CAD patients with a history of previous stroke, or diabetes are at high risk of IS. This high risk group of patients may benefit from dedicated education on stroke symptoms and the necessity to call immediately the emergency services. Improving prevention will be a societal challenge in the future.15 Even though our population received a very high rate of secondary prevention agents, we identified patients who were not treated with statins as a group at risk of developing an IS. The design of our study prevents any speculation on causal relationship between statins use and occurrence of IS, since there might be confounders associated with the risk of IS and the non-prescription of statins. Nevertheless, this result is in agreement with the reduction in CVEs observed in statins trials performed in stable CAD populations.16–18
Although a relatively rare event, ICrH remains the most disabling form of CVE. We observed a 45.2% mortality rate at 30 days after ICrH in patients with stable CAD, a result that is in line with population-based cohorts in Europe.19–21 Nevertheless, we found predictors that suggested a possible improvement. A significant proportion of patients with stable CAD are treated with oral anticoagulation, mainly because of AF. Although international guidelines recommend oral anticoagulation monotherapy for these patients when more than one year from last acute event,22,23 data in patients with stable CAD and modern management with wide use of coronary revascularisation including drug-eluting stents are lacking. This probably explains why the combination of oral anticoagulation with an antiplatelet drug is still very frequently prescribed by physicians.24,25 Of note, 12 patients out 34 ICrH had suffered from a traumatic subdural haematoma. Our results might contribute to raise awareness of the hazard of such a therapeutic strategy which is associated with a dramatic increase in the risk of intracranial bleeding.
Prevention of CVEs should be an integral part of management for patients with stable CAD. Although the risk of IS is much higher in absolute terms, the risk of ICrH is a concern when considering the field of development of new antithrombotic interventions with increased potency. While improving the prevention of future vaso-occlusive events should be our ultimate goal in CAD patients, the net clinical benefit of our treatments may differ from one patient to another. Beyond conventional risk factors, future research should try to identify structural brain biomarkers that may contribute to precisely evaluate the risk of ICrH. Screening for underlying silent brain biomarkers, such as brain microbleeds26 or cortical superficial siderosis,27 may contribute to tailor more precisely our preventive strategies. Some RCTs currently focus on this topic (RESTART trial ISRCTN71907627). A multidisciplinary approach of these high risk patients is urgently needed. Closer collaborations between cardiologists and neurologists should be promoted.
Supplemental Material
Supplemental material for Incidence and determinants of cerebrovascular events in outpatients with stable coronary artery disease by Charlotte Cordonnier, Gilles Lemesle, Barbara Casolla, Matthieu Bic, François Caparros, Nicolas Lamblin and Christophe Bauters in European Stroke Journal
Acknowledgements
CC and CB are members of the Institut Universitaire de France. We thank Michel Deneve for the monitoring of the CORONOR study.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CC: advisory boards (Bayer, Medtronics, Daiichi-Sankyo), investigator for clinical trials (Astra-Zeneca, Boehringer-Ingelheim, Daiichi-Sankyo, Pfizer). All fees were paid to ADRINORD or Lille University Hospital research account, no personal funding. GL: fees for lectures, consulting, travel grants (Amgen, Astra-Zeneca, Bayer, Biopharma, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, Eli-Lilly, MSD-Schering, Novartis, Pfizer, Sanofi-Aventis, Servier, The Medecine Company). MB: travel grants (Medtronics). NL: fees for lectures, consulting, travel grants (Actelion, Amgen, Astra-Zeneca, Bristol-Myers Squibb, GlaxoSmithKline, MSD-Schering, Novartis, Pfizer). CB: travel grants (Amgen, MSD-Schering). BC, FC: no disclosure. The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by Fédération Française de Cardiologie, Paris, France.
Informed consent
All patients consent to the study after being informed through a written document of the objectives of the study and on the treatment of data, as well as on their rights to object, of access and of rectification.
Ethical approval
This study was approved by the French medical data protection committee (CCTIRS) and authorized by the Commission Nationale de l’Informatique et des Libertés (CNIL) for the treatment of personal health data.
Guarantor
Cordonnier.
Contributorship
Charlotte Cordonnier: designed the study, acquired data, analysed data, wrote the first draft of the manuscript. Gilles Lemesle, Barbara Casolla, Matthieu Bic, François Caparros : acquired data, critically revised the manuscript. Nicolas Lamblin: designed the study, acquired data, analysed data, critically revised the manuscript. Christophe Bauters: designed the study, acquired data, performed statistical analysis, critically revised the manuscript.
References
- 1.Fihn SD, Gardin JM, Abrams J, et al. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012; 2012: e44–e164. [DOI] [PubMed] [Google Scholar]
- 2.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. [DOI] [PubMed] [Google Scholar]
- 3.Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69: 2149–2156. [DOI] [PubMed] [Google Scholar]
- 4.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003; 362: 782–788. [DOI] [PubMed] [Google Scholar]
- 5.Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364: 849–857. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351: 2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ducrocq G, Amarenco P, Labreuche J, et al. A history of stroke/transient ischemic attack indicates high risks of cardiovascular event and hemorrhagic stroke in patients with coronary artery disease. Circulation 2013; 127: 730–738. [DOI] [PubMed] [Google Scholar]
- 8.Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388: 2142–2152. [DOI] [PubMed] [Google Scholar]
- 9.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 11.Hamon M, Lemesle G, Tricot O, et al. Incidence, source, determinants, and prognostic impact of major bleeding in outpatients with stable coronary artery disease. J Am Coll Cardiol 2014; 64: 1430–1436. [DOI] [PubMed] [Google Scholar]
- 12.Bauters C, Deneve M, Tricot O, et al. Prognosis of patients with stable coronary artery disease (from the CORONOR study). Am J Cardiol 2014; 113: 1142–1145. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competitive risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 15.Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389. [PubMed] [Google Scholar]
- 17. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 18.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 19.Bamford J, Sandercock P, Dennis M, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project–1981-86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990; 53: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giroud M, Milan C, Beuriat P, et al. Incidence and survival rates during a two-year period of intracerebral and subarachnoid haemorrhages, cortical infarcts, lacunes and transient ischaemic attacks. The Stroke Registry of Dijon: 1985-1989. Int J Epidemiol 1991; 20: 892–899. [DOI] [PubMed] [Google Scholar]
- 21.Bejot Y, Grelat M, Delpont B, et al. Temporal trends in early case-fatality rates in patients with intracerebral hemorrhage. Neurology 2017; 88: 985–990. [DOI] [PubMed] [Google Scholar]
- 22.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 2011; 57: e101–e198. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 24.Lamberts M, Gislason GH, Lip GY, et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation 2014; 129: 1577–1585. [DOI] [PubMed] [Google Scholar]
- 25.Fauchier L, Greenlaw N, Ferrari R, et al. Use of anticoagulants and antiplatelet agents in stable outpatients with coronary artery disease and atrial fibrillation. International CLARIFY Registry. PLoS One 2015; 10: e0125164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 2010; 41: 1222–1228. [DOI] [PubMed] [Google Scholar]
- 27.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015; 138: 2126–2139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Incidence and determinants of cerebrovascular events in outpatients with stable coronary artery disease by Charlotte Cordonnier, Gilles Lemesle, Barbara Casolla, Matthieu Bic, François Caparros, Nicolas Lamblin and Christophe Bauters in European Stroke Journal