Abstract
Introduction
Deranged glycaemic control is common post-stroke, increasing risks of recurrent stroke and development of diabetes. The aim of the study is to examine glucose metabolism in relation to body composition, physical activity and sedentary time post-stroke.
Patients and methods
Observational study: Non-diabetic adults, unable to walk independently, were recruited within 2 weeks of first stroke. Primary outcome: 2-h glucose level (mmol/l, oral glucose tolerance test), assessed at baseline and 6 months. Homeostasis Model Assessment of Insulin Sensitivity, total body fat and lean mass (dual energy X-ray absorptiometry), sedentary time (lying or sitting), standing and walking (PAL2 accelerometer) were assessed at baseline, 1, 3 and 6 months. Generalised estimating equations were used to examine change over time and associations between outcome measures.
Results
Thirty-six participants (69.5 years (standard deviation 11.7), 13 (36.1%) female, moderate stroke severity (National Institute of Health Stroke Scale 11.5 (interquartile range 9.75, 16)). Within 6 months, adjusting for age and National Institute of Health Stroke Scale, every month 2-h glucose reduced by 4.5% (p < 0.001), Homeostasis Model Assessment of Insulin Sensitivity improved 3% (p = 0.04) and fat mass decreased 490 g (95% confidence interval 325, 655; p = 0.01). For every extra kilogram of body fat, 2-h glucose increased by 1.02 mmol/L (95% confidence interval 1.01, 1.02; p = 0.001); Homeostasis Model Assessment of Insulin Sensitivity reduced by 0.98% (95% confidence interval 0.97, 0.99; p = 0.001). Time spent sedentary reduced from 98.5% of measurement period (interquartile range 94.3, 99.8) to 74.3% (interquartile range 65.5, 88.6), by 2.8% monthly (95% confidence interval 1.8, 3.9, p < 0.001). For every additional 5% sedentary time, 2-h glucose increased by 1.05 mmol/L (95% confidence interval 1.04, 1.07; p < 0.001).
Conclusion
Reducing sedentary time and fat mass within 6 months of stroke may improve glucose tolerance and insulin resistance.
Keywords: Stroke, glycaemic control, physical activity, body composition
Introduction
Hyperglycaemia is common within hours of stroke onset,1 and dysglycaemia is prevalent in up to 80% of stroke survivors in sub-acute (3 months), and chronic (>6 months)2,3 recovery phases. Impaired glucose tolerance and insulin resistance following a stroke are independent risk factors for recurrent stroke4 and are also associated with the development of diabetes.4 Stress hyperglycaemia in the setting of stroke,1 insular cortical ischaemia and older age5 may contribute to post-stroke hyperglycaemia, and the emergence of insulin resistance after stroke is associated with younger age (<65 years), obesity, lacunar stroke and greater disability (modified Rankin grade > 1).6 Ischaemic neural damage and resultant neuroendocrine dysregulation and inflammatory cascades may contribute to the development of insulin resistance7 following stroke, however, the relationship between impaired glucose tolerance and insulin resistance in stroke survivors without diabetes is not fully understood.
In a recent randomised controlled study8 of 3876 people aged 63.5 years (standard deviation (SD) 10.6) with insulin resistance (>3.0 on the homeostasis model assessment of insulin resistance (HOMA-IR) index), treatment with the insulin sensitising drug Pioglitazone within 6 months of mild stroke or transient ischaemic attack reduced the 5-year risk for recurrent stroke or myocardial infarction, however fracture risk and weight gain increased. This study highlights insulin resistance as a potential target for cardiovascular disease risk reduction after stroke, for which non-pharmacological treatment methods may be worthy of further investigation.
Physical inactivity which is common after stroke9,10 may contribute to post-stroke insulin resistance via its associations with reduced muscle mass,11 changes in paretic leg muscle fibre type,12 reduced muscle capillarisation and peripheral blood flow13 and increased intramuscular fat and inflammatory markers.14 The timing of the changes in lean and fat masses in relation to glycaemic control after stroke is not well characterised.15,16 Furthermore, evidence suggests that sedentary behaviour (defined as waking behaviour that involves an energy expenditure of <1.5 metabolic equivalents (METs) such as sitting or lying) may be an independent risk factor for cardiovascular disease and mortality regardless of the amount of physical activity undertaken.17 Therefore, to study the relationship between glucose control and physical activity after stroke, sedentary behaviour also needs to be considered.
The aim of this study was to examine glucose metabolism in relation to body composition, physical activity and sedentary time following a stroke. We hypothesised that glucose tolerance and insulin sensitivity would decrease within 6 months of stroke. Further, we hypothesised that higher levels of physical activity and lower levels of sedentary time would be associated with improved glucose tolerance and insulin sensitivity.
Methods
This was a phase 1 pilot investigation that was a sub-study nested within a larger longitudinal observational study of post-stroke bone loss.18 Adults admitted to the acute stroke unit of two large metropolitan teaching hospitals in Melbourne (Australia) between March 2010 and August 2014 were screened for inclusion into the study. The inclusion criteria were diagnosis of a hemispheric stroke within the past week, and the ability to follow simple verbal commands. Exclusion criteria were: ability to walk independently, known diabetes, medical instability, previous stroke, other neurological or bone disease, other conditions significantly limiting function (e.g. limb amputation) and use of steroid or bone specific medication (e.g. bisphosphonates). Informed written consent was obtained for all participants as approved by Austin Health (H2008/03428), Northern Health (P4/13) and La Trobe University (09-065) Human Research Ethics Committees, in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Assessments occurred at baseline (within 2 weeks of stroke), 1 month later, and at 3 and 6 months post-stroke. Assessments were undertaken on the hospital ward, then at the research centre or at participants’ homes once they were discharged from hospital.
Outcomes
Participant demographics (age, sex, living arrangements, education level, smoking status, medical history), stroke severity (National Institute of Health Stroke Scale (NIHSS)) and stroke classification (Oxfordshire Classification) were recorded. The modified Rankin score (mRS) was used to assess disability at each assessment time point. Adverse medical events were prospectively recorded from patient reports and medical records: deaths, falls, neurological and cardiovascular events requiring hospitalisation, and any study related medical event.
Glucose metabolism
The primary outcome for this study was change in 2-h plasma glucose on oral glucose tolerance test (OGTT) assessed at baseline and 6 months. Glucose response was classified as normal, impaired or diagnostic of diabetes on the basis of plasma glucose value 2 h after ingestion of 75 g of glucose in a fasted state, according to the World Health Organization classifications.19 Glucose and insulin concentrations were measured at each time point before 9 am following an overnight fast, and were used to calculate insulin sensitivity (HOMA %S) by the Homeostasis Model Assessment of Insulin Sensitivity.20 Glycated haemoglobin A1c (HbA1c, coefficient of variation, CV, 2.2–3.4%) was measured to estimate glycaemic status during the preceding 3 months. C-peptide was measured as a marker of insulin production. Plasma glucose was measured using a hexokinase assay on the Roche Cobas 702, CV 2.0–3.2%. Insulin was measured by electrochemiluminescence immunoassay (ECLIA, CV 3.3–5.6%) on the Roche Cobas 602. HbA1c was measured by immunoassay on the Roche Integra 800, CV 2.2–3.4%.
Body composition
Total body fat and lean masses were estimated using dual-energy X-ray absorptiometry ((DXA), Lunar Prodigy DXA System, analysis software: 13.60), using the manufacturer’s procedures, CV 1–1.2%.21 No systematic long-term bias was evident in calibration data. All scans were undertaken on the same machine.
Physical activity
Physical activity was measured using a dual-axis accelerometer with switch tilt, sample rate 10 Hz (PAL2, positional activity logger 2, Gorman ProMed Pty Ltd, Melbourne, Australia). It is comprised of two parts: a control unit and an auxiliary switch, which are attached to the right leg above and below the knee by elasticised straps. PAL2 registered the number of changes in position and the amount of time that participants spent lying down, sitting, standing and walking.22 There is high agreement between PAL2 recordings and behavioural mapping of people with acute stroke: lying ICC 0.74 (95% confidence interval (CI) 0.46–0.89), sitting 0.68 (0.36–0.86), upright 0.72 (0.43–0.88).23 PAL2 is a modified version of the ‘Uptime’ device, which has high test–retest reliability (Pearson’s r = 0.84, p < 0.001).24
Consistent with previous studies of physical activity after stroke9 that have shown that activity levels do not differ between days in acute stroke units, the device was worn for 1 day at each time point from 8 am to 5 pm, representing the most active part of the day.25 Follow-up assessments were undertaken on a self-selected weekday that represented participants’ typical daily activity. Sedentary time was calculated as the sum of time lying and sitting as a proportion of total time wearing the device.
Anthropometry
Body mass was measured on an electronic scale (WB-100A, Tanita Corporation, Tokyo, Japan) with participants wearing light clothing. Height was measured using a wall-mounted standiometer (Holtain, Crosswell, UK). Participants unable to stand were weighed in their wheelchair on floor scales (Model 8000 Ranger, Wedderburn) or weight was taken from medical records; height was measured in supine from the top of head to heel using a standard measuring tape. Body mass index (BMI) was calculated (kg/m2). Waist and hip circumferences were measured in the standing position at the narrowest point between ribs and iliac crest, and at the widest part of the buttocks.
Walking ability
Walking ability was evaluated using the Functional Ambulation Category (FAC).26 This seven-point scale measures the ability to negotiate various terrains, with or without a gait aid. The scale ranged from “1: non-functional ambulation” to “6: independent ambulation on uneven surfaces.”
Statistical analysis
Given that this was a phase 1 pilot study, it is exploratory by design and was not powered to detect a change in glucose tolerance. Data for people who did not complete the study (deaths and drop outs) cannot be assumed to be missing-at-random27: these participants were not included in analyses and no results can be generalised for those participants. Missing data from all other participants were assumed to be missing-at-random.
Generalised estimating equations (GEE) were used to investigate change over time (months since stroke) of log transformed 2-h glucose and HOMA2 %S and secondary outcomes body composition, physical activity and sedentary time. This approach was also used to test the strength of associations between 2-h glucose and HOMA2 %S and secondary outcomes. Interpretation of the effect sizes produced by GEEs is the population-averaged change in the dependent variable (e.g. 2-h glucose or HOMA2 %S) per one unit change in an independent variable (e.g. every extra month after stroke) assuming other independent variables are held constant. For example, if the GEE coefficient between fasting glucose and time (months) after stroke was 0.993 and baseline glucose level was 8.7 mmol/L, after 1 month, it would be 8.7 × 0.993 = 8.64 mmol/L.
Models were then run adjusting for expected confounders stroke severity and age, which were a priori known to be associated with poorer recovery after stroke. Analyses were performed using STATA v 13 IC statistical software (StataCorp LP, College Station, TX, USA). A significance level of p = 0.05 was set for all statistical tests and no correction for multiplicity was undertaken due to the exploratory nature of this analysis. De-identified individual data are available from the corresponding author.
Results
Recruitment, retention and demographics
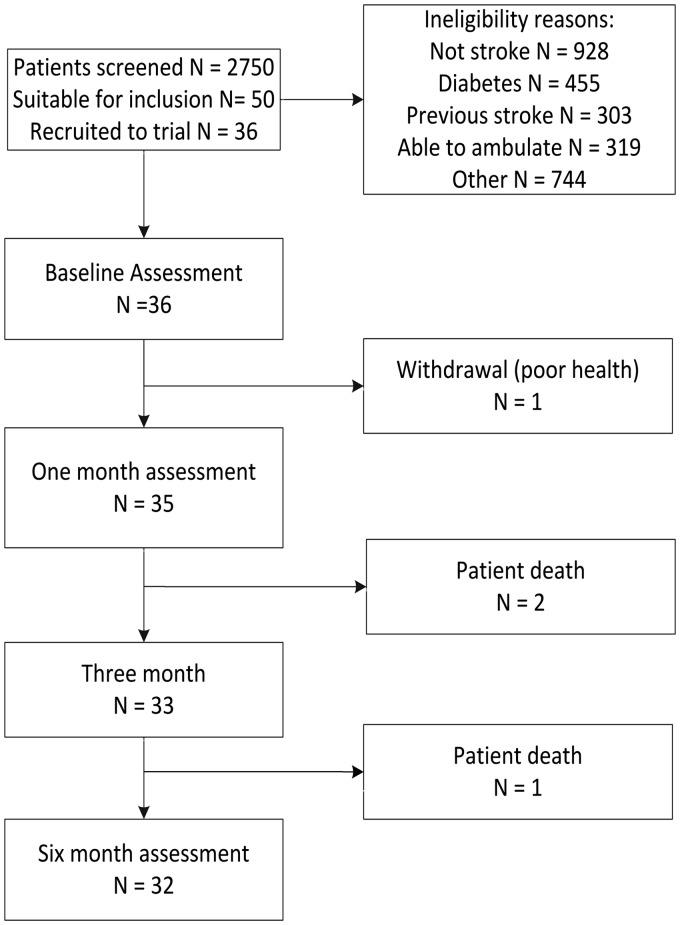
We screened 2749 patients on hospital admission. Almost one in five (17%) people had known diabetes and were therefore excluded (Figure 1). Consent was obtained for 36 patients (age 69.5 (SD 11.7), 13 (36.1%) female, NIHSS 12.8 (SD 4.7)). Four participants did not complete the study – three died and one withdrew due to deteriorating health (Figure 1).
Figure 1.
Participant recruitment and retention to 6 months of stroke.
There were no significant differences between participants who did and did not complete the study, except for the Oxfordshire stroke classification; people who completed the trial had fewer haemorrhagic strokes and more total anterior circulation stroke (χ2 = 9.9, p = 0.02) (Table 1).
Table 1.
Demographic and stroke characteristics.
| Characteristic | All recruits N = 36 | Completed study n = 32 | p a |
|---|---|---|---|
| Gender, female | 13 (36.1) | 12 (37.5) | 0.62 |
| Age, mean (SD) | 69.5 (11.7) | 68.9 (11.8) | 0.37 |
| Lived with others | 28 (77.8) | 25 (78.1) | 0.89 |
| Education | |||
| No formal education | 2 (5.6) | 2 (6.3) | 0.25 |
| Incomplete secondary | 7 (19.4) | 6 (18.8) | |
| Complete secondary | 4 (11.1) | 3 (9.4) | |
| Trade/apprentice | 7 (19.4) | 5 (15.6) | |
| Certificate/diploma | 9 (25.0) | 9 (28.1) | |
| University degree or higher | 7 (19.4) | 7 (21.9) | |
| Pre-stroke employment | |||
| Full or part time work | 14 (38.9) | 13 (40.6) | 0.83 |
| Home duties | 3 (8.3) | 3 (9.4) | |
| Student | 1 (2.8) | 1 (3.1) | |
| Retired | 18 (50.0) | 15 (46.9) | |
| Non-English speaking background | 7 (19.4) | 7 (21.9) | 0.30 |
| Previously walked no gait aid | 32 (88.9) | 29 (90.6) | 0.35 |
| Body mass index | 27.2 (5.4) | 27.6 (5.6) | 0.21 |
| Family history of diabetes | |||
| Yes | 3 (8.3) | 3 (9.4) | 0.33 |
| No | 18 (50.0) | 17 (53.1) | |
| Unknown | 9 (25.0) | 7 (21.9) | |
| Past medical history | |||
| Hypertension | 18 (50.0) | 15 (46.9) | 0.29 |
| High cholesterol | 12 (33.3) | 10 (31.3) | 0.45 |
| Ischaemic heart disease | 6 (16.7) | 4 (12.5) | 0.06 |
| Atrial fibrillation | 7 (19.4) | 6 (18.8) | 0.77 |
| Musculoskeletal | 15 (41.7) | 14 (43.8) | 0.47 |
| Number co-morbidities, mean (SD) | 4.1 (2.9) | 4.0 (2.7) | 0.24 |
| Smoking history | |||
| Current smoker | 12 (33.3) | 10 (31.3) | 0.57 |
| Never smoked | 13 (36.1) | 12 (37.5) | |
| Stopped in past 2 years | 2 (5.6) | 2 (6.3) | |
| Stopped > 2 years ago | 9 (25.0) | 9 (28.1) | |
| Stroke severity, NIHSS | |||
| Group median (IQR) | 11.5 (9.75, 16) | 12.5 (9.75, 16.5) | 0.26 |
| Mild | 8 (22.2) | 7 (21.9) | |
| Moderate | 20 (55.6) | 17 (53.1) | |
| Severe | 8 (22.2) | 8 (25.0) | |
| Stroke classification | |||
| TACI | 18 (50.0) | 18 (56.3) | 0.02a |
| PACI | 11 (30.6) | 10 (31.3) | |
| LACI | 3 (8.3) | 2 (6.3) | |
| Haemorrhage | 4 (11.1) | 2 (6.3) | |
Note. Data presented n (%) unless otherwise specified.
Independent sample T-test or Wilcoxon rank-sums dependent on distribution of continuous or ordinal data, Chi2 for nominal data.
NIHSS: National Institutes of Health Stroke Scale (mild < 8, moderate 8 to 16, severe > 16); TACI/PACI: total/partial anterior circulation infarct; LACI: lacunar circulation infarct.
Glycaemic control and insulin sensitivity
Due to swallowing impairment (dysphagia), 13 participants could not undertake OGTT at baseline. Six people subsequently completed OGTT at 1-month assessment; their OGTT results are included as “baseline” in Table 2 (median 9 days post stroke, interquartile range (IQR) 7, 18).
Table 2.
Measures of glycaemic control and body composition at baseline and 6 months after stroke.
| Baseline | Six months | Effect size (95% CI)a,b Change per month | p a | |
|---|---|---|---|---|
| Oral glucose tolerance test (OGTT) | ||||
| 2-h glucose (mmol/L) | 7.9 (6.7, 10.3), n = 24 | 5.9 (4.6, 7.7), n = 26 | 0.96 (0.94, 0.97) | <0.001 |
| Glucose responsec, N (%) | n = 23 | n = 26 | n/a | 0.033 |
| Normal | 12 (52.2) | 20 (76.9) | ||
| Impaired | 6 (26.1) | 6 (23.1) | ||
| Diabetic | 5 (21.7) | 0 | ||
| HOMA2 %S | 75.9 (50.5, 102.1), n = 30 | 92.2 (72.8,160.9), n = 29 | 1.03 (1.002, 1.06) | 0.04 |
| HbA1c | n = 31 | n = 29 | 0.18 | |
| mmol/mol | 40 (38, 41) | 41 (39, 42) | 1.001 (0.99, 1.02) | |
| % | 5.8 (5.6, 5.9) | 5.9 (5.7, 6.0) | ||
| HOMA2 %B | 115.7 (80.2, 136.4), n = 30 | 107.6 (86.5, 148.8), n = 29 | 2.51 (0.15, 41.89) | 0.52 |
| C-peptide | 1.04 (0.9, 1.32), n = 29 | 0.82 (0.67 1.06), n = 28 | 0.99 (0.95, 1.04) | 0.92 |
| Fat mass (kg) | 23.5 (16.5, 33.1), n = 28 | 20.9 (14.7, 27.9), n = 27 | 0.98 (0.97, 0.99) | 0.01 |
| Lean mass (kg) | 47.2 (40.8, 53.1), n = 28 | 49.1 (41.2, 54.8), n = 27 | 1.001 (0.999, 1.003) | 0.35 |
| Waist/hip ratio | 0.96 (0.86, 1.03), n = 24 | 0.93 (0.84, 1.01), n = 28 | 0.99 (0.98, 1.003) | 0.15 |
| Body mass index (kg/m2) | 26.5 (23.1, 30.3), n = 32 | 25.1 (22.9, 27.1), n = 26 | 0.82 (0.58, 1.16) | 0.26 |
Note. Data are presented median (IQR) unless otherwise specified.
GEE: generalised estimating equation; HbA1c: glycated haemoglobin A1c; HOMA2: updated Homeostasis Model Assessment; HOMA2 %S: insulin sensitivity; HOMA2 %B: beta cell function.
Adjusted for age and stroke severity (National Institute of Health Stroke Scale, NIHSS).
Normal fasting = <5.6 mmol/L; impaired glucose tolerance (IGT) = 2-h glucose 7.8–11.1 mmol/l; diabetes = 2-h glucose ≥ 11.1 mmol/l.19
Within the first 6 months of stroke, glucose tolerance improved i.e. for every month, 2-h glucose level was lower by 4.5% (95% CI 2.9, 6.1, p = 0.033). Insulin sensitivity also improved monthly by 2.9% (95% CI 0.2, 5.6, p = 0.03). Both associations remained significant when adjusting for age and stroke severity (Table 2).
Body composition: Muscle and fat mass, and BMI
Baseline DXA scans were undertaken at 10.7 (IQR 7.5, 13.9) days post stroke. World Health Organisation BMI classifications indicated that at baseline four people (12.5%) were underweight (BMI < 18.5), seven (21.9%) were normal weight (BMI 18.5–24.9), 13 (40.6%) were overweight (BMI 25–29.9) and eight people (25%) were obese (BMI ≥ 30) (Table 2). Between baseline and 6 months, total body fat mass decreased by 10.4% (95% CI 0.8, 21.1), which equated to 489 g per month (95 % CI 325, 655, p = 0.01), but no statistically significant change in lean mass was observed.
Physical activity
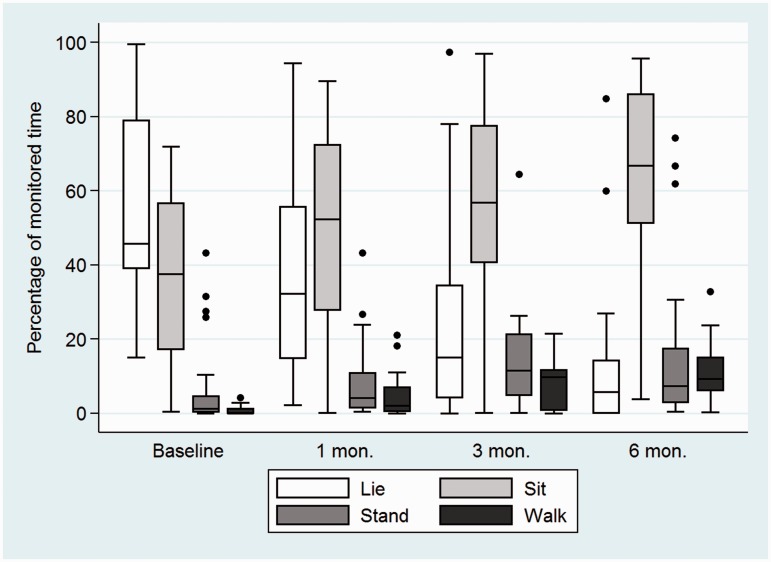
At baseline, almost all (median 98.5% (IQR 94.3, 99.8) of the day was spent sedentary, and only 1.5% (IQR 0.2, 5.8) of the time was spent upright, either standing or walking. Participants stood up on average 6 times (IQR 0, 13) throughout the day (Figure 2). By 6 months, most participants (21/32, 66%) were able to walk independently (FAC > 4), but were dependent on others for daily activities (mRS, median = 3 (IQR 2, 4)). All activity measures changed significantly between baseline and 6 months (p < 0.001). At 6 months, participants were sedentary 74.3% (IQR 65.5, 88.6, n = 27) of the day, mostly sitting (66.8% (IQR 39.9, 86.0), n = 27), and a quarter of the time was spent upright (25.7% (IQR 11.4, 34.5)). The number of times that participants stood up during activity monitoring increased to 24 (IQR 17, 35.5). Controlling for age and NIHSS, for every month, the percentage of time spent upright increased by a factor of 2.84 (95% 1.83, 3.85, p < 0.001).
Figure 2.
Physical activity between baseline and 6 months after stroke.
Associations between glycaemic control and physical activity and fat mass
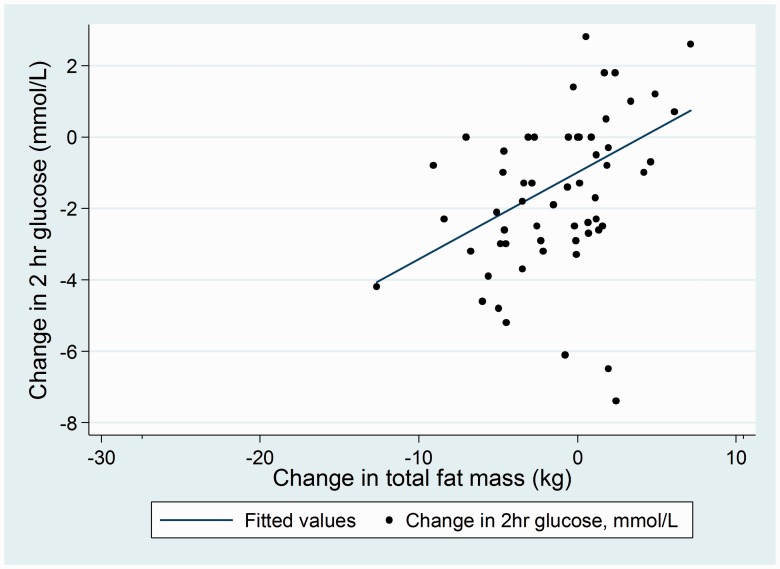
Better glycaemic control was significantly associated with lower total fat mass as shown in Figure 3: for every kilogram increase in total fat mass, when adjusting for age and stroke severity, 2-h glucose increased by 1.02 mmol/L (95% CI 1.01, 1.02). Likewise, for every 5% increase in sedentary time, 2-h glucose increased by 1.05 mmol/L (95% CI 1.04, 1.07, p < 0.001), after adjusting for number of times that participants stood up and total time that the accelerometer was worn, Table 3. Higher (better) insulin sensitivity was associated with lower total fat mass; for every kilogram increase in total fat mass, HOMA2 S% reduced by 0.98% (95% CI 0.97, 0.99, p = 0.001).
Figure 3.
Association between 6-month change in total body fat (kg) and 2-h glucose level (mmol/L) on oral glucose tolerance test.
Table 3.
Associations between glucose tolerance, insulin sensitivity, body composition and physical activity within 6 months of stroke.
| Independent variable | Glucose tolerance: 2-h glucose on OGTT (mmol/L) | Insulin sensitivity: HOMA2 S% |
|---|---|---|
| Total fat mass (kg) | 1.02 (1.01, 1.02) p = 0.001 | 0.98 (0.97, 0.99) p = 0.001 |
| Total lean mass (kg) | 0.003 (0.99, 1.01) p = 0.66 | 0.99 (0.97, 1.01) p = 0.11 |
| Body mass index (kg/m2) | 1.002 (0.99, 1.01) p = 0.57 | 0.99 (0.98, 1.01) p = 0.30 |
| % Sedentary time | 1.009 (1.007, 1.013) p < 0.001a | 0.997 (0.993, 1.003) p = 0.38 |
| Transitions | 0.999 (0.997, 1.001) p = 0.22 | 1.001 (0.99, 1.003) p = 0.22 |
Note. Cells contain generalised estimating equation effect size (95% CI), adjusting for age and stroke severity (NIHSS).
Adjusted for number of transitions and total wear time.
HOMA2 S%: homeostasis model assessment index measure of insulin sensitivity; OGTT: oral glucose tolerance test; Sedentary: lying or sitting; Transitions: number of times that participants stood up.
Discussion
This study was the first examination of glycaemia status and its associations with body composition, physical activity and sedentary time after stroke. The most important was that participants’ glucose tolerance and insulin sensitivity improved within 6 months of stroke, and fat mass reduced by ∼1/2 kg per month. At 6 months after stroke, no participants were classified as having diabetes following an OGTT (defined as 2-h glucose ≥ 11.1 mmol/l).19
Our findings are in contrast to previous studies in which glucose intolerance and insulin resistance were prevalent in chronic stroke populations.2,3 Contrasting findings may be related to differences in study populations and methodologies: in our study, participants who were unable to walk were recruited within 2 weeks of stroke and observed longitudinally. The studies by Kernan et al.2 and Ivey et al.3 were cross-sectional studies of people who were on average more than 3 months post-stroke or transient ischemic attack, who were able to walk. Loss of fat mass in our participants and related improvements in glycaemic control may be explained by increased energy costs of moving and walking, related to their higher level of impairment and inability to walk at baseline. Also, given that 13 participants in our study had swallowing difficulties (dysphagia) at baseline, they were at risk of malnutrition (inadequate protein and calories), which is common after stroke.28 Assessment of nutritional status is warranted in future studies.
In our study, better glucose tolerance and insulin sensitivity were independently associated with lower fat mass. These results reflect recommendations for weight loss in people with prediabetes to reduce their risk of progressing to diabetes, and for people with diabetes to improve their glycaemic control.29 Furthermore, we observed that improved glucose tolerance was associated with lower sedentary time when adjusted for the number of times that people stood up during activity monitoring. These results suggest that targeting reductions in sedentary time and fat mass after stroke may improve glucose tolerance and insulin sensitivity, and in turn cardiovascular health by reducing the risk of recurrent stroke and development of diabetes.
Our findings support recent Australian recommendations that regardless of the amount of physical activity undertaken, adults of all abilities should limit sedentary time due to its detrimental effects on cardiovascular health.30 Sedentary behaviour after stroke contributes to changes in muscle fibre type composition to more insulin resistant fast-twitch type II muscle fibre types,12 reduced muscle capillarisation and peripheral blood flow4,13 and increased intramuscular fat and inflammatory markers in paretic leg muscles,14 that have been related to glucose intolerance and insulin resistance.3
A high level of sedentary time observed in this study was not unexpected despite most participants regaining the ability to walk independently within 6 months. Less than 20% of monitored time consisted of standing or walking at 3 and 6 months. Similar results were demonstrated by Askim et al.9 who, using the same accelerometer as in this current study, observed that low levels of activity were maintained throughout 6 months of stroke. In the current study, participants lost body fat despite maintaining high levels of sedentary time within 6 months of stroke. This is in contrast to a study by Carin-Levy et al.31 who observed increases in DXA derived fat mass within 6 months of stroke: from 25.2 kg (IQR 20.2, 30.6) at baseline to 26.5 kg (20.7, 28.2), p = 0.01. Participants in the study by Carin-Levy et al.31 had similar BMI and mobility status at baseline compared to the current study, and they also observed no change in lean mass.
Moore et al.32 undertook a prospective investigation of post-stroke physical activity and glycaemic control.32 The study included 31 people aged 73 ± 9 years (45% female) within 7 days of mild stroke (NIHSS = 2 ± 2) who were able to walk 10 m independently.32 Participants underwent physical activity and fasting insulin and glucose tests at baseline and at 3 and 6 months. Physical activity increased by 3 months but plateaued by 6 months, at which time stroke survivors were sedentary for 22.5 h (94%) of the day. Fasting glucose and insulin sensitivity were within normal limits at baseline and did not change over time, and no association was observed between longitudinal changes in sedentary time and glycaemic control. As previously mentioned, the contrasting glycaemic control results between studies may be explained by higher energy demands of walking33 in participants in the current study due to their higher level of impairment compared to mild impairments in the group studied by Moore et al.32 Researchers are currently investigating the cardiovascular effects of breaking up sedentary time to better understand associations between energy costs and amount of physical activity undertaken by people with stroke.34
A limitation of the current study is that only 1 day of activity monitoring occurred at each time point, whereas it is recommended that physical activity monitoring be undertaken for 3 days in community dwelling non-stroke adult populations to accurately predict activity levels.35 However, physical activity does not differ between days on hospital wards,10 and daily variation in activity levels of community dwelling stroke survivors is currently being examined in more detail.36 Results of this examination will guide recommendations for future post-stroke activity monitoring periods. As this was a phase 1 study with a small sample, results are best interpreted cautiously and validated in a larger trial.
In summary, this was the first prospective study to examine glycaemic control, body composition, sedentary time and physical activity from within a week of moderately severe stroke. In contrast to our hypothesis, glucose tolerance and insulin sensitivity improved, likely due to reduced fat mass within 6 months of stroke, in part possibly due to higher energy demands of walking after stroke despite high levels of sedentary time. Results suggest that interventions aimed at reducing sedentary time and fat mass may improve glucose tolerance and insulin resistance, thereby reducing the risks of recurrent stroke and developing cardiovascular disease including diabetes.
Acknowledgements
The authors thank all participants and their families, and research nurse Kylie King for your dedication to this project. The Florey Institute of Neuroscience and Mental Health acknowledge the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Australian Research Council (ARCFT09901086), Austin Health Medical Research Fund and LaTrobe University Faculty Research Grant. EIE was supported by an NHMRC Early Career Research Fellowship (#1054312), Viertel Clinical Investigatorship, Sir Edward Weary Dunlop Medical Research Foundation grant and RACP fellowship. JB was supported by an NHMRC Established Researcher Fellowship. KB was supported by a La Trobe University Postgraduate Research Scholarship.
Ethical approval
This study was approved by Austin Health (H2008/03428), Northern Health (P4/13) and La Trobe University (09-065) Human Research Ethics Committees, and completed in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Informed consent
Informed written consent was obtained for all participants.
Guarantor
JB.
Contributorship
KB, EE, SI, MP and JB devised the study methods. KB coordinated the study, recruited participants, collected and analysed data, under the supervision of JB and SI. LC provided statistical advice. KB wrote the initial manuscript draft, all authors contributed comments and approved the final version.
References
- 1.Capes S, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke 2001; 32: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 2.Kernan W, Viscoli C, Inzucchi S, et al. Prevalence of abnormal glucose tolerance following a transient ischemic attack or ischemic stroke. Archiv Int Med 2005; 165: 227–233. [DOI] [PubMed] [Google Scholar]
- 3.Ivey F, Ryan A, Hafer-Macko C, et al. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovasc Dis 2006; 22: 368–371. [DOI] [PubMed] [Google Scholar]
- 4.Pyorala M, Miettinen H, Laakso M, et al. Hyperinsulinemia and the risk of stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Stroke 1998; 29: 1860–1866. [DOI] [PubMed] [Google Scholar]
- 5.Allport L, Baird T, Davis S. Hyperglycaemia and the ischaemic brain: continuous glucose monitoring and implications for therapy. Curr Diabet Rev 2008; 4: 245–257. [DOI] [PubMed] [Google Scholar]
- 6.Kernan W, Inzucchi S, Viscoli C, et al. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology 2003; 60: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 7.Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke 1989; 20: 871–875. [DOI] [PubMed] [Google Scholar]
- 8.Globus RK, Bikle DD, Morey-Holton E. The temporal response of bone to unloading. Endocrinology 1986; 118: 733–742. [DOI] [PubMed] [Google Scholar]
- 9.Askim T, Bernhardt J, Churilov L, et al. Changes in physical activity and related functional and disability levels in the first six months after stroke: a longitudinal follow-up study. J Rehabil Med 2013; 45: 423–428. [DOI] [PubMed] [Google Scholar]
- 10.Bernhardt J, Chitravas N, Meslo I, et al. Not all stroke units are the same: a comparison of physical activity patterns in Melbourne, Australia, and Trondheim, Norway. Stroke 2008; 39: 2059–2065. [DOI] [PubMed] [Google Scholar]
- 11.Ivey F, Hafer-Macko C, Macko R. Exercise training for cardiometabolic adaptation after stroke. J Cardiopulmon Rehab Prev 2008; 28: 2–11. [DOI] [PubMed] [Google Scholar]
- 12.De Deyne P, Hafer-Macko C, Ivey F, et al. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve 2004; 30: 209–215. [DOI] [PubMed] [Google Scholar]
- 13.Prior S, McKenzie M, Joseph L, et al. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation 2009; 16: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafer-Macko C, Ryan A, Ivey F, et al. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of evercise intervention strategies. J Rehab Res Dev 2008; 45: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.English C, McLennan H, Thoirs K, et al. Loss of skeletal muscle mass after stroke: a systematic review. Int J Stroke 2010; 5: 395–402. [DOI] [PubMed] [Google Scholar]
- 16.English C, Thoirs K, Coates A, et al. Changes in fat mass in stroke survivors: a systematic review. Int J Stroke 2012; 7: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorp A, Owen N, Neuhaus M, et al. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. Am J Prev Med 2011; 41: 207–215. [DOI] [PubMed] [Google Scholar]
- 18.Borschmann K, Pang M, Iuliano S, et al. Changes to volumetric bone mineral density and bone strength after stroke: A prospective study. Int J Stroke 2015; 10: 396–399. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization and International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF Consultation, Geneva, Switzerland, 2006.
- 20.Matthews D, Hosker J, Rudenski A, et al. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabeologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 21.Baim S, Wilson C, Lewiecki E, et al. Precision assessment and radiation safety for dualenergy X ray absorptiometry (DXA). J Clin Densitom 2005; 8: 371–378. [DOI] [PubMed] [Google Scholar]
- 22.Diggory P, Gorman M, Schwarz J, et al. An automatic device to measure time spent upright. Clin Rehab 1994; 8: 353–357. [Google Scholar]
- 23.Kramer S, Cumming T, Churilov L, et al. Measuring activity levels at an acute stroke ward: comparing observations to a device. Biomed Res Int 2013; 2013: 460482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran P, Schwarz J, Gorman M, et al. Validation of an automated up-timer for measurement of mobility in older adults. Med J Aust 1997; 167: 434–436. [DOI] [PubMed] [Google Scholar]
- 25.Bernhardt J, Dewey H, Thrift A, et al. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004; 35: 1005–1009. [DOI] [PubMed] [Google Scholar]
- 26.Holden M, Gill K, Magliozzi M. Gait assessment for neurologically impaired patients - standards for outcome assessment. Phys Therapy 1986; 66: 1530–1539. [DOI] [PubMed] [Google Scholar]
- 27.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. New Engl J Med 2016; 374: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley N, Martin R, Salter K, et al. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med 2009; 41: 707–713. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Standards of medical care in diabetes - 2012. Diabetes Care 2012; 35(Supplement 1): S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown W, Bauman A, Bull F, et al. Development of evidence-based physical activity recommendations for adults (18 - 64 years). Report prepared for the Australian Government Department of Health, Health AGDo (ed), 2012.
- 31.Carin-Levy G, Greig C, Young A, et al. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc Dis 2006; 21: 201–207. [DOI] [PubMed] [Google Scholar]
- 32.Moore S, Hallsworth K, Plotz T, et al. Physical activity, sedentary behaviour and metabolic control following stroke: a cross-sectional and longitudinal study. PLoS One 2013; 8: e55263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viscoli CM, Brass LM, Carolei A, et al. Pioglitazone for secondary prevention after ischemic stroke and transient ischemic attack: Rationale and design of the Insulin Resistance Intervention after Stroke Trial. Am Heart J 2014; 168: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen H, Dunstan DW, Bernhardt J, et al. Breaking up sitting time after stroke (BUST-Stroke). Int J Stroke Epub 2016; DOI: 10.1177/1747493016676616. [DOI] [PubMed] [Google Scholar]
- 35.Hart T, Swartz A, Cashin S, et al. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Activity 2011; 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fini N, Burge A, Bernhardt J, et al. Optimal duration of physical activity monitoring in stroke. Int J Stroke 2015; 10: 60. [Google Scholar]