Abstract
Introduction
Although several study protocols reported that vertebral artery hypoplasia can predispose to posterior circulation ischaemia, the role of vertebral artery hypoplasia in the risk of posterior circulation ischaemia still remains controversial. The aim of the present meta-analysis was to investigate the association of vertebral artery hypoplasia and posterior circulation ischaemia.
Patients and methods
We performed a systematic review and random effects meta-analysis of all eligible observational study protocols reporting prevalence rates of vertebral artery hypoplasia in patients with anterior circulation ischaemia and posterior circulation ischaemia.
Results
We identified eight study protocols including a total of 3875 acute ischemic stroke patients (mean age: 64.2 years, 61.3% males) and reporting a pooled prevalence of vertebral artery hypoplasia 18.6% (95%CI: 10.8–30.0%). In the overall analysis, a significantly higher probability of vertebral artery hypoplasia presence was found in posterior circulation ischaemia patients compared to patients with anterior circulation ischaemia (risk ratio = 2.12, 95%CI: 1.60–2.82, p < 0.001). In the subsequent sensitivity analysis, vertebral artery hypoplasia was again found to be significantly more prevalent in patients with posterior circulation ischaemia compared to anterior circulation ischaemia (risk ratio = 1.81, 95%CI: 1.58–2.06, p < 0.001), with no evidence of heterogeneity (I2 = 0%, p for Cochran Q = 0.55) between included studies.
Discussion
The present report is a meta-analysis of retrospective observational study protocols, with all the inherent limitations of included studies. The heterogeneity on the reported rates of vertebral artery hypoplasia could be attributed to differences in population age, sex, race, imaging protocols and vertebral artery hypoplasia definition between included studies.
Conclusion
Our meta-analysis provides further evidence for a possible causal relationship between vertebral artery hypoplasia and cryptogenic posterior circulation ischaemia, an association which undoubtedly deserves further investigation in future prospective study protocols.
Keywords: Vertebral artery hypoplasia, posterior circulation ischaemia, ischemic stroke
Introduction
Cryptogenic embolism has been reported to represent about 10% of the ischemic strokes in posterior circulation.1 Although several study protocols have reported that vertebral artery hypoplasia (VAH) can predispose to posterior circulation ischaemia (PCI) even in young, the role of VAH in the risk of cerebral ischaemia still remains controversial.2–4
In the present manuscript, we performed a systematic review and random effects meta-analysis of all available cohort studies investigating the association of VAH and cerebral ischaemia.
Methods
Eligible observational study protocols that reported prevalence rates of VAH in patients with anterior circulation ischaemia (ACI) and PCI were identified by searching MEDLINE and SCOPUS databases. The combination of search strings that was used in both database searches included the terms: ‘vertebral artery hypoplasia’, ‘ischemic stroke’ and ‘cerebral ischaemia’. No language or other restrictions were imposed. Last literature search was conducted on 4 July 2016. All retrieved studies were scanned independently by the two authors (AHK and SG), while any disagreement was resolved with consensus. We excluded from the quantitative/qualitative analysis all case series, case reports and studies not reporting VAH prevalence rates on either ACI or PCI.
We calculated the corresponding risk ratios (RRs) in each included study to express the relative risk of VAH presence in both the aforementioned subgroups. A random effects model (DerSimonian–Laird) was used to calculate the pooled RRs. The equivalent z-test was performed for each pooled RR, and if p < 0.05 it was considered statistically significant. We assessed heterogeneity between studies with the Cochran Q and I2 statistics. For the qualitative interpretation of heterogeneity, I2 values of at least 50% were considered to represent substantial heterogeneity, while values of at least 75% indicated considerable heterogeneity, as per the Cochrane Handbook.5 All statistical analyses were conducted using Review Manager (RevMan) Version 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and Comprehensive Meta-analysis Version 2 software (Borenstein M, Hedges L, Higgins J, et al. Englewood, NJ: Biostat, 2005).
Results
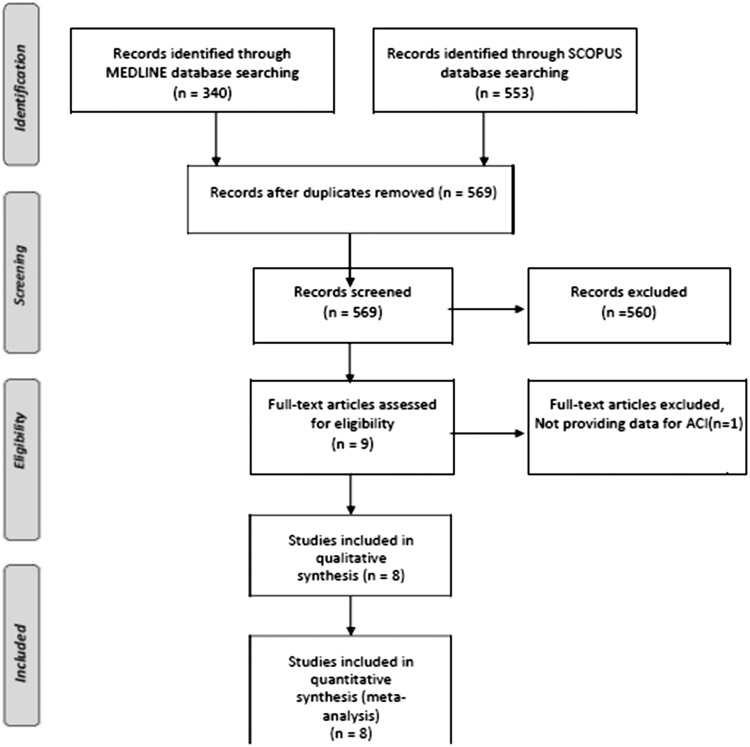
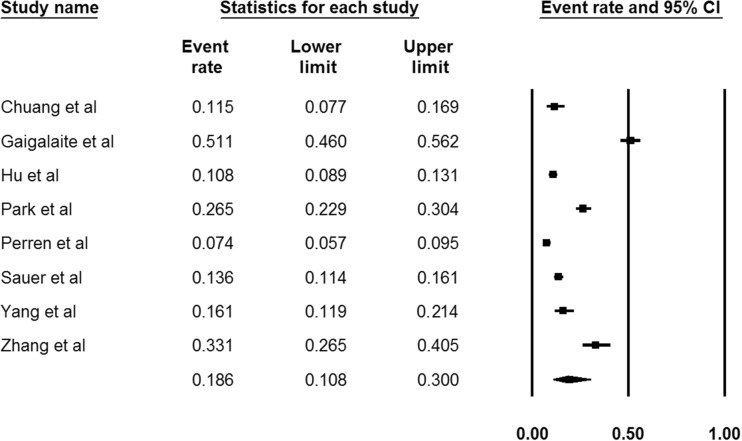
Our systematic literature search on MEDLINE and SCOPUS databases highlighted eight potential studies,6–13 reporting VAH prevalence rates in both patients with ACI and PCI (Figure 1). Included study protocols recruited a total of 3875 acute ischemic stroke patients (mean age: 64.2 years, 61.3% males; Table 1) and reported a pooled prevalence of VAH in 18.6% (95%CI: 10.8–30.0%; I2 = 98%, p for Cochran Q < 0.001; Figure 2).
Figure 1.
Flow chart presenting the selection of eligible studies.
Table 1.
Baseline characteristics of included studies.
| Study name | Year | Country | No of AIS patients | Mean age ± SD (years) | Males (%) | Total prevalence of VAH (%) | VAH/PCI (%) | VAH/ACI (%) | Vessel diameter for VAH definition | Imaging modality | Time from symptom onset to imaging |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chuang et al.6 | 2006 | Taiwan | 191 | 55.8 ± 14.0 | 68 | 11.51 | 54.5 | 2.5 | <2 mm | MRA | ≤72 h |
| Gaigalaite et al.7 | 2016 | Lithuania | 367 | – | – | 51.1 | 58.3 | 30.5 | <3 mm | MRA/CTA | – |
| Hu et al.8 | 2013 | China | 841 | 64.6 ± 13.5 | 68.6 | 10.8 | 17.0 | 8.5 | <2 mm | CE-MRA/(CTA) | – |
| Park et al.9 | 2007 | Korea | 529 | – | – | 26.5 | 45.6 | 27.1 | ≤2 mm | TOF-MRA | 2.9 ± 1.8 days |
| Perren et al.10 | 2007 | Switzerland | 725 | 67.1 | 61.1 | 7.4 | 13.0 | 4.6 | ≤2.5 mm or difference to contralateral side> 1:1.7 | CDU | – |
| Sauer et al.11 | 2016 | Germany | 815 | 70 ± 14 | 52.8 | 13.6 | 17.1 | 11.7 | ≤2.5 mm or difference to contralateral side > 1:1.7 | TOF-MRA | – |
| Yang et al.12 | 2015 | China | 235 | 42.9 ± 6.3 | 64.7 | 16.1 | 25.0 | 12.9 | <2 mm | DSA | – |
| Zhang et al.13 | 2016 | China | 172 | 61.3 | 54.6 | 33.1 | 44.8 | 27.2 | ≤2 mm or difference to contralateral side > 1:1.7 | CE-MRA | ≤5 days |
ACI: anterior circulation ischaemia; AIS: acute ischemic stroke; CDU: colour duplex ultrasound; CE: contrast enhanced; CTA: computed tomography angiography; DSA: digital subtraction angiography; MRA: magnetic resonance angiography; PCI: posterior circulation ischaemia; SD: standard deviation; TOF: time of flight; VAH: vertebral artery hypoplasia.
Figure 2.
Pooled prevalence of vertebral artery hypoplasia reported in the included study protocols.
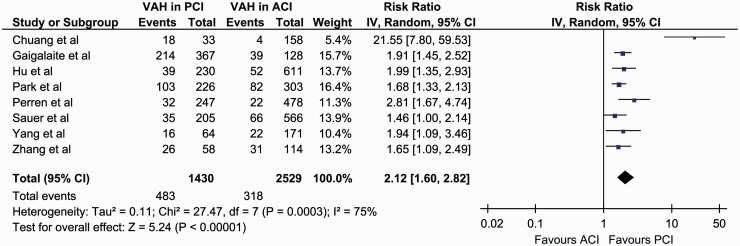
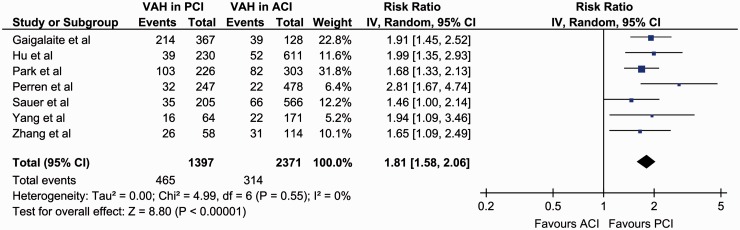
In the overall analysis of all included studies, significantly higher rates of VAH presence were found in patients with PCI (31.8% (95%CI: 19.2–48.0%)) compared to patients with ACI (12.8% (95%CI: 7.6–20.7%)) (RR = 2.12, 95%CI: 1.60–2.82, p < 0.001; Figure 3), with considerable heterogeneity among included studies (I2 = 75%, p for Cochran Q < 0.001). In the subsequent sensitivity analysis, after excluding a small-sized study reporting a considerably higher effect size compared to the other studies,6 VAH was found to be significantly more prevalent in patients with PCI (29.2% (95%CI: 16.6–46.2%)) compared to ACI (15.0% (95%CI: 9.0–24.0%)) (RR = 1.81, 95%CI: 1.58–2.06, p < 0.001; Figure 4), with no evidence of heterogeneity in remaining studies (I2 = 0%, p for Cochran Q = 0.55).
Figure 3.
Overall analysis on the reported prevalence of vertebral artery hypoplasia in patients with anterior cerebral ischaemia compared to patients with posterior cerebral ischaemia.
Figure 4.
Sensitivity analysis on the reported prevalence of vertebral artery hypoplasia in patients with anterior cerebral ischaemia compared to patients with posterior cerebral ischaemia.
Discussion
In our meta-analysis of available cohort studies, we found that VAH is present in approximately one out of five patients with ischemic stroke, while is almost twice more prevalent in patients with PCI compared to patients with ACI.
We found that the total prevalence of VAH varied significantly among the included studies. This heterogeneity could be attributed to the differences in mean population age across the included studies,6–13 as a higher prevalence of reported VAH-related PCI is anticipated in younger patients with cryptogenic stroke due to the increase of all other stroke aetiologies (large vessel disease, lacunar, cardioembolic) and stroke risk factors with age.14,15 Apart from age differences, racial (Asian versus Caucasian) and gender (female versus males) disparities across included studies could reflect imbalances in stroke subtypes and stroke mechanisms,16–18 and thus be partially responsible for the observed differences in the reported VAH prevalence rates.
Even though it has been reported that VAH can be reliably diagnosed and categorised on cervical MRI scans,19 the differentiation of VAH from both stenosis20,21 and occlusion22 still remains challenging. Moreover, taking also into account that contrast-enhanced magnetic resonance angiography has been associated with an increased rate of false positive results in the detection of VA stenosis23 and that CTA has been reported to have a higher sensitivity and positive predictive value than time-of-flight MRA for the detection of intracranial vessel stenosis and occlusion,24 the use of different imaging modalities in included studies and the lack of information on the time interval from stroke onset to vessel imaging for most study protocols (Table 1) should be regarded as significant limitations of the present meta-analysis and another potential source of heterogeneity on the reported prevalence rates of VAH. It is therefore evident that as all flow-dependent imaging studies bare a high risk of false positive results, the imaging has to be evaluated by a specialist to confirm the diagnosis of VAH. Although cerebrovascular ultrasound was used for the definition of VAH in only one of the included studies,10 the ultrasound’s flow profile could be used as an initial screening tool to differentiate between stenosis and hypoplasia,25 with other imaging modalities (CTA, MRA, DSA) confirming the final diagnosis at a later stage.
Finally, even though VAH is one of the most common congenital vascular variations there is still no standard definition.2 In the included studies, the threshold of VA diameter for the definition of VAH ranged from 2 to 3 mm (Table 1) and an additional criterion of VA asymmetry diameter ratio of more than 1:1.7 was applied in three out of eight studies.10,11,13 The disparities in VAH definition could therefore be at least partially responsible for the heterogeneity across included studies and for the strikingly high prevalence of VAH reported in one of the study protocols.7
The association between VAH and PCI has also been consistently highlighted in all included studies, except from one,11 suggesting thus a potentially increased risk of PCI in patients with VAH. Even though Sauer et al.11 reported that they found no clear evidence of a causal relationship between VAH and cerebral ischaemia, they reported that in their cohort VAH was associated with younger age (p = 0.037), stroke localisation in posterior circulation (p = 0.009) and with cerebrovascular ischemic events of ‘undetermined’ aetiology (p = 0.042, although non-significant after Bonferroni correction).11 Therefore, the only negative study so far on the association of VAH and PCI if interpreted from a different point of view might further enhance the possibility of a causal association, rather than reject it.
Apart from the included cohort studies, several imaging study protocols have also independently reported that VAH can predispose to posterior circulation regional hypoperfusion,26,27 and that when combined other risk factors can result in the clinical manifestation of PCI.28 This theory suggests that normally when blood flow on the one vertebral artery is temporarily reduced, the flow on the opposite vertebral artery is compensatory augmented to provide sufficient flow in the basilar artery, but in severe VAH the blood flow is reduced to a greater degree than the contralateral vertebral artery can reverse resulting thus in unbalanced haemodynamics and in inadequate blood supply to the brain.29
Small diameter arteries have also been reported to be more vulnerable to stenosis or occlusion, as its low flow velocity predisposes to prothrombotic or atherosclerotic processes in the presence of conventional vascular risk factors,30 and therefore PCI may occur as a result of artery-to-artery embolism from the low-flowed stenotic VA.31 VAH has also been associated with an increased risk of ipsilateral VA dissection compared to the normal counterpart, providing thus another possible stroke pathomechanism of artery-to-artery embolism from the hypoplastic vessel,32 while the increased vessel diameter of the contralateral to the hypoplastic VA could provide a route prone to the transfer of cardiac emboli due to its low resistance and increased blood flow.33
According to the aforementioned data, we consider that all patients with PCI and VAH should undergo a comprehensive diagnostic stroke workup to exclude both other VA abnormalities (dissection, stenosis or occlusion) and occult cardioembolism.34–36 Since no data are available to date on either the primary or secondary stroke prevention of patients with VAH, VAH patients with PCI and no other evident stroke aetiology should be treated according to the current guidelines for cryptogenic stroke.37,38
In conclusion, we considered that our meta-analysis of available retrospective cohort studies indicates a possible association between VAH and cryptogenic PCI. As only retrospective data are available to date, this association undoubtedly deserves further investigation in future prospective study protocols not only to elucidate further the potential causality but also to provide invaluable data on the underlying stroke mechanisms and potential therapeutic approaches.
Supplementary Material
Acknowledgements
None declared.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Not applicable (the present report is a review of already published studies).
Guarantor
AHK.
Contributorship
AHK and SG researched literature and conceived the study. AHK and SG wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript
References
- 1.Yamamoto Y, Georgiadis AL, Chang HM, et al. Posterior cerebral artery territory infarcts in the New England Medical Center Posterior Circulation Registry. Arch Neurol 1999; 56: 824–832. [DOI] [PubMed] [Google Scholar]
- 2.Katsanos AH, Kosmidou M, Kyritsis AP, et al. Is vertebral artery hypoplasia a predisposing factor for posterior circulation cerebral ischemic events? A comprehensive review. Eur Neurol 2013; 70: 78–83. [DOI] [PubMed] [Google Scholar]
- 3.Giannopoulos S, Markoula S, Kosmidou M, et al. Lateral medullary ischaemic events in young adults with hypoplastic vertebral artery. J Neurol Neurosurg Psychiatry 2007; 78: 987–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsanos AH, Kosmidou M, Giannopoulos S. Vertebral artery hypoplasia in posterior circulation cerebral ischemia. Clin Neurol Neurosurg 2013; 115: 1194–1195. [DOI] [PubMed] [Google Scholar]
- 5.Deeks JJ, Higgins JP and Altman DG. Chapter 9: analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions, http://handbook.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm (March 2011, accessed 4 February 2014).
- 6.Chuang YM, Huang YC, Hu HH, et al. Toward a further elucidation: role of vertebral artery hypoplasia in acute ischemic stroke. Eur Neurol 2006; 55: 193–197. [DOI] [PubMed] [Google Scholar]
- 7.Gaigalaite V, Vilimas A, Ozeraitiene V, et al. Association between vertebral artery hypoplasia and posterior circulation stroke. BMC Neurol 2016; 16: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu XY, Li ZX, Liu HQ, et al. Relationship between vertebral artery hypoplasia and posterior circulation stroke in Chinese patients. Neuroradiology 2013; 55: 291–295. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Kim JM, Roh JK. Hypoplastic vertebral artery: frequency and associations with ischaemic stroke territory. J Neurol Neurosurg Psychiatry 2007; 78: 954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perren F, Poglia D, Landis T, et al. Vertebral artery hypoplasia: a predisposing factor for posterior circulation stroke? Neurology 2007; 68: 65–67. [DOI] [PubMed] [Google Scholar]
- 11.Sauer T, Wolf ME, Ebert AD, et al. Vertebral artery hypoplasia does not influence lesion size and clinical severity in acute ischemic stroke. J Stroke Cerebrovasc Dis 2016; 25: 1770–1775. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Shen Z, Wen H, et al. The effect of vertebral artery hypoplasia in posterior circulation infarction in young patients. Int J Neurosci. 2016; 126: 1092–1096. [DOI] [PubMed]
- 13.Zhang DP, Ma QK, Zhang JW, et al. Vertebral artery hypoplasia, posterior circulation infarction and relative hypoperfusion detected by perfusion magnetic resonance imaging semiquantitatively. J Neurol Sci 2016; 368: 41–46. [DOI] [PubMed] [Google Scholar]
- 14.Yesilot Barlas N, Putaala J, Waje-Andreassen U, et al. Etiology of first-ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol 2013; 20: 1431–1439. [DOI] [PubMed] [Google Scholar]
- 15.Chatzikonstantinou A, Wolf ME, Hennerici MG. Ischemic stroke in young adults: classification and risk factors. J Neurol 2012; 259: 653–659. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez J, Koch S, Dong C, et al. Racial and ethnic disparities in stroke subtypes: a multiethnic sample of patients with stroke. Neurol Sci 2014; 35: 577–582. [DOI] [PubMed] [Google Scholar]
- 17.Sen S, Dahlberg K, Case A, et al. Racial-ethnic differences in stroke risk factors and subtypes: results of a prospective hospital-based registry. Int J Neurosci 2013; 123: 568–574. [DOI] [PubMed] [Google Scholar]
- 18.Hajat C, Heuschmann PU, Coshall C, et al. Incidence of aetiological subtypes of stroke in a multi-ethnic population based study: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2011; 82: 527–533. [DOI] [PubMed] [Google Scholar]
- 19.Peterson C, Phillips L, Linden A, et al. Vertebral artery hypoplasia: prevalence and reliability of identifying and grading its severity on magnetic resonance imaging scans. J Manipulative Physiol Ther 2010; 33: 207–211. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XJ, Wang W, Du B, et al. Wall imaging for unilateral intracranial vertebral artery hypoplasia with three-dimensional high-isotropic resolution magnetic resonance images. Chin Med J (Engl) 2015; 128: 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariani LL, Klein I, Pico F. Hypoplasia or stenosis: usefulness of high-resolution MRI. Rev Neurol (Paris) 2011; 167: 619–621. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi S, Wada K, Nagatani K, et al. Differentiation between vertebral artery hypoplasia and occlusion. Intern Med 2012; 51: 345. [DOI] [PubMed] [Google Scholar]
- 23.Choi HS, Kim DI, Kim DJ, et al. Accuracy of 3 T MR angioraphy in vertebral artery stenosis and coincidence with other cerebrovascular stenoses. Neuroradiology 2010; 52: 893–898. [DOI] [PubMed] [Google Scholar]
- 24.Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol 2005; 26: 1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 25.Jeng JS, Yip PK. Evaluation of vertebral artery hypoplasia and asymmetry by color-coded duplex ultrasonography. Ultrasound Med Biol 2004; 30: 605–609. [DOI] [PubMed] [Google Scholar]
- 26.Thierfelder KM, Baumann AB, Sommer WH, et al. Vertebral artery hypoplasia: frequency and effect on cerebellar blood flow characteristics. Stroke 2014; 45: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 27.Chen YY, Chao AC, Hsu HY, et al. Vertebral artery hypoplasia is associated with a decrease in net vertebral flow volume. Ultrasound Med Biol 2010; 36: 38–43. [DOI] [PubMed] [Google Scholar]
- 28.Szárazová AS, Bartels E, Bartels S, et al. Possible morphological pathomechanisms of ischemic stroke in the posterior circulation of patients with vertebral artery hypoplasia. J Neuroimaging 2015; 25: 408–414. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Cai A, Liu L. Sonographic diagnosis of congenital variations of the extracranial vertebral artery and assessment of its circulation. J Ultrasound Med 2009; 28: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 30.Caplan LR, Baker R. Extracranial occlusive vascular disease: does size matter? Stroke 1980; 11: 63–66. [DOI] [PubMed] [Google Scholar]
- 31.Caplan LR. Arterial occlusions: does size matter? J Neurol Neurosurg Psychiatry 2007; 78: 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou M, Zheng H, Gong S, et al. Vertebral artery hypoplasia and vertebral artery dissection: a hospital-based cohort study. Neurology 2015; 84: 818–824. [DOI] [PubMed] [Google Scholar]
- 33.Szárazová AS, Bartels E, Bartels S, et al. Possible morphological pathomechanisms of ischemic stroke in the posterior circulation of patients with vertebral artery hypoplasia. J Neuroimaging 2015; 25: 408–414. [DOI] [PubMed] [Google Scholar]
- 34.Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis 2013; 36: 1–5. [DOI] [PubMed] [Google Scholar]
- 35.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014; 13: 429–438. [DOI] [PubMed] [Google Scholar]
- 36.Katsanos AH, Bhole R, Frogoudaki A, et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology 2016; 87: 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 38.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.