Abstract
Introduction
Thrombolysis usage in ischaemic stroke varies across sites. Divergent advice from professional guidelines and product labels may contribute.
Patients and methods
We analysed SITS-International registry patients enrolled January 2010 through June 2016. We grouped sites into organisational tertiles by number of patients arriving ≤2.5 h and treated ≤3 h, percentage arriving ≤2.5 h and treated ≤3 h, and numbers treated ≤3 h. We assigned scores of 1–3 (lower/middle/upper) per variable and 2 for onsite thrombectomy. We classified sites as lower efficiency (summed scores 3–5), medium efficiency (6–8) or higher efficiency (9–11). Sites were also grouped by adherence with European product label and ESO guideline: ‘label adherent’ (>95% on-label), ‘guideline adherent’ (≥5% off-label, ≥95% on-guideline) or ‘guideline non-adherent’ (>5% off-guideline). We cross-tabulated site-efficiency and adherence. We estimated the potential benefit of universally selecting by ESO guidance, using onset-to-treatment time-specific numbers needed to treat for day 90 mRS 0–1.
Results
A total of 56,689 patients at 597 sites were included: 163 sites were higher efficiency, 204 medium efficiency and 230 lower efficiency. Fifty-six sites were ‘label adherent’, 204 ‘guideline adherent’ and 337 ‘guideline non-adherent’. There were strong associations between site-efficiency and adherence (P < 0.001). Almost all ‘label adherent’ sites (55, 98%) were lower efficiency. If all patients were treated by ESO guidelines, an additional 17,031 would receive alteplase, which translates into 1922 more patients with favourable three-month outcomes.
Discussion
Adherence with product labels is highest in lower efficiency sites. Closer alignment with professional guidelines would increase patients treated and favourable outcomes.
Conclusion
Product labels should be revised to allow treatment of patients ≤4.5 h from onset and aged ≥80 years.
Keywords: Alteplase, product label, professional guideline, thrombolysis
Background
Thrombolysis with intravenous recombinant tissue plasminogen activator (IV rt-PA) (alteplase; Actilyse or Activase) is effective and safe for patients with acute ischaemic stroke, and yet only a fraction of patients receive treatment.1–9 The product labels for IV rt-PA in Europe (EU) and the United States (US) are derived from early randomised controlled trials, which excluded important groups.10 The EU label restricts treatment to patients under 80 years, whilst the US label excludes patients greater than 3 h from symptom onset. IV rt-PA is effective and safe within 4.5 h of symptom onset,11–13 and there is clear treatment benefit in the elderly.9,14–18 Professional guidance from the European Stroke Organisation (ESO) and American Stroke Association (ASA) better reflects the evidence base for alteplase.19–21 The ESO recommend treatment within 4.5 h with no age limit, whilst ASA guidance in 2013 excluded patients aged >80 years beyond 3 h, although an update in 2016 acknowledged alteplase is effective within 4.5 h in the elderly.9,19–21 Thus, patients are often treated off-label,22 although this practice is not permitted in many countries and the current product labels therefore restrict the number of patients who can be treated.1,22
We aimed to assess variation in the use of IV rt-PA within the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Registry (SITS-ISTR), in relation to the principal criteria that differ between regional product labels and professional guidelines. Our objective was to assess whether centres’ expertise, measured in terms of efficient patient throughput and treatment logistics, is associated with closer adherence to the EU/US drug labels and professional guidelines and to estimate the potential impact on treatment rates and clinical outcomes if there were greater alignment of the product labels and professional guidelines. We hypothesised that centres which achieve excellent treatment logistics will adhere more closely with professional guidelines rather than strictly observing the product label for IV alteplase.
Methods
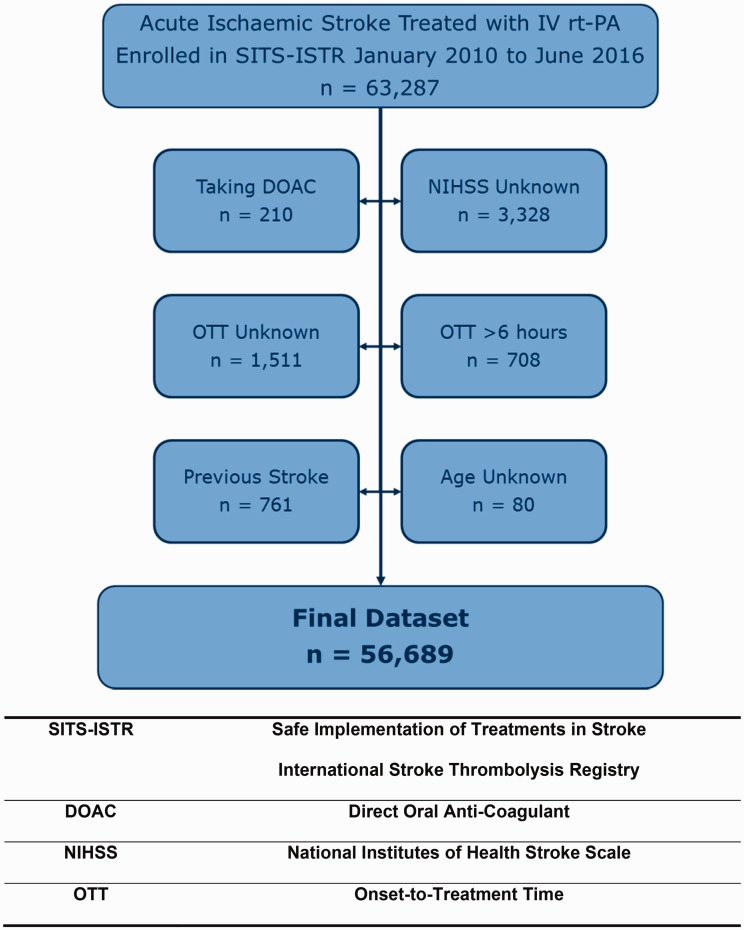
We conducted a retrospective analysis on individual patient data obtained from the SITS-ISTR between January 2010 and June 2016 (Figure 1). SITS-ISTR is a multinational open registry of patients with acute ischaemic stroke who received IV rt-PA.23 Patients from 597 participating centres were included, who had complete information on treating hospital and country, age, gender, onset-to-treatment time (OTT), total National Institutes of Health Stroke Scale (NIHSS), history of diabetes and stroke. We excluded patients on direct oral anticoagulants or with OTT recorded as >6 h. Baseline characteristics included data on pre-stroke modified Rankin Scale (mRS), medical history and medications. We also gathered data for onsite use of thrombectomy.
Figure 1.
Selection process of the study population.
We grouped sites according to their ‘selection adherence’ of alteplase use: label adherent (>95% of patients treated within label), guideline adherent (≥5% of patients treated off-label but ≥95% of patients treated within guideline) or guideline non-adherent (>5% of patients treated off-guideline). We assessed site quality using a tertile-based scoring algorithm. Sites were grouped into tertiles according to (i) the volume of patients arriving within 2.5 h and treated within 3 h, (ii) the percentage of patients arriving within 2.5 h and treated within 3 h, and (iii) the volume of patients treated within 3 h of stroke onset. We assigned sites a score for each variable: 1 point if the site was within the lower tertile, 2 points for the middle tertile or 3 points for the upper tertile. An additional 2 points were allocated for onsite use of thrombectomy. This resulted in a total score between 3 and 11 for each site. We classified sites with scores of 3 to 5 as ‘lower efficiency’, 6 to 8 as ‘medium efficiency’ and 9 to 11 as ‘higher efficiency’. We tested associations between site efficiency and selection adherence of alteplase use by cross-tabulation and Chi-squared analyses performed in SPSS version 22.0, with a significance level of 5%.
We estimated the potential for clinical benefit if treatment for all patients within our cohort was by the professional guideline versus product label for alteplase. We performed this analysis by applying guideline criteria for treatment with IV rt-PA to our entire cohort, and compared this to the number of patients who would have been treated if the product label criteria were applied. We conducted separate analyses for both European (ESO guideline and EU label) and American (ASA guideline and FDA label) criteria applied to the entire dataset. We calculated the number of patients for whom treatment would have been contraindicated by the product label but recommended by professional guideline. We stratified such patients according to OTT: within 90 min, 91 to 180 min or 181 to 270 min. We used OTT-specific numbers needed to treat (NNT) for a day 90-modified Rankin Scale (mRS) of 0–1 to estimate the number of additional patients who would achieve a favourable outcome if treatment was provided universally by professional guideline rather than by product label (NNT of 4.5 if OTT was 0 to 90 min, NNT of 9.0 if OTT 91 to 180 min and NNT of 14.1 if OTT 181 to 270 min). We divided the number of additional patients who would be treated within each time window by the corresponding NNT to estimate the number of patients who would achieve a favourable outcome.
Results
We analysed data from 56,689 patients treated at 597 sites during the study period. Baseline characteristics of the patients are shown in Table 1. By our predefined criteria, 163 sites (27%) were classified as ‘higher efficiency’, 204 sites (34%) as ‘medium efficiency’ and 230 sites (39%) as ‘lower efficiency’.
Table 1.
Baseline characteristics of the cohort.
| Characteristic | Measure | Entire cohort N = 56,689 |
|---|---|---|
| Age (years) | Mean (SD) | 69.8 (13.1) |
| Sex (male) | n (%) | 30,969 (55%) |
| Baseline NIHSS | Median (IQR) | 10 (6–16) |
| Onset-to-treatment time (min) | Median (IQR) | 151 (118–195) |
| Atrial fibrillation | n (%) | 11,947 (21%) |
| Hypertension | n (%) | 37,641 (66%) |
| Diabetes mellitus | n (%) | 10,604 (19%) |
| Hypercholesterolaemia | n (%) | 16,455 (29%) |
| Heart failure | n (%) | 4702 (8%) |
| Smoker | n (%) | 9347 (17%) |
| Previous stroke or TIA | n (%) | 9067 (16%) |
IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale; TIA: transient ischaemic attack.
When analysing selection adherence across all sites by the EU product label and ESO guideline, we found that 56 sites (9%) were label adherent, 204 sites (34%) were guideline adherent and 337 sites (56%) were guideline non-adherent. Site efficiency was strongly associated with selection adherence by European criteria (p < 0.001) (Figure 2). Among the 56 label-adherent sites, 55 (98%) were lower efficiency and one only (2%) was medium efficiency. Of the 204 guideline-adherent sites, 92 (45%) were lower efficiency, 75 (37%) were medium efficiency and 37 (18%) were higher efficiency. Among the 337 guideline non-adherent sites, 126 (37%) were higher efficiency, 128 (38%) were medium efficiency and 83 (25%) were lower efficiency. When we judged use in our mainly European dataset against US product label and ASA guideline criteria, a similar pattern emerged except that guideline non-adherence rose (see online Appendix).
Figure 2.
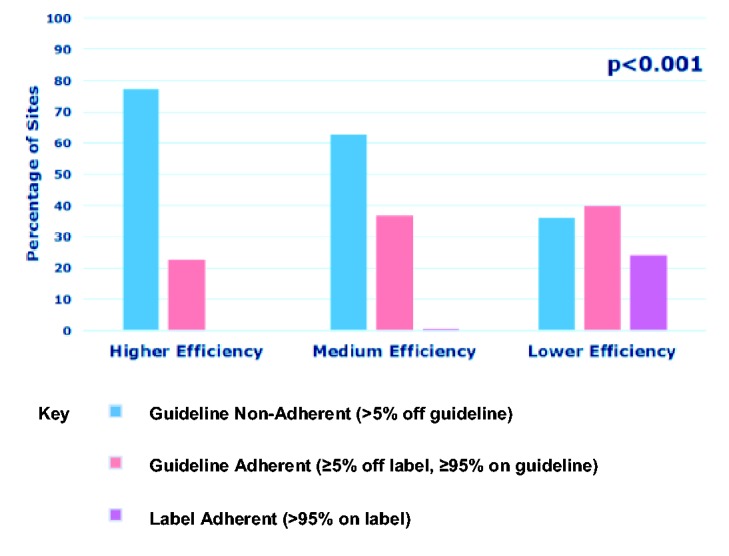
Site efficiency and selection adherence with EU Product Label and ESO Guideline.
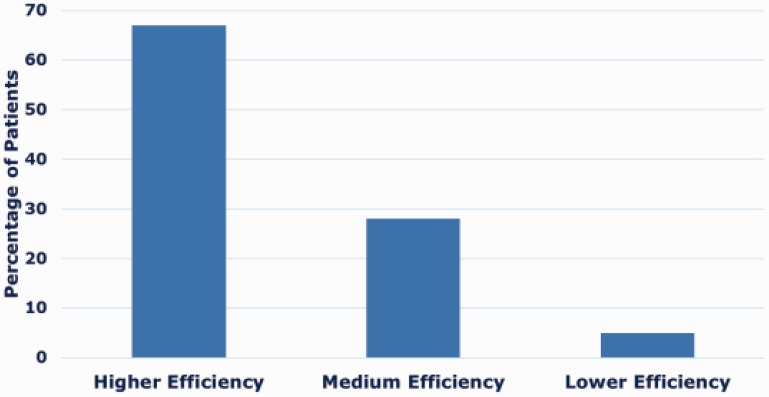
IV rt-PA was administered to 5770 patients (10%) beyond European guideline recommendations. This was due to patients treated with a BP greater than guideline recommendations in 4618 patients (8%), an OTT greater than 4.5 h in 1047 patients (2%) and a combination of elevated BP with an OTT greater than 4.5 h in 105 patients (0.2%). Among the 5770 patients administered IV rt-PA beyond European guideline recommendations, 3845 (67%) were treated in a higher efficiency site, 1644 (28%) in a medium efficiency site and 281 (5%) in a lower efficiency site (Figure 3).
Figure 3.
The percentages of patients treated off-guideline grouped by site efficiency, according to ESO guideline criteria (n = 5770).
Within our cohort, 50,919 patients (90%) would receive thrombolysis if treatment was universally delivered by the ESO guideline, compared to 33,888 patients (60%) by the European product label. Thus, an additional 17,031 patients (30%) would receive thrombolysis if treatment was universally delivered according to ESO guidance. This translates into 1922 patients who would achieve a favourable outcome when measured by OTT-specific NNT for a day 90 mRS of 0–1 (Figure 4).
Figure 4.
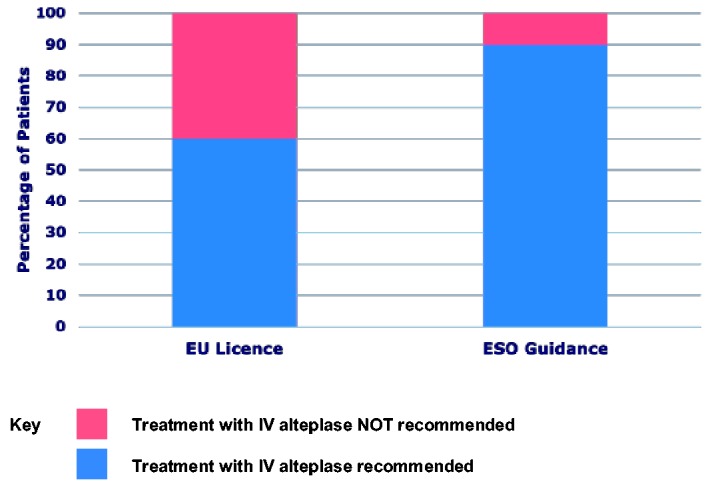
The percentage of patients who would be treated if the decision was based on ESO guidance versus EU product label: an additional 17,031 patients (30%) would be treated if the decision was based on ESO guidance, which translates into 1922 patients achieving favourable outcomes when estimated using OTT-specific NNT for a day 90 mRS of 0–1.
Discussion
We have demonstrated that strict adherence with the product label for IV rt-PA is greatest in sites that treat lower volumes of patients, have fewer facilities or achieve less impressive in-hospital timelines. Strict adherence with the product label restricts use of IV rt-PA, reducing the number of patients who can be treated and, by implication, who may achieve favourable outcomes. If treatment decisions within our cohort were based on ESO guidelines rather than the European drug label, an additional 2620 patients would be treated annually across the 6.5 years studied. This translates into an additional 296 patients each year with favourable outcomes. Evidence supporting the selection criteria described in the ESO and ASA professional guidelines is robust, and the conclusions of these organisations agree on all major points.19–21 The drug product labels for alteplase require review in both Europe and America, to reflect evidence highlighting the efficacy and safety of IV rt-PA in circumstances that were originally considered contraindications for thrombolysis.1,24,25 The key issue is that these labels, which simply control marketing activities and not prescribing per se, should permit the manufacturers to discuss and educate clinicians on the safe treatment of patients within 4.5 h of symptom onset or aged over 80 years. Revising the European and American product labels to this effect would deliver clinical outcomes consistent with those obtained when treating within the current drug labels, with no adverse effect on mortality.26 Alignment of educational messages is desirable and should be conveyed among the medical community.9
Our data demonstrate that less efficient sites have the lowest rates of treatment with alteplase off-label, which may in part be attributable to less developed regions being unable to treat off-label.27 This is consistent with findings from a previous study using SITS-ISTR data, which demonstrated that higher volume centres have the greatest rates of treatment with alteplase off-label.28 Improving the quality of treatment for every patient with acute stroke is a priority of the ESO and World Stroke Organisation (WSO), with the Angels Initiative recently introduced to help achieve this goal. Education of clinicians and revision of the product labels for alteplase will help our effort to deliver excellent care for patients with acute ischaemic stroke worldwide.
It is concerning that we observed high rates of treatment with alteplase beyond professional guidelines. Off-guideline treatment was administered to 10% of patients by European criteria, which was driven by treatment above BP recommendations and beyond 4.5 h. Treatment with alteplase off-guideline exposes patients to an increased risk of mortality that is not offset by potential for clinic benefit26 and clinicians should avoid this practice. Violations of pre-treatment BP parameters are associated with an increased risk of bleeding, and BP should be controlled before treatment with IV rt-PA to reduce the risk of symptomatic intracerebral haemorrhage.24,25 Most off-guideline treatments were in higher efficiency sites, and programmes discouraging this approach should include all the stroke community.
We designed a measure of site efficiency that acts as a marker of site quality. We allocated points for efficient treatment logistics, the volume and proportion of patients treated promptly and onsite use of thrombectomy. Our aim was to stratify sites according to treatment logistics, patient volume and delivery of comprehensive acute stroke care. Various indicators can be used to assess quality of acute stroke unit care,29 although not all of these data are available within SITS-ISTR. Our measure of site quality is arbitrary and uses objective information available within SITS-ISTR defined before we accessed the data, which is thus a weakness of our study. The criterion for site quality includes measures derived mainly from OTT and volume of patients, which may disadvantage centres with longer out-of-hospital transportation logistics and smaller sites. We defined BP based on that recorded at baseline within the SITS registry and cannot be certain that BP was not lowered prior to thrombolysis, which is a limitation.
A further limitation is the retrospective and observational design, although the large volume and accuracy of data collected within SITS-ISTR allow for robust statistical analyses.23 SITS-ISTR is a predominantly European cohort which is important when considering the generalisability of our findings. Patients managed outside Europe are often in countries with less experienced centres, and our results are relevant to these regions. Finally, SITS-ISTR includes patients voluntarily registered by participating centres which could contribute to selection bias, although data from SITS are robust and have been used in similar studies.14,23,28
Conclusion
We confirmed that strict adherence with the more restrictive product label for alteplase was concentrated among the least active or efficient hospitals, whereas more experienced sites offer treatment based on professional guideline criteria. However, we found that the busiest and most efficient sites are treating beyond even the professional guidelines, potentially exposing these patients to a risk of increased mortality that is not offset by potential for clinical benefit. We conclude that review and alignment of the marketing approvals for alteplase in acute ischaemic stroke with the current recommendations of the professional guidelines, to allow treatment of patients ≤4.5 h from onset and aged ≥80 years, should be coupled with enhanced education to operate within those guidelines to maximise the population safety and effectiveness of thrombolysis for stroke.
Supplementary Material
Acknowledgements
We would like to thank the Safe Implementation of Treatments in Stroke (SITS) Registry and Scientific Committee for their assistance with this research.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KRL is a member of the Stroke Thrombolysis Trialists’ Collaboration that has published pooled individual patient data analyses of the effects of rt-PA in acute ischaemic stroke; a member of the Scientific Committee of SITS; is Past President of the European Stroke Organisation that publishes guidelines on stroke management and coordinates the ESO-Angels project; and has received fees and expenses for data monitoring committees from Boehringer Ingelheim. WH reports no current conflicts. He declares that he served at the SCs of the ECASS 1–4 trials and has been compensated for work in the SCs and for lectures in the past. NA is the Vice Chairman and MM is a Researcher at SITS International, which receives an unrestricted grant from Boehringer Ingelheim for the SITS-International Stroke Thrombolysis Register.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SITS (Safe Implementation of Treatments in Stroke) is financed directly and indirectly by grants from Karolinska Institutet, Stockholm County Council, the Swedish Heart-Lung Foundation, the Swedish Order of St. John, Friends of Karolinska Institutet, and private donors, as well as from an unrestricted sponsorship from Boehringer-Ingelheim. SITS has previously received grants from the European Union Framework 7, the European Union Public Health Authority and Ferrer International. SITS is currently conducting studies supported by Boehringer-Ingelheim and EVER Pharma, as well as in collaboration with Karolinska Institutet, supported by Stryker, Covidien and Phenox. N Ahmed is supported by grants provided by the Stockholm County Council and the Swedish Heart-Lung Foundation. RM has been supported by the project no. LQ1605 from the National Program of Sustainability II (MEYS CR) and by the project FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 (OP VaVpI).
Informed consent
Not applicable.
Ethical approval
Ethical approval for this study was obtained from the Safe Implementation of Treatments in Stroke (SITS) Scientific Committee.
Guarantor
KRL.
Contributorship
KRL, ACC, JB and AHAR researched literature and conceived the study. KRL, ACC, JB and AHAR were involved in protocol development, gaining SITS Scientific Committee approval and data analysis. ACC, JB and AHAR wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Meretoja A, Putaala J, Tatlisumak T, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke 2010; 41: 1450–1458. [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1588. [DOI] [PubMed]
- 3.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Zoppo G, Yamaguchi T, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2003; 12: CD000213. [DOI] [PubMed] [Google Scholar]
- 6.Meretoja A, Tatlisumak T. Thrombolytic therapy in acute ischemic stroke – basic concepts. Curr Vasc Pharmacol 2006; 4: 31–44. [DOI] [PubMed] [Google Scholar]
- 7.Meretoja A, Tatlisumak T. Novel thrombolytic drugs: will they make a difference in the treatment of ischaemic stroke? CNS Drugs 2008; 22: 619–629. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher HC, Bateman BT, Boden-Albala B, et al. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med 2007; 50: 99–107. [DOI] [PubMed] [Google Scholar]
- 9.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals From the American Heart Association/American Stroke Association. Stroke 2016; 47: 581–641. [DOI] [PubMed] [Google Scholar]
- 10.Gumbinger C, Reuter B, Stock C, et al. Time to treatment with recombinant tissue plasminogen activator and outcome of stroke in clinical practice: retrospective analysis of hospital quality assurance data with comparison with results from randomised clinical trials. BMJ 2014; 348: g3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 12.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 372: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 13.Lees KR, Bluhmki E, Kummer von R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 14.Mishra NK, Ahmed N, Andersen G, et al. Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive. BMJ 2010; 341: c6046–c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karliński M, Kobayashi A, Litwin T, et al. Intravenous thrombolysis for acute ischaemic stroke in patients not fully adhering to the European licence in Poland. Neurol Neurochir Pol 2014; 46: 3–14. [DOI] [PubMed] [Google Scholar]
- 16.Ford GA, Ahmed N, Azevedo E, et al. Intravenous alteplase for stroke in those older than 80 years old. Stroke 2010; 41: 2568–2574. [DOI] [PubMed] [Google Scholar]
- 17.Meseguer E, Labreuche J, Olivot JM, et al. Determinants of outcome and safety of intravenous rt-PA therapy in the very old: a clinical registry study and systematic review. Age Ageing 2008; 37: 107–111. [DOI] [PubMed] [Google Scholar]
- 18.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients of > or =80 versus <80 years of age – a systematic review across cohort studies. Age Ageing 2006; 35: 572–580. [DOI] [PubMed] [Google Scholar]
- 19.European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed]
- 20.ESO Guideline Update (Abstract) – January 2009, European Stroke Organisation. www.congrex-switzerland.com/fileadmin/files/2013/eso-stroke/pdf/ESO_Guideline_Update_Jan_2009.pdf (accessed 28 August 2017).
- 21.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 22.Cappellari M, Moretto G, Micheletti N, et al. Off-label thrombolysis versus full adherence to the current European Alteplase license: impact on early clinical outcomes after acute ischemic stroke. J Thromb Thrombolysis 2013; 37: 549–556. [DOI] [PubMed] [Google Scholar]
- 23.Scheitz JF, Abdul-Rahim AH, MacIsaac RL, et al. Clinical selection strategies to identify ischemic stroke patients with large anterior vessel occlusion. Stroke 2017; 48: 290–297. [DOI] [PubMed] [Google Scholar]
- 24.Tsivgoulis G, Safouris A, Alexandrov AV. Safety of intravenous thrombolysis for acute ischemic stroke in specific conditions. Expert Opin Drug Saf 2015; 14: 845–864. [DOI] [PubMed] [Google Scholar]
- 25.Frank B, Grotta JC, Alexandrov AV, et al. Thrombolysis in stroke despite contraindications or warnings? Stroke 2013; 44: 727–733. [DOI] [PubMed] [Google Scholar]
- 26.Hacke W, Lyden P, Emberson J, et al. Effects of alteplase for acute stroke according to criteria defining the European Union and United States marketing authorizations: Individual-patient-data meta-analysis of randomized trials. Int J Stroke. Epub ahead of print 2017. doi: 10.1177/1747493017744464. [DOI] [PubMed]
- 27.Nomani AZ, Nabi S, Badshah M, et al. Review of acute ischaemic stroke in Pakistan: progress in management and future perspectives. Stroke Vasc Neurol 2017; 2: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anani N, Mazya MV, Bill O, et al. Changes in European Label and guideline adherence after updated recommendations for stroke thrombolysis: results from the safe implementation of treatments in stroke registry. Circ Cardiovasc Qual Outcomes 2015; 8: S155–S162. [DOI] [PubMed] [Google Scholar]
- 29.Wiedmann S, Norrving B, Nowe T, et al. Variations in quality indicators of acute stroke care in 6 European countries. Stroke 2012; 43: 458–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.