Abstract
We report a rare presentation of metastatic renal cell carcinoma (RCC) in a 71-year-old man who presented with persistent shoulder pain. MRI revealed widespread lytic lesions within the bones suggestive of metastatic disease but extensive imaging including CT chest, abdomen and pelvis with contrast and fluorodeoxyglucose-positron emission tomography did not identify a primary cancer. The diagnosis was ultimately made from a targeted bone and subsequently targeted liver biopsy, whereby immunohistochemistry was consistent with metastatic RCC (mRCC). While bone metastases in RCC are very common, it is extremely rare for patients to present with mRCC and no identifiable renal primary.
Keywords: urological cancer, pathology
Background
The differential diagnosis for a patient presenting with bone metastases is broad. When a primary carcinoma is readily identified, bone metastases most commonly originate from prostate (34%), breast (22%) and lung (20%) carcinoma.1 Despite the bone being the second most common site of metastatic spread in patients with renal cell carcinoma (RCC), it is extremely rare for RCC to present with bone metastases without evidence of a primary renal tumour.2
In patients whose initial presentation of a cancer diagnosis is from symptomatic metastases, the clinical history, examination, laboratory tests, noninvasive and invasive investigations are usually tailored to finding the primary tumour, since ultimately determining the primary will dictate future treatment options.
The main reason for presenting this case is to highlight the fact that patients presenting with metastatic disease in the absence of an unknown primary, if fit for investigation and treatment, should:
Have focused investigations.
Be spared investigations that are unlikely to progress the case.
Have tissue diagnosis expedited.
In this patient’s case despite having multiple investigations over a 10-month period, ultimately it was the targeted bone and liver biopsies that provided the diagnosis.
Case presentation
A 71-year-old man initially presented with right shoulder and chest wall pain. Following attendance at his local accident and emergency department, a diagnosis of costochondritis was made, and a prescription for analgesia given. Three months later, however, due to persistent pain, he visited his general practitioner who organised an MRI of the spine which revealed multiple, lytic vertebral lesions suggestive of metastatic disease.
On further questioning, he reported drenching night sweats, unintentional weight loss and malaise. There was no history of haematuria, melaena, gastrointestinal or genitourinary symptoms. He is an ex-smoker with a 30-year pack history but drinks minimal alcohol and no known exposure to cadmium. Medical history includes type 2 diabetes, hypertension and benign prostatic hyperplasia. There is a strong family history of cancer; two sisters and a brother were diagnosed with bowel cancer, another brother, bladder cancer and further, sister, cancer of unknown primary (CUP), all aged 60–70.
Examination revealed sarcopenia but no evidence of clubbing, pallor, icterus or lymphadenopathy. He was euthyroid with no palpable thyroid masses. Respiratory, cardiovascular and breast examinations were normal. There was no organomegaly or masses palpable on abdominal examination, and neurological examination was normal.
Extensive investigations were performed to identify the primary cancer site including multiple radiological modalities but also several invasive investigations (table 1, figure 1).
Table 1.
Summary of patient’s investigations, imaging, serology and invasive procedures
| Imaging/time postinitial MRI investigation | ||
| MRI whole spine | 0 week | Multiple sclerotic and mostly lytic bone lesions suspicious of metastases |
| Nuclear medicine bone scan | 6 weeks | Increased isotype uptake in the pelvis, spine, ribs and right shoulder suggestive of widespread bone metastases |
| CT chest, abdomen and pelvis with contrast | 6 weeks | Multiple metastatic bone lesions. No obvious primary identified. No abnormality within kidneys |
| MRI pelvis/prostate | 9 weeks | No evidence of malignancy within prostate |
| 18F-FDG-PET (figure 1) | 12 weeks | Intense uptake in bony deposits throughout the axial skeleton and proximal long bones. No uptake in major organs or lymph nodes |
| Ultrasound thyroid | 26 weeks | Normal |
| Invasive procedures | ||
| Flexible cystoscopy | 9 weeks | Non-occlusive prostate and no urothelial abnormality |
| Gastroscopy | 11 weeks | Normal |
| Transrectal ultrasound-guided prostate biopsy | 11 weeks | Benign prostate tissue |
| Bone marrow aspirate and trephine | 15 weeks | Appearances are those of carcinoma but immunohistochemistry unhelpful in determining primary |
| CT-guided targeted bone biopsy | 22 weeks | Metastatic carcinoma—immunohistochemistry strongly supports metastasis from renal cell carcinoma |
| Ultrasound-guided targeted liver biopsy | 42 weeks | Metastatic carcinoma—immunoprofile confirms renal cell carcinoma; racemase positive suggests high-grade papillary subtype |
F-FDG-PET, fluorodeoxyglucose F 18-positron emission tomography.
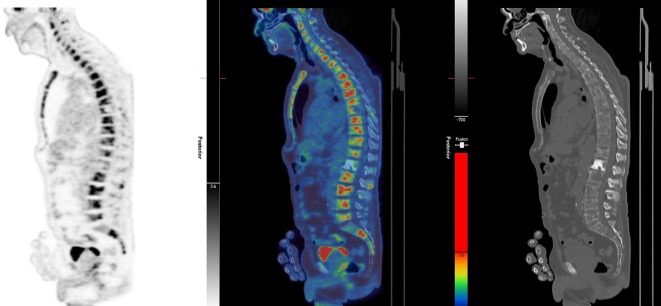
Figure 1.
18F-FDG-PET images demonstrating multiple bone metastases. Maximal intensity projection images demonstrating intense hypermetabolism in the bone metastases and sagittal spine images again demonstrating the multiple bone lesions. 18F-FDG-PET, fluorodeoxyglucose F 18-positron emission tomography.
In particular, due to a previous history of a mildy raised prostate-specific antigen (PSA) and prostatic hypertrophy, he had an MRI prostate and ultrasound-guided prostate biopsies which excluded prostate cancer. The patient subsequently went on to have a bone marrow aspiration and trephine biopsy which was reported as metastatic carcinoma, and the reporting histopathologist commented that the immunohistochemistry had been unhelpful in determining a primary. Five months after the initial MRI spine demonstrating likely bone metastases, the patient went onto have a targeted bone biopsy which was reported as metastatic carcinoma with immunohistochemistry highly suggestive of a renal cell carcinoma (figure 2).
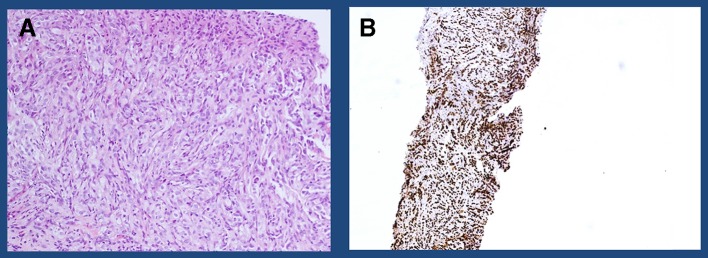
Figure 2.
Immunohistochemical analysis of targeted bone biopsy (A) H&E stain of the tumour composed of nests of epithelioid cells with granular to clear cytoplasm consistent with renal cell carcinoma and (B) PAX 8—positive in 90% of renal cell cancers.
The patient’s case was subsequently discussed in the renal cancer specialist multidisciplinary meeting, and the consensus was to treat as metastatic bone disease from an occult renal cancer.
Differential diagnosis
Bone metastases are not an uncomon presentation of patients with cancer. Cancers that can commonly present as a consequence of symptoms related to bone metastases are:1
Prostate cancer.
Lung cancer.
Breast cancer.
Renal cancer.
Thyroid cancer.
Multiple myeloma.
Outcome and follow-up
The patient commenced treatment with a tyrosine-kinase inhibitor (sunitinib), the current standard of care for metastatic renal cell cancer and had an initial good symptomatic response. However, unfortunately after approximately 6 months of treatment, his CT scans demonstrated new liver metastases. At this stage, in view of the unususal presentation together with the slightly unclear histology from the bone biopsy, the decision was made to carry out a targeted liver biopsy.
The liver biopsy was initially compared with the previous bone marrow and targeted bone biopsies, and importantly all three demonstrated similar morphology suggesting metastases from the same primary tumour. Again, diffuse nuclear PAX8 staining was consistent throughout the tumour in all three biopsies. However, since PAX8 can be seen in other tumours, including thyroid carcinoma and tumours of Mullerian origin, further immunohistochemistry was carried out. RCC, which is a relatively specific marker for renal cell carcinomas, showed patchy positivity in all three biopsies, and furthermore, racemase was also positive which would be expected to be positive in a papillary renal cell carcinoma. Finally, further staining with CK7 and TTF-1 was carried out to exclude a thyroid cancer, and these were negative.
In view of the liver biopsy results, the patient was subsequently switched to a second-line tyrosine kinase inhibitor (axitinib). He tolerated this relatively well but an assessment CT scan after 3 months of treatment demonstrated disease progression. As it stands, he is currently being considered for immunotherapy.
Discussion
We have presented the case of a 70-year old patient presenting with bone metastases in the absence of an obvious primary for two reasons.
The first is to highlight the importance of focused investigations to find the primary tumour. In this case, it allowed the patient to receive molecular-targeted treatment for his metastatic renal cell cancer rather than generic combination chemotherapy which would be the standard of care for CUP. Interestingly, in this particular instance, targeted bone and liver biopsies provided the diagnosis of metastatic RCC (mRCC) despite no radiological evidence of a renal mass. To the best of our knowledge, we are aware of only one other description of such a case worldwide.3 In these cases, the mechanism of metastasis is not clear, but one possibility is that the primary tumour is too small to be accurately located with current imaging modalities.
The second reason for presenting this case is to highlight the number of unnecessary investigations the patient underwent over a 5-month period prior to his targeted bone biopsy, thus highlighting the role of the ‘CUP’ teams. It is recognised that patients presenting with metastatic disease in the absence of an obvious primary often undergo multiple investigations, some unnecessarily, and as a consequence, National Institute for Health and Care Excellence (NICE) published guidance in 2010 entitled ‘Metastatic malignant disease of unknown primary origin in adults: diagnosis and management’.4 In this document, it states that patients fit enough for, and who want, investigation should have them carried out in an efficient manner. It also outlines the core initial tests including CT scan chest, abdomen, pelvis but also recommends, for example, endoscopy only if symptoms warrant this. One could argue that in this patient’s particular case with a normal PSA and negative myeloma screen, once his CT had confirmed bone only disease that the next investigation should have been a targeted bone biopsy. Fortunately, in this case, our patient did not clinically deteriorate during his 5 months of investigations and remained a good enough performance status to receive treatment for mRCC. However, many patients with metastatic disease would have deteriorated during this time and thus potentially have not gone to receive treatment that could have at least improved their quality of life and possibly survival.
The NICE guidance recommends that patients presenting in this manner be referred to the hospital’s CUP team to enable the patient to access, prior to tissue confirmation, an oncology consultant assessment, palliative care input and a clinical nurse specialist to support them through this journey. This team will take responsibility for ensuring patients have appropriate investigations, symptom control, psychological support and information. It would also be the role of the CUP team to ensure prompt diagnosis and onward referral to the correct team for long-term management.
Learning points.
Bone metastases are common and require focused imaging and systematic investigations.
Targeted bone biopsy should be expedited in patients with no identifiable primary carcinoma.
Accurate histological diagnosis influences treatment choices and provides prognostic information required for care planning.
Cancer of unknown primary teams can expedite targeted investigations and coordinate subsequent care with specialist teams.
Footnotes
Contributors: LW wrote the manuscript and researched the patient’s clinical history and literature. RG runs the CUP service for the RFH hospital. She managed this patient, investigated and made the diagnosis as well as edited the final draft. SN provided the nuclear medicine figures and approved the final manuscript. JW was the histopathologist who manged the patient’s case, provided the figures and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open 2017;7:e016022 10.1136/bmjopen-2017-016022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Data were provided by the Office for National Statistics on request, July 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/previousReleases.
- 3. Kumar RM, Aziz T, Jamshaid H, et al. Metastatic renal cell carcinoma without evidence of a primary renal tumour. Curr Oncol 2014;21:521–4. 10.3747/co.21.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute for Clinical Excellence. Metastatic malignant disease of unknown primary origin in adults: diagnosis and management. NICE guideline (CG104), 2010. [PubMed] [Google Scholar]