Abstract
Objective
To assess adoption of World Health Organization (WHO) guidance into national policies for prevention of mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV) and to monitor implementation of guidelines at facility level in rural Malawi, South Africa and the United Republic of Tanzania.
Methods
We summarized national PMTCT policies and WHO guidance for 15 indicators across the cascades of maternal and infant care over 2013–2016. Two survey rounds were conducted (2013–2015 and 2015–2016) in 46 health facilities serving five health and demographic surveillance system populations. We administered structured questionnaires to facility managers to describe service delivery. We report the proportions of facilities implementing each indicator and the frequency and durations of stock-outs of supplies, by site and survey round.
Findings
In all countries, national policies influencing the maternal and infant PMTCT cascade of care aligned with WHO guidelines by 2016; most inter-country policy variations concerned linkage to routine HIV care. The proportion of facilities delivering post-test counselling, same-day antiretroviral therapy (ART) initiation, antenatal care and ART provision in the same building, and Option B+ increased or remained at 100% in all sites. Progress in implementing policies on infant diagnosis and treatment varied across sites. Stock-outs of HIV test kits or antiretroviral drugs in the past year declined overall, but were reported by at least one facility per site in both rounds.
Conclusion
Progress has been made in implementing PMTCT policy in these settings. However, persistent gaps across the infant cascade of care and supply-chain challenges, risk undermining infant HIV elimination goals.
Résumé
Objectif
Évaluer la transposition des recommandations de l'Organisation mondiale de la Santé (OMS) dans les politiques nationales de prévention de la transmission mère-enfant (PTME) du virus de l'immunodéficience humaine (VIH) et contrôler l'application de ces politiques dans les centres de santé de zones rurales d'Afrique du Sud, du Malawi et de République-Unie de Tanzanie.
Méthodes
Nous avons répertorié les politiques nationales de PTME et les recommandations de l'OMS pour 15 indicateurs, sur toute la chaîne de soins de santé de la mère et du nourrisson, sur la période comprise entre 2013 et 2016. Deux séries d'enquêtes ont été réalisées (2013-2015 et 2015-2016) dans 46 centres de santé au service des populations de cinq systèmes de surveillance démographique et de santé. Nous avons interrogé les responsables de ces centres à l'aide de questionnaires directifs afin d'obtenir une description de la prestation des soins. Nous avons calculé la proportion de centres ayant appliqué chaque indicateur ainsi que la fréquence et la durée des ruptures de stock de fournitures, pour chaque zone étudiée et chaque série d'enquêtes.
Résultats
En 2016, dans tous les pays étudiés, les lignes directrices de l'OMS avaient été prises en compte dans les politiques nationales relatives à la chaîne des soins de PTME du VIH; la plupart des différences constatées entre les politiques de ces différents pays concernaient la liaison avec les soins de routine contre le VIH. La proportion des centres offrant des conseils après dépistage, proposant de débuter une thérapie antirétrovirale (TAR) le jour même, fournissant dans un même endroit des soins prénataux et des TAR et appliquant l'Option B+ a augmenté ou est restée à 100% dans toutes les zones étudiées. Les progrès dans l'application des politiques en matière de diagnostic et de traitement du nourrisson ont été variables d'une zone à une autre. Les ruptures de stock de kits de dépistage du VIH ou de médicaments antirétroviraux au cours de l'année précédente ont généralement diminué, mais dans chaque zone, sur les deux périodes étudiées, au moins une structure a été confrontée à ce problème.
Conclusion
Des progrès ont été faits dans l'application des politiques de PTME dans ces régions. Néanmoins, des manquements persistants dans la chaîne de soins de santé du nourrisson et les problèmes des chaînes d'approvisionnement risquent de compromettre l'atteinte des objectifs d'élimination du VIH chez le nourrisson.
Resumen
Objetivo
Evaluar la adopción de las directrices de la Organización Mundial de la Salud (OMS) en las políticas nacionales de prevención de la transmisión del virus de la inmunodeficiencia humana (VIH) de madre a hijo y supervisar la aplicación de las directrices a nivel de las instalaciones sanitarias en las zonas rurales de Malawi, la República Unida de Tanzanía y Sudáfrica.
Métodos
Resumimos las políticas nacionales de PTMI y las directrices de la OMS para 15 indicadores en toda la serie de servicios de atención maternoinfantil durante el período 2013-2016. Se realizaron dos rondas de encuestas (2013-2015 y 2015-2016) en 46 instalaciones sanitarias que atienden a cinco poblaciones del sistema de vigilancia sanitaria y demográfica. Se administraron cuestionarios estructurados a los gestores de las instalaciones para describir la prestación de servicios. Informamos las proporciones de las instalaciones que aplican cada indicador y la frecuencia y duración de la falta de existencias de suministros, por emplazamiento y ronda de encuestas.
Resultados
En todos los países, las políticas nacionales que influyen en la serie de servicios de atención maternoinfantil de la PTMI se ajustaron a las directrices de la OMS para 2016; la mayoría de las variaciones de las políticas entre países se referían a la vinculación con la atención habitual de la infección por el VIH. La proporción de instalaciones que ofrecen asesoramiento posterior a la prueba, iniciación de la terapia antirretrovírica en el mismo día, atención prenatal y suministro de terapia antirretrovírica en el mismo edificio, y la Opción B+ aumentaron o se mantuvieron en el 100 % en todos los emplazamientos. El progreso en la aplicación de las políticas de diagnóstico y tratamiento del lactante varió de un emplazamiento a otro. Las existencias de kits de pruebas del VIH o de medicamentos antirretrovirales se redujeron en general en el último año, pero en ambas rondas se informó de la existencia de al menos una instalación por emplazamiento.
Conclusión
Se ha progresado en la aplicación de la política de PTMI en estos ámbitos. Sin embargo, las persistentes brechas en la serie de servicios de atención infantil y los desafíos de la cadena de suministro pueden socavar los objetivos de eliminación del VIH infantil.
ملخص
الغرض
تقييم اعتماد منظمة الصحة العالمية (WHO) في السياسات الوطنية للوقاية من انتقال العدوى من الأم إلى الطفل (PMTCT) لفيروس نقص المناعة البشرية (HIV) ومراقبة تنفيذ المبادئ التوجيهية على مستوى المرافق في المناطق الريفية في جنوب أفريقيا وجمهورية تنزانيا المتحدة وملاوي.
الطريقة
لقد قمنا بتلخيص السياسات الوطنية للوقاية من انتقال العدوى من الأم إلى الطفل (PMTCT) وتوجيهات منظمة الصحة العالمية من أجل 15 مؤشر عبر سلسلة أجهزة رعاية الأمومة والطفولة خلال الفترة ما بين 2013 و2016. أُجريت جولتا مسح (2013-2015 و2015-2016) في 46 مرفقاً صحياً يخدم خمسة مجتمعات نظام مراقبة صحية وديموغرافية. قمنا بإدارة استبيانات منظمة لمديري المرافق لوصف تقديم الخدمة. وقمنا بالإبلاغ عن نسب التسهيلات المطبقة لكل مؤشر وتكرار ومدد مخزون اللوازم حسب الموقع وجولة المسح.
النتائج
في جميع البلدان، اهتمت السياسات الوطنية التي تؤثر على سلسلة الرعاية للأمهات والرضع للوقاية من انتقال العدوى من الأم إلى الطفل (PMTCT) والمتوافقة مع المبادئ التوجيهية لمنظمة الصحة العالمية بحلول عام 2016؛ ومعظم التغيرات في السياسات بين البلدان أيضًا بالارتباط بالرعاية الروتينية لفيروس نقص المناعة البشرية (HIV). وارتفعت نسبة المرافق التي تقدم المشورة بعد الاختبار، والبدء في العلاج بمضادات الفيروسات الرجعية (ART) في نفس اليوم، والرعاية السابقة للولادة، وتوفير العلاج بمضادات الفيروسات الرجعية في نفس المبنى، والخيار ب + الذي زاد أو بقى بنسبة 100٪ في جميع المواقع. وقد تفاوت التقدم في تنفيذ السياسات المتعلقة بتشخيص الرضع وعلاجهم بين المواقع. كما انخفض مخزون مجموعات اختبار فيروس نقص المناعة البشرية (HIV) أو العقاقير المضادة للفيروسات الرجعية في العام الماضي بشكل عام، ولكن تم الإبلاغ عن ذلك من قبل مرفق واحد على الأقل لكل موقع في كلتا الجولتين.
الاستنتاج
تم إحراز تقدم في تنفيذ سياسة الوقاية من انتقال العدوى من الأم إلى الطفل (PMTCT) في هذه الظروف. ومع ذلك، فإن الثغرات المستمرة عبر سلسلة رعاية الرضع وتحدّيات سلسلة التوريد تقوّض أهداف القضاء على فيروس نقص المناعة البشرية لدى الرضع.
摘要
目的
旨在评估将世界卫生组织 (WHO) 的指导方针纳入艾滋病毒 (HIV) 母婴传播预防 (PMTCT) 政策,并监测马拉维、南非和坦桑尼亚联合共和国的农村地区医疗机构层面指导方针的实施情况。
方法
我们总结了国家艾滋病毒母婴传播预防政策和世界卫生组织指南自 2013-2016 年为孕产妇和婴儿护理联动提供的 15 项指标。在 2013 年至 2015 年和 2015 年至 2016 期间分别对 46 个医疗机构、服务于五大医疗和人口监控系统的人群进行了两轮调查。我们对机构管理者进行了结构式问卷调查,以描述服务的提供情况。我们根据地点和调查轮次,报告实施各项指标的机构比例以及缺货的频率和持续时间。
结果
所有国家中,影响产妇和预防艾滋病毒母婴传播的国家政策应符合截至 2016 年的世界卫生组织的指导方针;大多数国家间政策的变化都与常规艾滋病毒护理有关。在同一栋楼内提供检测后咨询、当日启动抗逆转录病毒疗法 (ART)、产前护理并提供抗逆转录病毒疗法,以及在所有站点增加“Option B+”计划或保持 100% 覆盖。各站点在实施婴儿诊断和治疗政策方面的进展各不相同。过去一年,艾滋病毒检测试剂盒或抗逆转录病毒药物的缺货量整体下降,但在这两轮调查中,每个站点至少有一个机构存在缺货现象。
结论
此类情况下,实施艾滋病毒母婴传播预防政策取得进展。然而,婴儿联动护理和供应链挑战之间的持续差距有可能破坏消除婴儿感染艾滋病毒的目标。
Резюме
Цель
Оценка включения рекомендаций Всемирной организации здравоохранения (ВОЗ) в национальные стратегии профилактики передачи вируса иммунодефицита человека (ВИЧ) от матери ребенку (РМТСТ) и отслеживание внедрения таких рекомендаций на уровне объектов здравоохранения в сельских районах Малави, Объединенной Республики Танзания и Южной Африки.
Методы
Авторы суммировали национальные стратегии в отношении PMTCT и рекомендации ВОЗ по 15 индикаторам в цепочке мероприятий по оказанию помощи матери и ребенку на протяжении 2013–2016 гг. Исследование проводилось в виде двух раундов опросов (2013–2015 гг. и 2015–2016 гг.) в 46 учреждениях здравоохранения, которые обслуживали пять популяций систем надзора за здоровьем и демографической ситуацией. Руководителям учреждения здравоохранения были выданы структурированные анкеты для описания оказания услуг. В статье приведены сведения о доле учреждений, внедривших каждый из индикаторов, а также о частоте и продолжительности случаев нехватки ресурсов с разбивкой по зонам оказания услуг и раунду опросов.
Результаты
Во всех странах национальные стратегии, влияющие на цепочку предоставления услуг в отношении материнского и детского РМТСТ, были приведены в соответствие с рекомендациями ВОЗ к 2016 г. Большинство вариантов стратегий в разных странах касались привязки к плановому лечению ВИЧ-инфицированных. Доля медицинских учреждений, предоставляющих возможность консультации после тестирования, начала антиретровирусной терапии (АРТ) в тот же день, дородового лечения и АРТ в том же здании, а также предоставляющих вариант В+, выросла или осталась на уровне 100% во всех обследованных зонах. Прогресс во внедрении стратегий диагностики и лечения младенцев различался в зависимости от зоны исследования. Дефицит тест-систем для выявления антител к ВИЧ или антиретровирусных препаратов за последний год в целом уменьшился, но сообщения о нехватке поступали по меньшей мере из одного учреждения в каждой зоне в течение обоих опросов.
Вывод
Наблюдается прогресс во внедрении стратегий PMTCT в указанных условиях. Однако постоянные недочеты в цепочке предоставления услуг младенцам и проблемы с поставками могут поставить под угрозу цели по устранению ВИЧ у младенцев.
Introduction
In recent years, ambitious global commitments have been made to improve programmes for the prevention of mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV). In 2011, the Global Plan was launched to “eliminate infant HIV infections by 2015 and keep their mothers alive”.1 While the target was not reached, the initiative contributed to a 60% decline in infant infections from 270 000 to 110 000 between 2009 and 2015 across 21 priority countries in sub-Saharan Africa.2 More recently, the Joint United Nations Programme on HIV/AIDS (acquired immune deficiency syndrome) set targets to reach 95% of pregnant women living with HIV with sustained, lifelong HIV treatment, and to reduce the annual number of newly infected children to less than 40 000 by the end of 2018 globally.3
Option B+ is the lifelong provision of antiretroviral therapy (ART) for all HIV-positive pregnant and breastfeeding women regardless of immune status. The Global Plan has been credited with accelerating implementation of Option B+2,4 and improving coverage of PMTCT services for mothers and infants. By 2014, antenatal HIV testing exceeded 90% in many eastern and southern African countries.5 By the end of 2015, more than 80% of pregnant women living with HIV in the 21 Global Plan countries were receiving ART for PMTCT, and of an estimated 1.2 million HIV-exposed infants, 51% (612 000) were receiving an HIV test within two months of birth. 6 However, despite this progress, retention of women in HIV care during the pregnancy and postpartum period continues to be challenging.7 Higher dropout rates have been documented after delivery and at two years of follow-up, suggesting that service integration and linking mothers to routine ART services are important determinants of retention in care.8–10 As well as being detrimental to their own health, losing mothers from care contributes to higher HIV transmission risks to their infants, and poorer uptake of services across the cascade of infant care.11
The timing of adoption and roll-out of PMTCT policies, and the extent to which policies are implemented within health facilities, will be important in determining whether countries meet global targets for mothers living with HIV and their infants. Furthermore, how well-prepared health systems are to cope with the changes is crucial to the success of these evolving programmes. This is particularly so in rural areas where most pregnant women in sub-Saharan Africa reside, and where policy implementation may be delayed or patchy.12 As policy changes lead to increasing client numbers, PMTCT programmes have faced challenges in ensuring sufficient supplies of HIV test kits and antiretroviral (ARV) drugs, particularly in rural areas.13 Stock-outs of supplies may undermine client confidence and affect programme coverage. Increased patient numbers are not always offset by the recruitment of additional trained personnel, contributing to concerns around the quality of care.13,14
To address the programmatic challenges which are preventing countries from reaching global PMTCT targets, a better understanding is needed of the extent to which national policies align with World Health Organization (WHO) guidance. We also need data about implementation gaps at the facility level and the consequences for managing supplies of drugs and diagnostic tests. Although previous research has investigated HIV policy adoption and implementation in sub-Saharan Africa,12,15–19 few studies have documented implementation of PMTCT policies, especially after implementation of Option B+, and in rural settings. This paper used data from policy reviews and health-facility surveys conducted with health workers between 2013 and 2016 in five health and demographic surveillance system sites in rural Malawi, South Africa and the United Republic of Tanzania. The aim was to assess whether the WHO guidance on PMTCT has been adopted in national policies and implemented by rural health facilities. As a secondary objective, we assessed the frequency and duration of stock-outs of HIV test kits and drug supplies over the same period.
Methods
Study settings
We purposively selected three out of six countries participating in a wider mortality study being conducted in health and demographic surveillance system sites by the network for Analysing Longitudinal Population HIV/AIDS data in Africa.20,21 These countries were chosen to represent a range of adoption dates of Option B+ (Malawi: 2011; South Africa: 2015; United Republic of Tanzania: 2013) and mother-to-child transmission rates (8.9% in Malawi; 5.3% in South Africa; 12.2% in the United Republic of Tanzania).22–24 The five sites are served by 46 health facilities providing HIV services to approximately 400 000 residents21 (Table 1).
Table 1. Characteristics of the five study sites included the study of the prevention of mother-to-child transmission of HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013−2016.
| Variable | Malawi |
South Africa |
United Republic of Tanzania |
||||
|---|---|---|---|---|---|---|---|
| Karonga | Agincourt | uMkhanyakude | Ifakara | Kisesa | |||
| Size of site, km2 | 135 | 420 | 438 | 2 400 | 150 | ||
| Population of site | 39 045 | 90 000 | 90 000 | 169 000 | 30 486 | ||
| No. (%) of HIV positive residentsa,b | 1 943/32 983 (5.9) | 775/2 670 (29.0) | 2 719/8 365 (32.5) | 112/2 435 (4.6) | 381/5 754 (6.6) | ||
| Total no. of health facilitiesc | 7 | 11 | 27 | 19 | 11 | ||
| Health facility survey | |||||||
| No. of facilities in analysisd | 5 | 6 | 17 | 11 | 7 | ||
| Survey dates | |||||||
| Round 1 | 12 – 18 Dec 2013 | 21 Aug – 27 Nov 2013 | 13 – 21 Jan 2015 | 22 Oct 2013 – 3 Jun 2014 | 4 Oct 2013 – 22 Jan 2014 | ||
| Round 2 | 26 – 29 May 2015 | 16 Jul – 25 Nov 2015 | 20 May – 3 Jun 2016 | 22 Sep – 8 Oct 2015 | 22 Jul – 4 Aug 2015 | ||
| Service use | |||||||
| No. of HIV tests provided at antenatal care visits | |||||||
| Round 1 | 302 | 231 | 1 940 | 1 551 | 694 | ||
| Round 2 | 375 | 422 | 1 196 | 1 891 | 1 034 | ||
| No. of visits for PMTCT services | |||||||
| Round 1 | 10 | 31 | 94 | 21 | 108 | ||
| Round 2 | 31 | 46 | 1 079 | 1 115 | 1 318 | ||
HIV: human immunodeficiency virus; PMTCT: prevention of mother-to-child transmission.
a All estimates are for adults aged 15–45 years old except Kisesa, where estimates are for adults 15–49 years old.
b Data from 2007–2012 Karonga; 2010–2011 Agincourt; 2014–2015 Ifakara; 2015 uMkhanyakude; 2016 Kisesa.
c Serving the population at each site.
d Facilities offering antenatal care and PMTCT services that were surveyed in both rounds 1 and 2.
HIV policy review
In 2013, we conducted a review of WHO guidance and national HIV policies from 2003 to 2013, covering HIV testing, PMTCT and ART provision.25 In 2016, we updated the review for the period 2013 to 2015. The first phase involved a review of the literature and consultation with 28 HIV researchers and practitioners to define a conceptual framework with five main areas of health-service factors relating to delivery of HIV testing, PMTCT and ART services (service access and coverage; quality of care; coordination of care and patient tracking; support to people living with HIV; and medical management).25 We devised 54 associated policy indicators and included all 15 that pertained to PMTCT in this study.
The second phase involved reviewing WHO guidelines and national policy documents. We retrieved these through online searches of websites of health ministries and national HIV organization or through email communications or in-person visits with representatives of organizations. Documents were included if they were nationally relevant; contained programmatic or clinical guidance on PMTCT services; and were published between January 2003 and June 2015. Information from the documents was summarized in an Excel spreadsheet (Microsoft Corp. Redmond, United States of America) that tracked policy content, source, year of adoption and policy changes over time.
Health-facility surveys
We conducted surveys of health facilities between August 2013 and January 2015 (round 1), and between May 2015 and June 2016 (round 2; Table 1). The questionnaire was informed by the WHO service availability and readiness assessment tool,26 and covered the delivery of HIV testing, PMTCT and ART services, as described previously.17 We conducted survey questionnaires face-to-face, in English, with the staff in charge at each facility. Interviewers observed the availability of treatment guidelines and consulted pharmacy records for drug stocks and availability of test kits.
All health facilities providing HIV services to the health and demographic surveillance system populations were surveyed, except one small private clinic in Karonga, one public facility in Agincourt and facilities serving fewer than 100 patients per month in Ifakara. In uMkhanyakude and Kisesa, we also included facilities outside the site area, but used by health and demographic surveillance system residents.17 For this analysis, we only included facilities that participated in both survey rounds and offered PMTCT services.
We conducted all analysis in Stata, version 15 (Stata Corp., College Station, USA). We recoded categorical variables as binary variables to demonstrate the proportion of facilities that were fully compliant with each policy (versus partial or non-compliance). We then used descriptive statistics to show the proportion of facilities implementing each policy, by survey round and site. HIV test kit and drug stock-outs were recorded for the previous year with median durations for the longest stock-out during this period recorded in days.
Ethical approval was obtained locally for each site and from the London School of Hygiene and Tropical Medicine (no. 8891–1). Survey participants provided written informed consent.
Results
Policy review
We reviewed 10 WHO guidelines and 47 national policy documents (Box 1). By 2016, national policies influencing the maternal and infant cascades of care were in line with WHO guidelines in all three countries, despite substantial variation in the years of adoption (Tables 2 and Table 3).
Box 1. Policy documents and guidance included in the study of implementation of policies for the prevention of mother-to-child transmission of HIV, Malawi, South Africa and United Republic of Tanzania, 2013–2016.
South Africa
The South Africa antiretroviral treatment guidelines. Pretoria: National Department of Health, South Africa; 2010.
Clinical guidelines: PMTCT (prevention mother to child transmission). Pretoria: National Department of Health and South African National AIDS Council, South Africa; 2010.
National HIV counselling and testing policy guidelines. Pretoria: National Department of Health, South Africa; 2010.
The South African antiretroviral treatment guidelines. Pretoria: National Department of Health, South Africa; 2013.
South African prevention of mother to child guidelines. Pretoria: National Department of Health, South Africa; 2015.
National HIV counselling and testing policy guidelines. Pretoria: National Department of Health, South Africa; 2015.
National consolidated guidelines for the prevention of mother to child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria: National Department of Health, South Africa; 2015.
Guidelines for maternity care in South Africa, A manual for clinics, community health centres and district hospitals. Pretoria: National Department of Health, South Africa; 2015.
National HIV testing services policy. Pretoria: National Department of Health, South Africa; 2016.
Malawi
Guidelines for the use of ART in Malawi. 1st ed. Lilongwe: Ministry of Health, Malawi; 2003.
Prevention of mother to child transmission guidelines. Lilongwe: Ministry of Health, Malawi; 2004.
Guidelines for the use of ART in Malawi. 2nd ed. Lilongwe: Ministry of Health, Malawi; 2006.
Management of STIs, using syndromic management approach. 3rd ed. Lilongwe: Ministry of Health, Malawi; 2007.
Paediatric HIV testing and counselling. Lilongwe: Ministry of Health, Malawi; 2007.
Management of HIV associated diseases. 2nd edition. Lilongwe: Ministry of Health, Malawi; 2008.
Paediatric HIV testing and counselling. Lilongwe: Ministry of Health, Malawi; 2008.
Guidelines for the use of ART in Malawi. 3rd ed. Lilongwe: Malawi Ministry of Health; 2008.
HCT guidelines. Lilongwe: Ministry of Health, Malawi; 2009.
Prevention of mother to child transmission of HIV and paediatric HIV care guidelines. Lilongwe: Ministry of Health, Malawi; 2010.
Guidelines for the use of ART in Malawi. 3rd ed. Lilongwe: Malawi Ministry of Health; 2010.
Clinical management of HIV in children and adults: Malawi integrated guidelines. Lilongwe: Ministry of Health, Malawi; 2011.
National HIV and AIDS Strategic Plan 2011–2016. Lilongwe: Ministry of Health, Malawi; 2011.
Consolidated guidelines for the use of ART for treating and preventing HIV infection. Lilongwe: Ministry of Health, Malawi; 2013.
Malawi guidelines for clinical management of HIV in children and adults. Lilongwe: Ministry of Health, Malawi; 2014.
Viral load strategic scale up plan: increasing access to viral load testing in Malawi. Lilongwe: Ministry of Health, Malawi; 2015.
National strategic plan for HIV and AIDS: 2015–2020. Lilongwe: Ministry of Health, Malawi; 2015.
Consolidated guidelines on HIV testing services. Lilongwe: Ministry of Health, Malawi; 2015.
Consolidated strategic information guidelines. Lilongwe: Ministry of Health, Malawi; 2015.
National health information system policy. Lilongwe: Ministry of Health, Malawi; 2016.
Consolidated guidelines for the use of ART for treating and preventing HIV infection. Lilongwe: Ministry of Health, Malawi; 2016.
Consolidated guidelines for the prevention, diagnosis, treatment and care for key populations. Lilongwe: Ministry of Health, Malawi; 2016.
Guidelines on HIV self-testing and partner notification. Lilongwe: Ministry of Health, Malawi; 2016.
Guidelines for the clinical management of HIV. 3rd ed. Lilongwe: Ministry of Health, Malawi; 2016.
Guidelines on patient-centered HIV patient monitoring and case surveillance. Lilongwe: Ministry of Health, Malawi; 2017.
United Republic of Tanzania
National guidelines for the clinical management of HIV and AIDS. 2nd ed. Dar es Salaam: Tanzanian National AIDS Control Program; 2005.
Guidelines for HIV testing and counselling in clinical settings. Dar es Salaam: Tanzanian National AIDS Control Program; 2007.
National health policy. Dar es Salaam: Ministry of Health and Social Welfare, United Republic of Tanzania; 2007.
The national road map strategic plan to accelerate reduction of maternal, newborn and child deaths in United Republic of Tanzania. Dar es Salaam: Ministry of Health and Social Welfare, United Republic of Tanzania; 2008.
National guidelines for the clinical management of HIV and AIDS. 3rd ed. Dar es Salaam: Tanzanian National AIDS Control Program; 2009.
National guidelines for home based care services. Dar es Salaam: Tanzanian National AIDS Control Program; 2010.
National guidelines for the clinical management of HIV and AIDS. 4th ed. Dar es Salaam: Tanzanian National AIDS Control Program; 2012.
Antenatal care guidelines. Dar es Salaam: Ministry of Health and Social Welfare, United Republic of Tanzania; 2014.
National guidelines for comprehensive care of prevention of mother-to-child transmission of HIV. 3rd ed. Dar es Salaam: Ministry of Health and Social Welfare, United Republic of Tanzania; 2012.
National guidelines for comprehensive care services for prevention of mother-to-child transmission of HIV and keeping mothers alive. Dar es Salaam: Ministry of Health and Social Welfare, United Republic of Tanzania; 2013.
Third national multi-sectoral strategic framework for HIV and AIDS. Dar es Salaam: Prime Minister’s Office, United Republic of Tanzania; 2013.
Health sector strategic plan 2015–2020; HSSP IV: Reaching all households with quality health care. Dar es Salaam: Ministry of Health, United Republic of Tanzania; 2015.
National guidelines for the management of HIV and AIDS. Dar es Salaam: Ministry of Health, Community Development, Gender, Elderly and Children; United Republic of Tanzania; 2017.
World Health Organization
The right to know: new approaches to HIV testing and counselling. Geneva: World Health Organization; 2003.
Scaling-up HIV testing and counselling services: a toolkit for programme managers. Geneva: World Health Organization; 2003.
Patient monitoring guidelines for HIV care and ART. Geneva: World Health Organization; 2006.
Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. Geneva: World Health Organization; 2006.
Task shifting: global recommendations and guidelines. Geneva: World Health Organization; 2008.
Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. Geneva: World Health Organization; 2009.
Delivering HIV test results and messages for re-testing and counselling in adults. Geneva: World Health Organization; 2010.
Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: World Health Organization; 2010.
Program update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva: World Health Organization; 2012.
Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013.
AIDS: acquired immune deficiency syndrome; ART: antiretroviral therapy; HIV: human immunodeficiency virus.; HCT: HIV Counselling and Testing; HSSP: Health Sector Strategic Plan; STIs: Sexually Transmitted Infections.
Table 2. Timing of publication of WHO recommendations on PMTCT of HIV and their adoption as policy by countries: maternal care, Malawi, South Africa and United Republic of Tanzania, 2013–2016 .
| WHO recommendation | Study indicator | Year guideline published | Year guideline adopted as national policy |
||
|---|---|---|---|---|---|
| Malawi | South Africa | United Republic of Tanzania | |||
| HIV testing | |||||
| Provider-initiated testing and counselling is standard for all clients, including in antenatal care | Provider-initiated testing and counselling implemented in antenatal care | 2004 | 2006 | 2010 | 2007 |
| Individual as well as group pre-test counselling is recommended | Pre-test counselling always given | 2003 | 2006 Individual or group |
2010 Individual or group |
2005 |
| Post-test counselling always given | |||||
| Treatment initiation | |||||
| ART should be initiated in all pregnant and breastfeeding women living with HIV regardless of WHO clinical stage and at any CD4 cell count and be continued lifelong (Option B+) | Option B+ implemented | 2013 | 2011 | 2015 | 2013 |
| HIV treatment (for the mothers’ own health) should be given on same day as antenatal care services | HIV treatment given on same day as antenatal care services | 2015 | 2011 | 2015 | 2013 |
| In generalized epidemic settings, ART should be initiated and maintained at maternal and child health-care settings for eligible pregnant and postpartum women and for infants | ART for pregnant women testing HIV-positive provided in the same building as antenatal care | 2013 | 2011 | 2015 | 2012 |
| Linkage to care | |||||
| Clear guidance should be given on when HIV-positive pregnant women are referred to ART clinic | Women are referred to ART clinics from PMTCT during antenatal care or by 6 weeks post-delivery | 2006 | 2011 | 2010 | 2013 When child is 24 months old |
| Streamlined interventions should be done to reduce time between diagnosis and engagement in care, including (i) enhanced linkage with case management, (ii) support for HIV disclosure, (iii) patient tracing, (iv) training staff to provide multiple services and (v) streamlined services | Maternal referrals to HIV care and treatment services from PMTCT are documented | 2013 | Not explicit | 2016 | 2009 |
| Health worker accompanies woman during initial referral to routine ART services | Not stated | 2015 Active referral but not explicitly accompanied |
Not stated | ||
| Check if woman arrives in routine ART services | 2004 | Not explicit | Not explicit | 2010 | |
ART: antiretroviral therapy; HIV: human immunodeficiency virus; PMTCT: prevention of mother-to-child transmission; WHO: World Health Organization.
Table 3. Timing of publication of WHO recommendations on PMTCT of HIV and their adoption as policy by countries: infant care, Malawi, South Africa and United Republic of Tanzania, 2013–2016.
| WHO recommendation | Study indicator | Year guideline published | Year guideline or study indicator adopted as national policy |
||
|---|---|---|---|---|---|
| Malawi | South Africa | United Republic of Tanzania | |||
| Testing | |||||
| All HIV-exposed infants have HIV virological testing at 4–6 weeks of age or at the earliest opportunity thereafter | Early infant diagnosis offered by 6 weeks or as early as possible thereafter | 2010 | 2007 | 2004 | 2012 |
| Infant prophylaxis | |||||
| Infants of mothers who are receiving ART and are breastfeeding should receive 6 weeks of infant prophylaxis with daily nevirapine. If infants are receiving replacement feeding, they should be given 4–6 weeks of infant prophylaxis with daily nevirapine (or twice-daily zidovudine). Infant prophylaxis should begin at birth or when HIV exposure is recognized postpartum | Infant prophylaxis provided for 4–6 weeks postpartum | 2010 | 2011 | 2015 | 2012 |
| Infant prophylaxis provided until cessation of breastfeeding | 2009 | 2011 | 2010 | 2012 | |
| National authorities should decide whether health services will principally counsel mothers to either exclusively breastfeed and receive ART interventions or avoid all breastfeeding, as the strategy that will most likely give infants the greatest chance of HIV-free survival. Where breastfeeding is judged to be the best option: exclusively breastfeed for the first 6 months, introduce appropriate complementary food thereafter, and continue breastfeeding for 12 months; wean gradually within 1 month | Counselling on infant feeding provided | 2010 | 2016 | 2015 | 2012 |
| Infant care | |||||
| Not addressed | Infant and paediatric ART provided in all facilities which offer ART for adults | NA | 2011 | 2015 | 2005 |
ART: antiretroviral therapy; HIV: human immunodeficiency virus; NA: not applicable; WHO: World Health Organization.
Guidance stipulating that pre- and post-test counselling should be provided was first released by WHO in 2003, and then adopted by Malawi in 2006, by the United Republic of Tanzania in 2005 and by South Africa in 2010. Policies on provider-initiated testing and counselling in antenatal care were first adopted by Malawi in 2006, two years before it was recommended by WHO, and adopted by the United Republic of Tanzania in 2007 and South Africa in 2010. Malawi also adopted same-day ART initiation and Option B+ policies before WHO first issued its guidance. WHO recommendations from 2006 relating to linkage of mothers to routine ART care specify the need for clear guidance on when HIV-positive pregnant women should be referred from antenatal care to ART clinics, without indicating when it should occur. We noted variation in the timing of referral of pregnant or postpartum women to ART clinics in the study countries, with Malawi and South African policies stipulating that this should occur within 42 days after delivery. In the United Republic of Tanzania, the policy stipulating referral within 42 days was replaced in 2013 to allow ART provision for mothers in reproductive health clinics until their child reaches 2 years old. WHO guidance on infant diagnosis, prophylaxis and treatment were released in 2010. All countries aligned themselves with the WHO recommendations, with variations in the year of adoption.
Health facility survey
We included survey data from 46 health facilities serving the populations of the five health and demographic surveillance system sites (Table 4).
Table 4. Characteristics of facilities included in the study of implementation of PMTCT guidelines for HIV, Malawi, South Africa and United Republic of Tanzania, 2013–2016.
| Facility type | No. (%) of health facilities |
||||||
|---|---|---|---|---|---|---|---|
| Malawi |
South Africa |
United Republic of Tanzania |
|||||
| Karonga (n = 5) | Agincourt (n = 6) | uMkhanyakudea (n = 17) | Ifakara (n = 11) | Kisesab (n = 7) | |||
| Government-run | 3 (60) | 6 (100) | 17 (100) | 10 (91) | 7b (100) | ||
| Faith-based organization-run | 2 (40) | 0 (0) | 0 (0) | 1 (< 1) | 0 (0) | ||
| Dispensary | 0 (0) | 0 (0) | 0 (0) | 2 (18) | 3 (43) | ||
| Small clinic | 0 (0) | 6 (100) | 17 (100) | 0 (0) | 0 (0) | ||
| Large clinic or small health centre | 0 (0) | 0 (0) | 0 (0) | 3 (27) | 0 (0) | ||
| Large health centre or small sub-district hospital | 5 (100) | 0 (0) | 0 (0) | 4 (36) | 1 (14) | ||
| District hospital or large hospital | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 1 (14) | ||
| Referral hospital | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 2 (29) | ||
HIV: human immunodeficiency virus.
a In uMkhanyakude 10 facilities were outside that system and in Kisesa 3 facilities were outside the health and demographic surveillance system.
b In Kisesa 1 of the 7 facilities were government-run, but managed by a faith-based organization.
Note: n is the total number of facilities offering antenatal care and prevention of mother-to-child transmission services participating in health and demographic surveillance system surveys at that site.
Maternal care cascade
Provider-initiated testing and counselling was offered by all facilities in all sites for both rounds (Table 5). The greatest increases in the proportion of facilities always offering both pre- and post-test counselling were seen in Kisesa: from 29% (2/7) to 86% (6/7) for pre-test counselling and from 57% (4/7) to 100% (7/7) for post-test counselling. In the Malawian and South African sites, all facilities implemented Option B+ during round 1. Implementation of Option B+ was later in the United Republic of Tanzania, with 55% (6/11) of facilities in Ifakara and 71% (5/7) of facilities in Kisesa delivering Option B+ by round 1, increasing to 100% in both sites by round 2. The proportion of facilities offering women ART services on the same day as receiving an HIV diagnosis at antenatal care increased or remained stable over the two rounds, reaching at least 91% by round 2 in all sites.
Table 5. Facility-level provision of maternal care in the study of implementation of PMTCT guidelines for HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016.
| Maternal care indicator | No. (%) of health facilities |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malawi |
South Africa |
United Republic of Tanzania |
||||||||||
| Karonga (n = 5) |
Agincourt (n = 6) |
uMkhanyakude (n = 17) |
Ifakara (n = 11) |
Kisesa (n = 7) |
||||||||
| Round 1 | Round 2 | Round 1 | Round 2 | Round 1 | Round 2 | Round 1 | Round 2 | Round 1 | Round 2 | |||
| HIV testing | ||||||||||||
| Provider-initiated testing and counselling provided in antenatal care | 5 (100) | 5 (100) | 6 (100) | 6 (100) | 17 (100) | 17 (100) | 11 (100) | 11 (100) | 7 (100) | 7 (100) | ||
| Pre-test counselling always given | 4 (80) | 5 (100) | 6 (100) | 6 (100) | 17 (100) | 17 (100) | 10 (91) | 9 (82) | 2 (29)a | 6 (86)a | ||
| Post-test counselling always given | 5 (100) | 5 (100) | 6 (100) | 6 (100) | 17 (100) | 16 (94) | 8 (73)a | 10 (91) | 4 (57) | 7 (100) | ||
| Treatment initiation | ||||||||||||
| Option B+ implemented | 5 (100) | 5 (100) | 6 (100) | 6 (100) | 17 (100) | 17 (100) | 6 (55) | 11 (100) | 5 (71) | 7 (100) | ||
| HIV treatment services given on same day as antenatal care services | 5 (100) | 5 (100) | 6 (100) | 6 (100) | 16 (94) | 17 (100) | 6 (56) | 10 (91) | 6 (86) | 7 (100) | ||
| ART for pregnant women testing HIV-positive provided in the same building as antenatal care | 3 (60) | 4 (80) | 6 (100) | 6 (100) | 11 (65) | 15 (88) | 5 (46) | 11 (100) | 6 (86) | 6 (86)a | ||
| Linkage to care | ||||||||||||
| Women are referred to ART clinics from PMTCT during antenatal care or by 6 weeks post-delivery | 5 (100) | 5 (100) | 5 (83) | 5 (83) | 15 (88) | 14 (82) | 6 (56) | 0 (0) | 7 (100) | 1 (14) | ||
| Maternal referrals to HIV care and treatment services from PMTCT are documented | 5 (100) | 5 (100) | 6 (100) | 4 (67) | 17 (100) | 16 (94) | 10 (91) | 11 (100) | 6 (86) | 7 (100) | ||
| Health worker accompanies woman during initial referral to routine ART services | 4 (80) | 4 (80) | 5 (83) | 4 (67) | 5 (29) | 4 (24) | 9 (82) | 3 (27) | 3 (43) | 1 (14) | ||
| Check if woman arrives in routine ART services | 5 (100) | 5 (100) | 6 (100) | 5 (83)a | 17 (100) | 13 (77) | 10 (91) | 1 (9)a | 5 (71) | 7 (100) | ||
ART: antiretroviral therapy; HIV: human immunodeficiency virus; PMTCT: prevention of mother-to-child transmission.
a Missing data from 1 facility in survey round.
Note: n is the total number of facilities offering antenatal care and prevention of mother-to-child transmission services participating in health and demographic surveillance system surveys at that site.
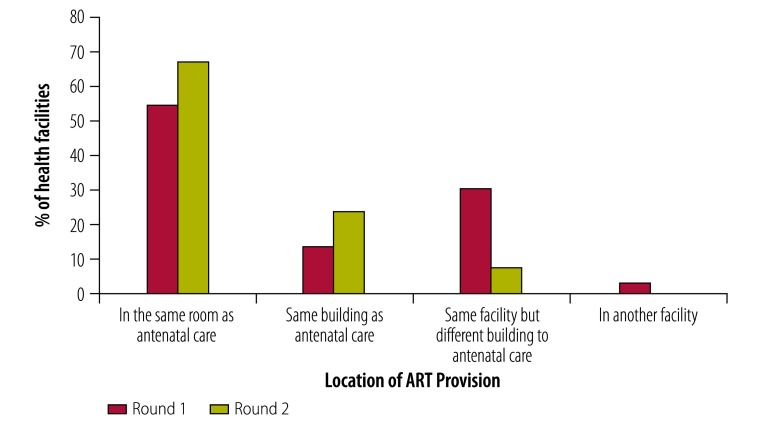
All five facilities in Karonga and 87% (20/23) in the South African sites reported making referrals by 6 weeks postpartum in round 1 with little change in round 2. In the Tanzanian sites, 72% (13/18) of health facilities reported making maternal referrals within this timeframe in round 1, dropping to 6% (1/18) by round 2. The proportion of facilities reporting that health workers accompanied women to routine ART services declined by round 2 in all sites except Karonga. The location of ART provision for PMTCT also changed over the two rounds (Fig. 1).
Fig. 1.
Location of ART provision during antenatal care in the study of implementation of PMTCT guidelines for HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016
ART: antiretroviral therapy; HIV: human immunodeficiency virus.
Note: Total number of facilities: Karonga (n = 5); Agincourt (n = 6); uMkhanyakude (n = 17); Ifakara, (n = 11); Kisesa (n = 7).
Infant care cascade
Facility-level implementation of early infant diagnosis between 4–6 weeks by round 2 was high with little variation between sites and countries (Table 6). However, the provision of infant prophylaxis was different between sites. In the South African sites, the proportion of facilities providing prophylaxis at 4–6 weeks decreased from 87% (20/23) to 61% (14/23), with a similar decrease in the proportion of facilities providing prophylaxis until the cessation of breastfeeding, from 70% (16/23) to 61% (14/23). In the Malawian and Tanzanian sites, all facilities provided prophylaxis at 4–6 weeks by round 2.
Table 6. Facility-level provision of infant care in the study of implementation of PMTCT guidelines for HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016.
| Infant care indicator | No. (%) of health facilities |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malawi |
South Africa |
United Republic of Tanzania |
||||||||||
| Karonga (n = 5) |
Agincourt (n = 6) |
uMkhanyakude (n = 17) |
Ifakara (n = 11) |
Kisesa (n = 7) |
||||||||
| Round 1 | Round 2 | Round 1 | Round 2 | Round 1 | Round 2 | Round 1 | Round 2 | Round 1 | Round 2 | |||
| HIV testing | ||||||||||||
| Early infant diagnosis offered by 6 weeks or as early as possible thereafter | 5 (100) | 4 (80) | 6 (100) | 6 (100) | 17 (100) | 17 (100) | 11 (100) | 10 (91) | 5 (70) | 7 (100) | ||
| Infant prophylaxis | ||||||||||||
| Infant prophylaxis provided 4–6 weeks postpartum or until cessation of breastfeeding | 5 (100) | 5 (100) | 5 (83) | 2 (33) | 15 (88) | 12 (71) | 6 (55) | 11 (100) | 5 (71) | 7 (100) | ||
| Infant prophylaxis provided until cessation of breastfeeding | 1 (20) | 5 (100) | 5 (83) | 2 (33) | 11 (65) | 12 (71) | 4 (36) | 11 (100) | 4 (57) | 6 (86) | ||
| Counselling on infant feeding provided | 2 (40) | 4 (80) | 6 (100) | 6 (100) | 16 (94) | 17 (100) | 11 (100) | 9 (82) | 7 (100) | 7 (100) | ||
| Routine care | ||||||||||||
| Infant and paediatric ART provided in all facilities which offer ART for adults | 5 (100) | 4 (80) | 6 (100) | 6 (100) | 17 (100) | 17 (100) | 9 (82) | 10 (90) | 4 (57) | 3 (43) | ||
ART: antiretroviral therapy; HIV: human immunodeficiency virus.
Note: n is the total number of facilities offering antenatal care and prevention of mother-to-child transmission services participating in health and demographic surveillance system surveys at that site.
The provision of infant feeding counselling was consistently high in all sites over both rounds. Infant care was offered in all facilities across both rounds in the two South African sites, increasing in Ifakara from 82% (9/11) to 90% (10/11), but decreasing in Karonga (100%; 5/5 to 80%; 4/5) and Kisesa (57%; 4/7 to 43%; 3/7).
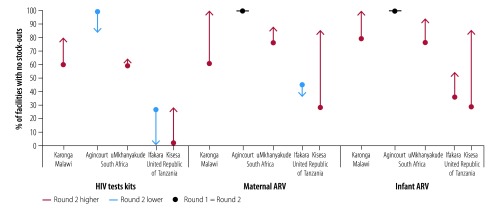
Supply stock-outs
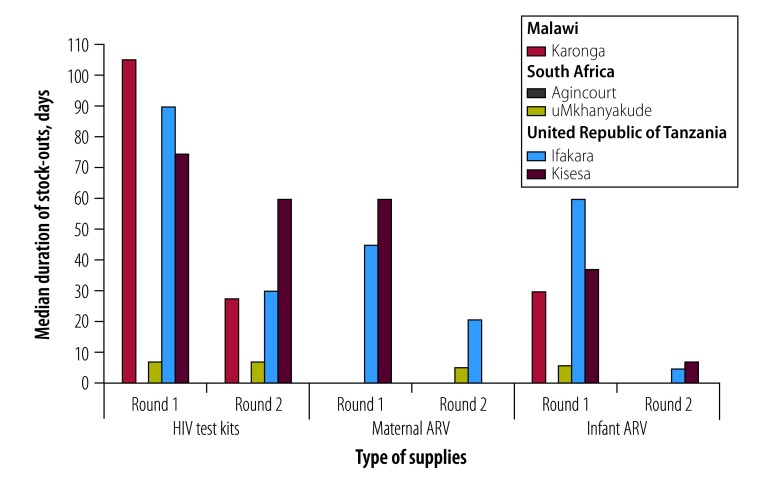
The proportion of facilities reporting no stock-outs of HIV test kits in the year before the survey varied by round and site (Fig. 2). The Malawian and South African sites had the highest proportion of facilities reporting no stock-outs across both rounds. In both Tanzanian sites, the proportion of facilities experiencing no stock-outs of HIV test kits in the past year was low, ranging from 0% to 30% in the two rounds. The median duration of the longest HIV test kit stock-outs in both South African sites was less than 2 weeks in both rounds (Fig. 3). In Karonga, the median duration of the longest stock-outs decreased from 105 days to 28 days between round 1 and 2. In the United Republic of Tanzania, the median duration of the longest stock-outs was 90 days and 75 days in Ifakara and Kisesa, respectively, in round 1, decreasing to between 30 days and 60 days, respectively, by round 2.
Fig. 2.
Proportion of health facilities with no stock-outs in the past year for HIV test kits and maternal and infant antiretroviral drugs in the study of implementation of PMTCT guidelines for HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016
ARV: antiretroviral drugs; HIV: human immunodeficiency virus.
Note: Total number of facilities: Karonga (n = 5); Agincourt (n = 6); uMkhanyakude (n = 17); Ifakara, (n = 11); Kisesa (n = 7).
Fig. 3.
Median length of the longest stock-out in the past year for HIV test kits and maternal and infant antiretroviral drugs in the study of implementation of PMTCT guidelines for HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016
ARV: antiretroviral drugs; HIV: human immunodeficiency virus.
Note: Data were missing from Kisesa in round 2 and Agincourt in round 1.
A similar pattern was reported for maternal ARV drugs, with all sites having a higher proportion of facilities reporting no stock-outs by round 2 (or maintaining 100% of facilities reporting no stock-outs) except Ifakara. In Ifakara fewer facilities reported no maternal drug stock-outs by round 2; however, the median duration of the longest stock-out declined from 45 to 21 days over the two rounds.
In all sites, the proportion of facilities reporting no stock-outs of infant ARV drugs increased or remained at 100% in all sites over the two rounds. The median duration of the longest stock-outs declined over the two rounds from 60 to 5 days in Ifakara and from 37 to 7 days in Kisesa.
Discussion
Our study shows overall progress in delivering PMTCT services in five rural settings in Africa in line with WHO guidance, despite some implementation gaps and persistent supply-chain challenges. We found that in all study countries, national policies influencing the maternal and infant PMTCT cascade aligned with WHO guidelines by 2016, despite considerable variation in the year of adoption. Malawi was notable in having adopted several policies before their recommendation by WHO, including same-day ART initiation and Option B+, and other countries have drawn lessons from Malawi’s experience.27 Over the study period, all sites improved the proportion of facilities that were implementing policies designed to improve ART coverage among pregnant women. These policies include integration of ART services into antenatal care, same-day initiation of ART and documentation of transfers from PMTCT into routine HIV care. These findings provide further evidence of progress achieved in these countries during the Global Plan years, contributing to ART coverage among pregnant women of 65%, 90% and > 95% in Malawi, the United Republic of Tanzania and South Africa respectively.11
Our findings suggest that most of the variation across countries in the adoption and implementation of PMTCT policy concerned the delivery of HIV care within antenatal care services, including the timing of the mothers’ transfer to routine HIV care from antenatal clinics. Timings varied from 42 days following delivery in Malawi and South Africa, to 2 years after delivery in the United Republic of Tanzania. This variation is likely explained by the broad guidance provided by WHO, and perhaps illustrates the lack of evidence on the relative merits of each strategy.28 In particular, the extent to which these policy differences influence mothers’ retention in care is unclear, with high drop-out rates after transfer to routine care reported in some settings, regardless of the timing of the referral.8,29 Further research should investigate the impact of service integration on PMTCT outcomes, and explore the perspectives of service providers and users on different models to inform future policy refinements.
Although infant testing, prophylaxis and treatment policies were well-established in all three countries, implementation was not universal across the surveyed facilities, with variation within and between countries, and over survey rounds. Patchy implementation of these policies may be explained by insufficient capacity or resources, particularly in rural areas, and may help to explain why improvements in infant outcomes have lagged behind those in maternal outcomes in these countries.2 Our findings align with other studies that suggest that greater focus is needed on service implementation across the infant cascade of care if progress towards global targets for infants is to be accelerated.30
The introduction of Option B+ led to concerns that health systems may be insufficiently prepared for the resulting increase in client numbers. Our findings suggest that supplying sufficient HIV test kits to meet demand in these rural areas was a persistent challenge, with all sites still reporting stock-outs by round 2. Stock-outs of HIV test kits may undermine women’s confidence in the HIV services being offered,31 and may help to explain the persistently low uptake of HIV testing in some settings, including the United Republic of Tanzania.32 The overall reduction in maternal and infant ARV drug stock-outs between survey rounds, as well as in the median duration of the longest stock-out in the past 12 months, may indicate the success of Option B+ in simplifying drug forecasting, procurement and stock monitoring.33 These findings also suggest that concerns that Option B+ may over-burden health systems through increased client numbers have not been realized with regards to drug supply chains.34 Nevertheless, further evidence is needed to understand the impacts of Option B+ on other aspects of health systems including on the workforce and service delivery mechanisms such as integration.35
Our study has various limitations, including potential reporting bias given that survey responses were obtained from facility staff who may be inclined to overestimate some measures of service delivery. Reporting bias was minimized for some indicators by triangulating responses with observations in the facilities, including for availability of treatment guidelines. We also consulted pharmacy records to validate reports of drug stocks and test availability. Our data cover the period from 2013 to 2016, and the accelerated roll-out of universal Test and Treat28 since 2016 may have led to changes in implementation of Option B+ policies. Furthermore, the timing of survey rounds differed between countries, making it difficult to compare the findings across settings. We selected facilities because they served the health and demographic surveillance system site populations; therefore, facilities were not nationally representative and this limits the generalizability of our findings. However, the facilities can be considered typical of those found in rural areas in each country. Using a similar analytical approach, data from nationally representative health-facility surveys (e.g. those using service availability and readiness assessment methods) could be used for future assessments of HIV policy implementation. Finally, further investigations are needed to understand why gaps occur and how these may be addressed, as well as to assess the proportion of clients that receive care in line with national guidelines and the impact of policy implementation on patient outcomes, including retention in care, mortality or ART coverage.
In conclusion, we found general alignment of national PMTCT policies with WHO guidance, and substantial progress in their facility-level implementation in five rural African settings between 2013 and 2016. However, gaps in implementation of infant care policies persisted in all sites, threatening to undermine efforts to eliminate new infant HIV infections by 2020. Concerns that supply chains could not cope with additional client numbers from PMTCT policy changes have not been met, although occurrences of stock-outs may undermine progress if the causes are not addressed.
Acknowledgements
This work was supported by the Medical Research Council [grant number MR/P014313/1]. JR is supported by DELTAS Africa Initiative grant number DEL-15–011 to THRiVE-2. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust grant number 107742/Z/15/Z and the government of the United Kingdom of Great Britain and Northern Ireland. MM also holds a post at the Africa Health Research Institute and acknowledges their support.
Competing interests:
None declared.
References
- 1.Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive: 2011–2015 [internet]. Geneva: Joint United Nations Programme on HIV/AIDS; 2011. Available from: http://www.unaids.org [cited 2018 April 18].
- 2.Haroz D, von Zinkernagel D, Kiragu K. Development and impact of the Global Plan. J Acquir Immune Defic Syndr. 2017 May 1;75(1) Suppl 1:S2–6. doi: 10.1097/QAI.0000000000001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A super-fast-track framework for ending AIDS among children, adolescent and young women by 2020. Geneva: Joint United Nations Programme on HIV/AIDS; 2015. Available from: http://www.unaids.orghttp://[cited 2018 April 24].
- 4.Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants [internet]. Geneva: World Health Organization; 2010. Available from: http://www.who.int/en/http://[cited 2018 Nov 8]. [PubMed]
- 5.Global health sector response to HIV, 2000–2015: focus on innovations in Africa: progress report. Geneva: World Health Organization/Joint United National Programme on HIV/AIDS; 2015. Available from: http://apps.who.int/iris/bitstream/handle/10665/198065/9789241509824_eng.pdf;jsessionid=050FDD3D813E3F8F2987A68CC924F90A?sequence=1 [cited 2018 Nov 9].
- 6.On the fast-track to an AIDS-free generation. Geneva: Joint United Nations Programme on HIV/AIDS; 2016. Available from: http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdfhttp://[cited 2019 Jan 7].
- 7.Gamell A, Luwanda LB, Kalinjuma AV, Samson L, Ntamatungiro AJ, Weisser M, et al. KIULARCO Study Group Prevention of mother-to-child transmission of HIV Option B+ cascade in rural Tanzania: the One Stop clinic model. PLoS One. 2017 Jul 12;12(7):e0181096. doi: 10.1371/journal.pone.0181096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knettel BA, Cichowitz C, Ngocho JS, Knippler ET, Chumba LN, Mmbaga BT, et al. Retention in HIV care during pregnancy and the postpartum period in the Option B+ era: systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr. 2018 Apr 15;77(5):427–38. doi: 10.1097/QAI.0000000000001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV. 2016 Apr;3(4):e175–82. doi: 10.1016/S2352-3018(16)00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiragu K, Collins L, Von Zinkernagel D, Mushavi A. Integrating PMTCT into maternal, newborn, and child health and related services: experiences from the global plan priority countries. J Acquir Immune Defic Syndr. 2017 May 1;75(Suppl 1):S36–42. doi: 10.1097/QAI.0000000000001323. [DOI] [PubMed] [Google Scholar]
- 11.2015 progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva: Joint United Nations Programme on HIV/AIDS; 2015. Available from: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf [cited 2018 April 18].
- 12.Cawley C, McRobie E, Oti S, Njamwea B, Nyaguara A, Odhiambo F, et al. Identifying gaps in HIV policy and practice along the HIV care continuum: evidence from a national policy review and health facility surveys in urban and rural Kenya. Health Policy Plan. 2017 Nov 1;32(9):1316–26. doi: 10.1093/heapol/czx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenthani L, Haas AD, Egger M, Van Oosterhout JJ, Jahn A, Chimbwandira F, et al. Brief report: HIV testing among pregnant women who attend antenatal care in Malawi. J Acquir Immune Defic Syndr. 2015 Aug 15;69(5):610–4. doi: 10.1097/QAI.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MH, Ahmed S, Hosseinipour MC, Giordano TP, Chiao EY, Yu X, et al. Implementation and operational research: the impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2015 Apr 15;68(5):e77–83. doi: 10.1097/QAI.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambia J, Renju J, Wringe A, Todd J, Geubbels E, Nakiyingi-Miiro J, et al. From policy to practice: exploring the implementation of antiretroviral therapy access and retention policies between 2013 and 2016 in six sub-Saharan African countries. BMC Health Serv Res. 2017 Nov 21;17(1):758. doi: 10.1186/s12913-017-2678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McRobie E, Wringe A, Nakiyingi-Miiro J, Kiweewa F, Lutalo T, Nakigozi G, et al. HIV policy implementation in two health and demographic surveillance sites in Uganda: findings from a national policy review, health facility surveys and key informant interviews. Implement Sci. 2017 Apr 5;12(1):47. doi: 10.1186/s13012-017-0574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church K, Machiyama K, Todd J, Njamwea B, Mwangome M, Hosegood V, et al. Identifying gaps in HIV service delivery across the diagnosis-to-treatment cascade: findings from health facility surveys in six sub-Saharan countries. J Int AIDS Soc. 2017 Jan 12;20(1):21188. doi: 10.7448/IAS.20.1.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta ANZ, Wringe A, Crampin AC, Chisambo C, Koole O, Makombe S, et al. HIV policy and implementation: a national policy review and an implementation case study of a rural area of northern Malawi. AIDS Care. 2016 Sep;28(9):1097–109. doi: 10.1080/09540121.2016.1168913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mwangome MN, Geubbels E, Wringe A, Todd J, Klatser P, Dieleman M. A qualitative study of the determinants of HIV guidelines implementation in two south-eastern districts of Tanzania. Health Policy Plan. 2017 Jul 1;32(6):825–34. doi: 10.1093/heapol/czx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slaymaker E, McLean E, Wringe A, Calvert C, Marston M, Reniers G, et al. The network for analysing longitudinal population-based HIV/AIDS data on Africa (ALPHA): data on mortality, by HIV status and stage on the HIV care continuum, among the general population in seven longitudinal studies between 1989 and 2014. Gates Open Res. 2017 Nov 6;1:4. doi: 10.12688/gatesopenres.12753.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reniers G, Wamukoya M, Urassa M, Nyaguara A, Nakiyingi-Miiro J, Lutalo T, et al. Data resource profile: network for analysing longitudinal population-based HIV/AIDS data on Africa (ALPHA network). Int J Epidemiol. 2016 Feb;45(1):83–93. doi: 10.1093/ije/dyv343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malawi: HIV country profile: 2017 [internet]. Geneva: World Health Organization; 2017. Available from: http://www.who.int/hiv/data/ [cited 2018 Nov 9].
- 23.South Africa: HIV country profile: 2017 [internet]. Geneva: World Health Organization; 2017. Available from: http://www.who.int/hiv/data/ [cited 2018 Nov 9].
- 24.United Republic of Tanzania: HIV country profile: 2017 [internet]. Geneva: World Health Organization; 2017. Available from: http://www.who.int/hiv/data/http://[cited 2018 Nov 9].
- 25.Church K, Kiweewa F, Dasgupta A, Mwangome M, Mpandaguta E, Gómez-Olivé FX, et al. A comparative analysis of national HIV policies in six African countries with generalized epidemics. Bull World Health Organ. 2015 Jul 1;93(7):457–67. doi: 10.2471/BLT.14.147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Service Availability and Readiness Assessment (SARA): an annual monitoring system for service delivery. Geneva: World Health Organization; 2014. Available from: http://apps.who.int/iris/bitstream/handle/10665/149025/WHO_HIS_HSI_2014.5_eng.pdf;jsessionid=E067D9726572D0E5B9C6F41E6D702B04?sequence=1 [cited 2018 Jan 2].
- 27.Kalua T, Tippett Barr BA, van Oosterhout JJ, Mbori-Ngacha D, Schouten EJ, Gupta S, et al. Lessons learned from option B+ in the evolution toward test and start from Malawi, Cameroon, and the United Republic of Tanzania. J Acquir Immune Defic Syndr. 2017 May 1;75(Suppl 1):S43–50. doi: 10.1097/QAI.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 29.Gamell A, Luwanda LB, Kalinjuma AV, Samson L, Ntamatungiro AJ, Weisser M, et al. KIULARCO Study Group Prevention of mother-to-child transmission of HIV Option B+ cascade in rural Tanzania: the One Stop clinic model. PLoS One. 2017 Jul 12;12(7):e0181096–0181096. doi: 10.1371/journal.pone.0181096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gumede-Moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: a systematic literature review. Medicine (Baltimore) 2017 Oct;96(40):e8055. doi: 10.1097/MD.0000000000008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013 Jul 19;16(1):18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.90 90 90 An ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 33.Modi S, Callahan T, Rodrigues J, Kajoka MD, Dale HM, Langa JO, et al. Overcoming health system challenges for women and children living with HIV through the Global Plan. J Acquir Immune Defic Syndr. 2017 May 1;75(Suppl 1):S76–85. doi: 10.1097/QAI.0000000000001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieffer MP, Mattingly M, Giphart A, van de Ven R, Chouraya C, Walakira M, et al. EGPAF Technical Directors Forum Lessons learned from early implementation of option B+: the Elizabeth Glaser Pediatric AIDS Foundation experience in 11 African countries. J Acquir Immune Defic Syndr. 2014 Dec 1;67(Suppl 4):S188–94. doi: 10.1097/QAI.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutabazi JC, Zarowsky C, Trottier H. The impact of programs for prevention of mother-to-child transmission of HIV on health care services and systems in sub-Saharan Africa – a review. Public Health Rev. 2017 Dec 5;38(1):28. doi: 10.1186/s40985-017-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]