Abstract
We present the case of a 62-year-old African-American woman with medical history of hypertension and hyperlipidaemia who presented to dermatology clinic for ‘bug bites’. Skin examination showed resolving bullae on the shins and postinflammatory pigment changes. Histopathology showed eosinophilic spongiosis and direct immunofluorescence (DIF) was negative for IgG, IgM, IgA and C3. After returning to clinic with recurrent severe bullous eruptions, the patient presented with anaemia, lymphocytosis, posterior cervical lymphadenopathy and weight loss. An exuberant bite reaction in the setting of lymphoma was suspected. Further workup with haematology revealed elevated IgG level and total protein levels. Flow cytometry showed a B cell lymphoma subtype. Extensive imaging was positive for diffuse lymphadenopathy, with accompanying evidence of Ebstein-Barr virus infection. Our case highlights the importance of considering exuberant arthropod bite reaction in the setting of undiagnosed lymphoma in a patient with bullous eruption and negative DIF.
Keywords: dermatology, haematology (drugs and medicines), skin, haematology (incl blood transfusion)
Background
Mantle cell lymphoma (MCL) is a type of CD5+ B cell lymphoma that accounts for 3%–6% of all non-Hodgkin’s lymphoma cases.1 MCL can be distinguished from small lymphocytic lymphoma/chronic lymphocytic leukaemia (SLL/CLL), as MCL is CD23− and FMC7+, and SLL/CLL is CD23+ and FMC7−.2 In addition, the chromosomal translocation t(11;14)(q13;q32) is characteristic of MCL.1 2
Severe insect bite reactions and insect bite-like reactions can be observed in patients with haematological malignancies, most commonly CLL,3–9 and less commonly MCL.10–18 In 1965, an ‘exaggerated reaction to insect bites’ was observed in eight adult patients with CLL as a characteristic cutaneous eruption at the site of a known insect bite with intense pruritus, erythema, oedema and induration that sometimes progressed into a large bulla. An exaggerated delayed hypersensitivity type of cutaneous response to insect bites and mosquito antigen was noted, and the lesions resolved spontaneously within 2–14 days.3 A separate phenomenon, referred to as ‘insect bite-like reactions’ is a non-specific recurrent rash that resembles arthropod bites, described as pruritic red papules and plaques, occurring without recollection of insect bites or outdoor activities.11
Hypersensitivity to mosquito bites (HMB) is a separate condition characterised by a more severe reaction induced by mosquito bites, including systemic symptoms such as fevers, malaise and hypotension in addition to cutaneous lesions. However, without systemic symptoms, repeated episodes of intense cutaneous eruptions following insect bites are referred to as an exaggerated reaction to insect bites.3 10 19–21 Bullous reactions to bedbug bites may also suggest an underlying vasculitis like Churg-Strauss.22
In this report, we describe the clinical presentation, histology, immunohistochemistry and molecular characteristics of exaggerated skin reactions to insect bites that preceded the diagnosis of MCL.
Case presentation
A 62-year-old African-American woman with medical history of hypertension and hyperlipidaemia presented to dermatology clinic for recurrent cutaneous bullous eruptions following ‘bug bites’. The patient did not have a family history of autoimmune disorders, connective tissue disease or sarcoidosis. She also did not have any risk factors for HIV infection and denied drug use.
These bullous eruptions occurred for about 3 years, mostly during the summertime. The patient recalled getting insect bites while outdoors, which evolved over several days into large pruritic tense bullae filled with transparent fluid, without necrosis or haemorrhage. The bullous eruptions started approximately 3 years ago, and each episode resolved within 3 weeks. Systemic symptoms, such as fever and hypotension, were not observed at first. Joint involvement was not noted.
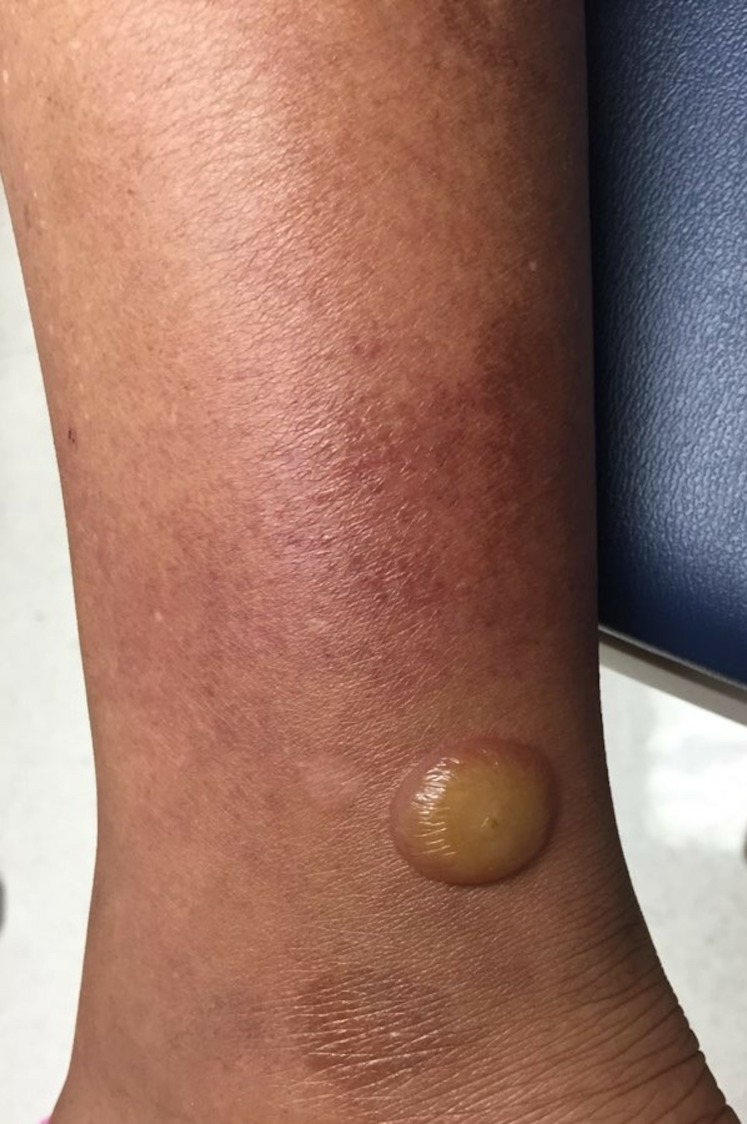
At initial presentation, the patient did not have any active lesions. Several weeks later, she returned with recurrent active lesions associated with outdoor exposure and presumed insect bites (figures 1 and 2). She had no ulcerations in her mouth and Nikolsky’s sign was negative. With initial punch biopsy results showing ‘eosinophilic spongiosis’, we favoured a bullous arthropod eruption versus insect bite-like reaction. Over several emergency department visits and clinic visits, the patient was treated with supportive care, in addition to insect-bite avoidance, insect repellent, antihistamines, and topical, intralesional and systemic steroids.
Figure 1.
Tense bulla with clear fluid and surrounding pink, severely indurated 8–10 cm plaque on left thigh.
Figure 2.
Tense bulla on right lower extremity.
The patient was seen a year later, and was noted to have new tense bullae on upper and lower extremities. In addition, clinical examination showed cervical lymphadenopathy, and the patient reported a 15-pound weight loss; thus, the patient was referred to haematology and oncology. Peripheral blood flow cytometry analysis revealed a differential diagnosis that included CLL/SLL and MCL.
After discussing the diagnosis of an indolent CD5+ B cell lymphoma (MCL vs CLL with an 11:14 translocation), the patient opted for clinical monitoring. However, she returned a few months later with left upper quadrant abdominal discomfort, additional weight loss and progressive anaemia. The patient also had significant leucocytosis and lymphocytosis. Additionally, the patient developed ophthalmoplegia with complete right eye ptosis and partial left eye ptosis, but without diplopia. Eye examination was consistent with bilateral CNIII (cranial nerve 3) palsy.
The patient was diagnosed with MCL, stage IVB, with an MCL International Prognostic Index in the high-risk category based on the study by Hoster et al.1
Investigations
On initial presentation, punch biopsy of a bullous lesion showed eosinophilic spongiosis (figure 3), with a histological differential diagnosis of arthropod bite reaction, eosinophilic cellulitis, drug reaction and hypersensitivity eruption. No flame figures were identified. Perilesional tissue direct immunofluorescence (DIF) testing was unremarkable except for ‘rare colloid bodies’.
Figure 3.
(10×) H&E stain. Numerous eosinophils are present in the papillary dermis.
On return to clinic 1 year later, a biopsied lesion on the distal lower extremity was diagnosed by pathology as a ‘subepidermal blister with eosinophils’ (figure 4). DIF did not reveal immunopathological abnormality.
Figure 4.
(20×) H&E stain. There is a subepidermal bulla with numerous eosinophils present within the split.
After referral to haematology and oncology, peripheral blood flow cytometry analysis revealed a CD5-positive B-cell lymphoproliferative disorder (described as an atypical B-cell population expressing CD5, CD19, CD20, CD22, CD45 and kappa surface light chain, but not CD10 or CD23), with a differential diagnosis that included CLL/SLL and MCL. Additional analysis of peripheral blood by fluorescence in-situ hybridization indicated the presence of a translocation between t(11;14)(q13;q32) with fusion of CCND1/IGH (cyclin D1/Immunoglobulin heavy) in 11% of cells examined.
A PET/CT study showed diffuse lymphadenopathy associated with mildly dilated spleen consistent with lymphoma (figure 5). Epstein-Barr virus (EBV) DNA was detected as 8300 copies/mL in the patient’s peripheral blood. Serum protein electrophoresis was normal pattern; however, serum immunofixation electrophoresis identified an IgM kappa monoclonal immunoglobulin. She had elevated serum free kappa and lambda light chains with a normal kappa/lambda ratio of 0.83. She also had an elevated IgG, IgA and IgM.
Figure 5.

From left to right: CT, PET and fused PET/CT showing (A) cervical, (B) axillary, (C) mediastinal, (E) iliac and (F) inguinal lymphadenopathy associated with intense FDG (fluoro-deoxyglucose) uptake in the (D) mildly dilated spleen consistent with lymphoma.
A few months later, biopsies of an enlarged lymph node, lung nodule and bone marrow showed CD5+ mature B-cell lymphoma with kappa restriction: expressing similar phenotype to peripheral blood flow cytometry results; with immunohistochemistry positive for CD20 and cyclin D1, and variably positive for MIB-1. PET/CT findings were concerning for worsening disease. Orbital MRI showed enhancement of bilateral cavernous sinuses, concerning for lymphomatous infiltration.
Differential diagnosis
Histopathological differential diagnoses included bullous arthropod reaction, drug reaction, eosinophilic cellulitis, hypersensitivity eruption and Churg-Strauss syndrome. Bullous arthropod reaction was favoured, given no systemic findings.
Treatment
The patient was admitted to the hospital for chemotherapy initiation. She received a total of 6 cycles of bendamustine plus rituximab with significant response to treatment, including regression of adenopathy, normalisation in spleen size, decreased lymphocytosis, improvement of haemoglobin/platelets and less severe skin reactions to arthropod bites. In addition, EBV DNA was no longer detected in the patient’s serum after completing chemotherapy.
Outcome and follow-up
At dermatology follow-up months later, the patient continued to have cutaneous reactions to arthropod bites, but they were less severe. Subsequently, PET/CT showed disease in the nasal cavity, which was later confirmed to be refractory MCL on biopsy of the nasal cavity.
The patient subsequently completed 3 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone and cytarabine chemotherapy, followed by 3 cycles of rituximab and Ara-c chemotherapy. She later developed central nervous system relapse with leptomeningeal disease and persistent leg paralysis with cauda equina syndrome. She was treated with high-dose methotrexate, and has now been transitioned to treatment with ibrutinib.
Discussion
In the above case, the recurrent cutaneous eruptions preceded the diagnosis of a haematological malignancy by almost a year. Prior cases of cutaneous lesions, including exaggerated reaction to insect bites, HMB and insect bite-like reaction, preceding the diagnosis of haematological malignancy have been reported.4 11 14 16–18 Thus, we demonstrate the importance of recognising these cutaneous lesions as a potential sign of underlying MCL and other haematological malignancies, and need for screening for an underlying malignancy.
Several cases in East Asia have been reported that demonstrate an association among MCL, EBV and intense skin reactions to insect bites, including exaggerated reaction to insect bites and HMB.13–16 In addition, three cases of insect bite-like reactions (without known insect bites) in patients with MCL reported no EBV association.11 12 The pathogenesis of the skin eruption may be related to the release of different cytokines that also trigger an IgE elevation and the presence of dermal eosinophils. Of note, several patients with MCL classified as stage IV have extracutaneous signs associated with lymph nodes, the bone marrow and/or the gastrointestinal tract. Patients with widespread MCL who developed skin lesions often had an unfavourable course.23
There is a potential association between EBV and MCL.13 24 25 EBV infection may contribute or be related to the malignant phenotype of B-cell non-Hodgkin’s lymphomas, or alternatively EBV infection may occur after malignant transformation.13 26 27 Moreover, the systemic symptoms and HMB may be due to host immune responses to EBV reactivation.28 Although the role of the virus on the development of MCL or the cutaneous eruptions is unclear, our case demonstrates a potential correlation between intense skin reactions to mosquito bites and MCL in the setting of EBV infection.
With chemotherapy, serum EBV DNA was undetectable and the cutaneous reactions to mosquito bites improved. Many treatment modalities have been used for the treatment of insect bite-like reactions and exaggerated reactions to insect bites associated with haematological malignancies.13 27 These include topical corticosteroids, antipruritics, systemic antihistamines, systemic glucocorticoids, IVIG and chemotherapy.4 11 12 14 Variable improvement in skin lesions has been reported with chemotherapy.4 11 14 18
In our patient, recurrent bullous eruptions treated with intralesional triamcinolone injections and topical clobetasol ointment showed a partial response to treatment. In addition, the patient subsequently had improvement of bullous arthropod-bite eruptions following chemotherapy treatment with bendamustine plus rituximab for MCL stage IVB.
In summary, we describe an adult patient who presented with an exaggerated skin reaction to insect bites and later diagnosed with MCL in the setting of EBV viraemia, all three of which improved after treatment with bendamustine plus rituximab. In addition, we demonstrate the importance of screening for an underlying haematological malignancy in the setting of recurrent bug bite reactions.
Patient’s perspective.
This journey has been tough for me. Not knowing why the mosquito bites appeared so severely was frustrating. I hope that this case helps others in an educational way.
Learning points.
Severe insect bite reactions and insect bite-like reactions are occasionally observed in patients with haematological malignancies, most commonly in chronic lymphocytic leukaemia and less commonly in mantle cell lymphoma.
Various cases of cutaneous lesions, including exaggerated reaction to insect bites, HMB and insect bite-like reaction, occurring before the diagnosis of haematological malignancy have been previously reported.
Our case demonstrates a potential correlation between intense skin reactions to mosquito bites and mantle cell lymphoma in the setting of Epstein-Barr virus infection.
In the setting of recurrent bug bites, appropriate screening and therapy initiation can allow for faster diagnosis of haematological malignancies and subsequent improvement of cutaneous symptoms.
Footnotes
Contributors: KD and EB-a are joint first authors of this case report. KD contributed to chart review, data collection, manuscript writing and editing, manuscript formatting and submission, patient consent, inclusion of patient photographs and follow-up data, and manuscript revisions. EB-a contributed to chart review, data collection, and manuscript writing, editing, and revisions. MD contributed to chart review; data collection; manuscript writing, editing, revisions, and review; and mentorship. MG contributed to manuscript editing, revisions, and comprehensive review, overall mentorship, and is the corresponding author. All authors agreed on the final version of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558–65. 10.1182/blood-2007-06-095331 [DOI] [PubMed] [Google Scholar]
- 2. Schlette E, Fu K, Medeiros LJ. CD23 expression in mantle cell lymphoma: clinicopathologic features of 18 cases. Am J Clin Pathol 2003;120:760–6. 10.1309/XV4AG7EMWQU7ER67 [DOI] [PubMed] [Google Scholar]
- 3. Weed RI. Exaggerated delayed hypersensitivity to mosquito bites in chronic lymphocytic leukemia. Blood 1965;26:257–68. [PubMed] [Google Scholar]
- 4. Davis MD, Perniciaro C, Dahl PR, et al. Exaggerated arthropod-bite lesions in patients with chronic lymphocytic leukemia: a clinical, histopathologic, and immunopathologic study of eight patients. J Am Acad Dermatol 1998;39:27–35. 10.1016/S0190-9622(98)70398-6 [DOI] [PubMed] [Google Scholar]
- 5. Rosen LB, Frank BL, Rywlin AM. A characteristic vesiculobullous eruption in patients with chronic lymphocytic leukemia. J Am Acad Dermatol 1986;15:943–50. 10.1016/S0190-9622(86)70254-5 [DOI] [PubMed] [Google Scholar]
- 6. Kolbusz RV, Micetich K, Armin AR, et al. Exaggerated response to insect bites. An unusual cutaneous manifestation of chronic lymphocytic leukemia. Int J Dermatol 1989;28:186–7. 10.1111/j.1365-4362.1989.tb02460.x [DOI] [PubMed] [Google Scholar]
- 7. Pedersen J, Carganello J, van der Weyden MB. Exaggerated reaction to insect bites in patients with chronic lymphocytic leukemia. Clinical and histological findings. Pathology 1990;22:141–3. 10.3109/00313029009063552 [DOI] [PubMed] [Google Scholar]
- 8. Houston JG, Keene WR. Exaggerated delayed hypersensitivity to mosquito venom in association with lymphoproliferative disorders. J Fla Med Assoc 1970;57:15–17. [PubMed] [Google Scholar]
- 9. Asakura K, Kizaki M, Ikeda Y. Exaggerated cutaneous response to mosquito bites in a patient with chronic lymphocytic leukemia. Int J Hematol 2004;80:59–61. 10.1532/IJH97.04021 [DOI] [PubMed] [Google Scholar]
- 10. Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci 2016;82:145–52. 10.1016/j.jdermsci.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 11. Barzilai A, Shpiro D, Goldberg I, et al. Insect bite-like reaction in patients with hematologic malignant neoplasms. Arch Dermatol 1999;135:1503–7. 10.1001/archderm.135.12.1503 [DOI] [PubMed] [Google Scholar]
- 12. Dodiuk-Gad RP, Dann EJ, Bergman R. Insect bite-like reaction associated with mantle cell lymphoma: a report of two cases and review of the literature. Int J Dermatol 2004;43:754–8. 10.1111/j.1365-4632.2004.02145.x [DOI] [PubMed] [Google Scholar]
- 13. Shigekiyo T, Ohmori H, Chohraku M, et al. Unusual skin reactions after mosquito bites and Epstein-Barr virus reactivation in a patient with mantle cell lymphoma. Intern Med 2004;43:986–9. 10.2169/internalmedicine.43.986 [DOI] [PubMed] [Google Scholar]
- 14. Mori T, Okamoto S, Kuramochi S, et al. An adult patient with hypersensitivity to mosquito bites developing mantle cell lymphoma. Int J Hematol 2000;71:259–62. [PubMed] [Google Scholar]
- 15. Konuma T, Uchimaru K, Sekine R, et al. Atypical hypersensitivity to mosquito bites without natural killer cell proliferative disease in an adult patient. Int J Hematol 2005;82:441–4. 10.1532/IJH97.05019 [DOI] [PubMed] [Google Scholar]
- 16. Kunitomi A, Konaka Y, Yagita M. Hypersensitivity to mosquito bites as a potential sign of mantle cell lymphoma. Intern Med 2005;44:1097–9. 10.2169/internalmedicine.44.1097 [DOI] [PubMed] [Google Scholar]
- 17. Khamaysi Z, Dodiuk-Gad RP, Weltfriend S, et al. Insect bite-like reaction associated with mantle cell lymphoma: clinicopathological, immunopathological, and molecular studies. Am J Dermatopathol 2005;27:290–5. [DOI] [PubMed] [Google Scholar]
- 18. Chassine AF, Dadban A, Charfi S, et al. [Eosinophilic dermatosis associated with hematological disorders: a clinical, histopathological and immunohistochemical study of six observations]. Ann Dermatol Venereol 2010;137:181–8. 10.1016/j.annder.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 19. Tokura Y, Ishihara S, Tagawa S, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol 2001;45:569–78. 10.1067/mjd.2001.114751 [DOI] [PubMed] [Google Scholar]
- 20. Ishihara S, Yabuta R, Tokura Y, et al. Hypersensitivity to mosquito bites is not an allergic disease, but an Epstein-Barr virus-associated lymphoproliferative disease. Int J Hematol 2000;72:223–8. [PubMed] [Google Scholar]
- 21. Asada H. Hypersensitivity to mosquito bites: a unique pathogenic mechanism linking Epstein-Barr virus infection, allergy and oncogenesis. J Dermatol Sci 2007;45:153–60. 10.1016/j.jdermsci.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 22. deShazo RD, Feldlaufer MF, Mihm MC, et al. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012;125:688–94. 10.1016/j.amjmed.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 23. Kalińska-Bienias A, Ziarkiewicz-Wróblewska B, Kowalewski C, et al. Mantle cell lymphoma with skin involvement. Postepy Dermatol Alergol 2015;32:229–34. 10.5114/pdia.2014.44028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hummel M, Anagnostopoulos I, Korbjuhn P, et al. Epstein-Barr virus in B-cell non-Hodgkin’s lymphomas: unexpected infection patterns and different infection incidence in low- and high-grade types. J Pathol 1995;175:263–71. 10.1002/path.1711750303 [DOI] [PubMed] [Google Scholar]
- 25. Daibata M, Kubonishi I, Miyoshi I. Differential tumorigenicity between Epstein-Barr virus genome-positive and genome-negative cell lines with t(11;14)(q13;q32) derived from mantle cell lymphoma. J Virol 1996;70:9003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roh EJ, Chung EH, Chang YP, et al. A case of hypersensitivity to mosquito bite associated with Epstein-barr viral infection and natural killer cell lymphocytosis. J Korean Med Sci 2010;25:321–3. 10.3346/jkms.2010.25.2.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higuchi M, Muta T, Karube KN, et al. Epstein-Barr virus-positive blastoid variant of mantle cell lymphoma in an adult with recurrent infectious mononucleosis-like symptoms: a case report. Int J Hematol 2007;85:219–22. 10.1532/IJH97.06178 [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto T, Hirai Y, Miyake T, et al. Epstein-Barr virus reactivation is induced, but abortive, in cutaneous lesions of systemic hydroa vacciniforme and hypersensitivity to mosquito bites. J Dermatol Sci 2016;82:153–9. 10.1016/j.jdermsci.2016.03.001 [DOI] [PubMed] [Google Scholar]