Abstract
Immune checkpoint inhibitors (ICPis) have revolutionised survival outcomes for cancer patients by bolstering anti-tumour immunity. However, immune activation also occurs in non-cancer tissue, and a significant proportion of patients develop immune-mediated colitis, which can be fatal if not promptly recognised and managed. Diagnosis is often made by inflammation observed during lower gastrointestinal endoscopy. Little is known about microscopic inflammation (histological findings of inflammation in the absence of overt mucosal injury). Management strategies beyond the use of systemic corticosteroids, which incur a high burden of deleterious side effects, have not been extensively explored. We describe the cases of two cancer patients with ICPi-induced colitis who had isolated histoloigical features of colitis in the absence of macroscopic disease. Sustained clinical and histological remission was induced with the topical steroid preparation, beclometasone dipropionate (Clipper), with no adverse effects.
Keywords: immunology, inflammatory bowel disease, oncology
Background
Immune check point inhibitors (ICPis) including anti-PD-1 and anti-CTLA-4 antibodies, have transformed outcomes for cancer patients, however, global augmentation of the immune response is associated with an increased risk oftriggering immune mediated toxicity, including ICPi-induced colitis. ICPi-colitis, resembling some aspects of inflammatory bowel disease (IBD), is the most common reason for discontinuing ICPi therapy (up to 39% of patients),1 the most common cause of severe toxicity2 and ICPi-related death.3 ICPi use is anticipated to increase exponentially, rendering an urgent need for the development of evidence-based treatment algorithms.
Management of ICPi-colitis typically involves systemic corticosteroids, with biologics (most commonly anti-TNF), used in refractory disease. However systemic corticosteroids are fraught with side effects including life-threatening infections.4 There is an unmet need to identify effective anti-inflammatory agents with more favourable side effect profiles, which is especially pertinent in patients with additional comorbidities. Furthermore, there are theoretical concerns that prolonged, high-dose immunosuppression could hamper anti-tumour immunity, although retrospective review of clinical trial data has failed to realise these concerns.5
We describe the clinical, endoscopic and histological features of two ICPi treated patients who developed ICPi-colitis, but with isolated microscopic disease, which is an emerging phenotype that is poorly characterised. Importantly, this is also the first description of successful treatment with Clipper (beclometasone dipropionate), which is a second-generation topical corticosteroid with fewer systemic side effects. The low cost, low toxicity and promising efficacy of second generation topical corticosteroids renders them highly attractive treatment options, and makes a case for assessing their potential further in prospective ICPi-colitis trials.
Case presentation
Patient 1
A 64-year-old woman diagnosed with metastatic melanoma had wide local excision of the cancer in her left cheek and lymph node dissection in the neck, but experienced recurrence 2 years later. Her medical history also included insulin dependent type 2 diabetes, hypertension and hypothyroidism. She was started on anti-PD-1 therapy (pembrolizumab) and experienced drug-induced gastrointestinal (GI) toxicity 10 weeks after onset of therapy. Her symptoms included occasional mild lower cramping abdominal pain and intermittent non-bloody loose stool occurring up to eight times/day. Apart from fatigue, she had no other associated symptoms.
Patient 2
A 78-year-old woman with a recent diagnosis of metastatic non-small cell lung cancer, who also had a history of hypertension, diverticular disease and chronic obstructive pulmonary disease (COPD). She was started on combination anti-PD-1 and anti-CTLA-4 immunotherapy (nivolumab and ipilimumab) as part of the Checkmate 817 trial and experienced GI symptoms after 12 weeks. This included left-sided cramping abdominal pain and intermittent loose non-bloody stool up to four times/day. She had a reduced appetite but no weight loss and no other associated symptoms.
Patient 1
Blood tests at the time of her GI symptoms were unremarkable. Stool tests were negative for bacterial infection. A faecal calprotectin was mildly raised at 62 µg/g.
A flexible sigmoidoscopy was normal to the point of insertion (figure 1). Four rectosigmoid biopsies were taken which showed mild chronic inflammation of the lamina propria and cryptitis (figure 2A,B), with inflammation seen in all biopsies. There was no evidence of cytomegalovirus infection (CMV).
Figure 1.

Endoscopic image of the sigmoid colon showing a normal appearance of the mucosa.
Figure 2.
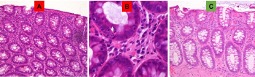
H&E stain of rectosigmoid mucosa in patient 1 showing mild acute and chronic inflammation with expansion of neutrophils and lymphocytes in the lamina propria (A) and infiltration of neutrophils into the crypts-cryptitis (B). Resolution of inflammation post-Clipper therapy (C).
Patient 2
Blood tests showed a normal full blood count, and a mildly raised CRP of 11 (which was also present before ICPi therapy was commenced). Stool tests were negative for bacterial infection, Clostridium difficile and parasites. A faecal calprotectin was raised at 346 µg/g. An abdominal X-ray showed some prominent small bowel loops but was otherwise unremarkable.
A flexible sigmoidoscopy was normal to the point of insertion. Four rectosigmoid biopsies showed moderate chronic inflammation of the lamina propria, with flattening of the surface epithelium and an increase in number of surface apoptotic bodies (figure 3A). Again, inflammation was seen in all biopsies and there was no evidence of CMV.
Figure 3.

H&E stain of rectosigmoid mucosa in patient 2 showing moderate acute and chronic inflammation with expansion of neutrophils and lymphocytes in the lamina propria, flattening of the surface epithelium (black arrow) and an increase in number of surface apoptotic bodies (A). Resolution of inflammation post-Clipper therapy (B).
Treatment
Both patients received 5 mg of oral Clipper (beclometasone dipropionate) once daily, for 4 weeks. They were not on any other immunosuppressive therapy.
Outcome and follow-up
Following initiation of Clipper therapy, clinical remission (cessation of diarrhoea and abdominal pain) occurred on day 3 in patient 1, and day 7 in patient 2.
Repeat flexible sigmoidoscopy was performed two weeks after completion of Clipper therapy, with four biopsies taken from the rectosigmoid junction. This showed resolution of histological inflammation in both patients (figure 2C and figure 3B).
There were no treatment-related adverse events. Moreover, patient 1 who had a history of insulin dependent diabetes, reported stable blood sugar levels in contrast to a previous experience with systemic corticosteroids which had necessitated medical input and alterations to her insulin regimen.
At 6 months follow-up, both patients remained in remission from colitis. Outcomes following resumption of ICPi post-ICPi-colitis is an area of research still in its infancy. However, ICPi therapy was not resumed in either of our patients. Patient 1 also had a history of previous ICPi-induced skin toxicity, and in the context of a good response to immunotherapy from a cancer point of view, ICPi therapy was suspended. Patient 2 had previously experienced other immune-mediated toxicities, including hepatitis and pneumonitis, and in view of stable disease from a cancer perspective, she was kept under surveillance.
Discussion
Immune checkpoints such as CTLA-4 and PD-1, are regulatory molecules expressed by immune cells responsible for ‘switching off’ the immune system and are important for maintaining tissue homeostasis. Antagonism of these inhibitory checkpoints with ICPis, trigger hyperactivation of immune cells with exaggerated effector responses, including significant augmentation of anti-tumour immune responses. Consequently, ICPis have revolutionised the treatment landscape for several cancers.6 Although ‘turbo-charged’ immune responses stimulate anti-tumour immunity, this comes at the cost of triggering severe immune-mediated injury to non-cancer tissues. The most frequently occurring serious and life threatening of these includes severe diarrhoea/colitis, which affects up to 44% of ICPi-treated patients.7 This is particularly common in combination therapy (anti-CTLA-4 and anti-PD-1),7 which offers the best chance of long-term survival for some cancers.1 Manifestations include severe gut damage (mucosal ulceration, intestinal perforation), and disabling symptoms (diarrhoea, faecal incontinence, pain).8 There is a wide spectrum of histopathological findings including presence of intraepithelial neutrophilic lymphocytes, crypt distortion, cryptitis, crypt abscesses, lymphoplasmacytosis, ulceration and apoptotic bodies.9 10 The presence of epithelial apoptotic bodies, as evident in patient 2, also bears some resemblance to graft versus host disease (GvHD)11 although the density of apoptotic bodies are more pronounced in GvHD and is typically accompanied by a mononuclear, rather than mixed inflammatory infiltrate.
We report an emerging phenotype in two ICPi-treated patients who had macroscopically normal appearnces of the colon, but microscopic (histological) evidence of inflammation. This is distinct to endoscopically visible inflammation8 9 and microscopic colitis (encompassing collagenous colitis and lymphocytic colitis), which have been described in this context.12 13 Our findings reinforce the importance of performing colonic biopsies in all patients with suspected ICPi-colitis who have normal looking colonic mucosa at endoscopy. Further work is needed to determine if microscopic inflammation is a distinct phenotype, or an event that precedes development of macroscopically evident inflammation.
A limitation of this study was that patients were investigated with flexible sigmoidoscopy, and therefore could have missed isolated right sided colitis. However, in one observational study, 38/39 patients with ICPi-colitis had inflammation affecting the rectosigmoid junction.8 Furthermore, the relative ease of accessing flexible sigmoidoscopy (cheaper, quicker without the need for oral bowel preparation) makes this investigation a pragmatic and straightforward first line inverstigation. Nevertheless, persistent symptoms despite anti-inflammatory treatment should prompt consideration of full colonoscopic evaluation (which was not necessary in our patients due to their excellent response to treatment).
Currently, management of ICPi-colitis is guided by the European Society of Medical Oncology (ESMO), which uses the standardised Common Terminology Criteria for Adverse Events (CTCAE) system to determine severity of GI toxicity and guide subsequent management.14 Accordingly, both patients would have normally received high-dose oral corticosteroids with a slow tapering regimen, associated with a high likelihood of side effects. However, we report clinical and histological remission induced by Clipper, a drug which is currently licensed for management of mild to moderate flares in ulcerative colitis.15It is worth noting that our patients did not exhibit features of severe colitis and until emerging data informs otherwise, systemic corticosteroids are likely to remain the therapy of choice in this context.
Clipper is an oral controlled release preparation of beclometasone dipropionate, a topical steroid that is slowly released in the distal small bowel and proximal colon where it provides coverage to the entire colon, and has minimal systemic absorption. This obviates the need for concurrent prescription of bone and gastric protection medication, as is often necessary with systemic corticosteroids.
In the context of diabetes, Clipper had added value for patient 1, given previous challenges in managing her blood glucose during corticosteroid treatment. The potential to circumvent corticosteroids is highly appealing—not only because of the promise in reducing systemic corticosteroid-related morbidity and mortality, but the shorter treatment course associated with Clipper may permit timely resumption of anti-cancer therapy if required.
Learning points.
The use of immune check point inhibitors (ICPis) is rising exponentially and clinicians will be increasingly faced with the management of their associated toxicities, including ICPi-colitis.
ICPi-colitis is an emerging mucosal gut disease which resembles some aspects of inflammatory bowel disease.
Every patient undergoing endoscopy for suspected ICPi-colitis, should have colonic biopsies taken regardless of endoscopic findings.
ICPi-induced microscopic colitis may be an under recognised and very treatable entity.
There may be a role for Clipper in inducing clinical and histological remission, thus obviating the need for systemic corticosteroids.
Footnotes
Contributors: HI: substantially contributed to write-up of the manuscript. MG: obtained and annotated the histology images, drafted the work. SP: drafted the work and revised it critically. NP: drafted the work and revised it critically.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 2017;119:1–12. 10.1016/j.critrevonc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 3. De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res 2017;5:312–8. 10.1158/2326-6066.CIR-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Castillo M, Romero FA, Argüello E, et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis 2016;63:1490–3. 10.1093/cid/ciw539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–8. 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8. Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with Anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. 10.1093/ecco-jcc/jjv227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geukes Foppen MH, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 2018;3:e000278 10.1136/esmoopen-2017-000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coutzac C, Adam J, Soularue E, et al. Colon immune-related adverse events: Anti-CTLA-4 and Anti-PD-1 blockade induce distinct immunopathological entities. J Crohns Colitis 2017;11:1238–46. 10.1093/ecco-jcc/jjx081 [DOI] [PubMed] [Google Scholar]
- 11. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–9. 10.1200/JCO.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baroudjian B, Lourenco N, Pagès C, et al. Anti-PD1-induced collagenous colitis in a melanoma patient. Melanoma Res 2016;26:308–11. 10.1097/CMR.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 13. Choi K, Abu-Sbeih H, Samdani R, et al. Can Immune Checkpoint Inhibitors Induce Microscopic Colitis or a Brand New Entity? Inflamm Bowel Dis 2019;25:385–93. 10.1093/ibd/izy240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haanen JB, Thienen H, Blank CU. Toxicity patterns with immunomodulating antibodies and their combinations. Semin Oncol 2015;42:423–8. 10.1053/j.seminoncol.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 15. Stenke E, Hussey S. Ulcerative colitis: management in adults, children and young people (NICE Clinical Guideline CG166). Arch Dis Child Educ Pract Ed 2014;99:194–7. 10.1136/archdischild-2013-305512 [DOI] [PubMed] [Google Scholar]


