Abstract
Adrenal infarction is a rare event, especially in pregnancy. The diagnosis is challenging because patients present with acute abdomen and initial workup are usually unrevealing. We present a case of unilateral adrenal infarction in a pregnant young woman without any other causes of thrombophilia, who presented with acute abdominal pain and an unremarkable initial workup. MRI and contrast-enhanced CT scan revealed a non-haemorrhagic infarct of the right adrenal gland. Our case highlights the importance of considering this rare diagnosis in the differential for a pregnant woman with acute abdomen without any obvious surgical cause.
Keywords: adrenal disorders, haematology (incl blood transfusion), pregnancy, radiology
Background
Adrenal glands are essential endocrine organs responsible for maintaining the haemodynamic stability, electrolyte balance and the stress response of the human body. Any injury to these organs leading to adrenal insufficiency can be life-threatening. Adrenal gland infarction is usually haemorrhagic and bilateral.1 Unilateral adrenal gland infarction in pregnancy is extremely rare, with eight cases reported in medical literature to date.2–6 Most of these infarcts were associated with an underlying thrombophilic syndrome, like antiphospholipid syndrome, Factor VIII abnormality4 or methylenetetrahydrofolatereductase gene mutation.2
Case presentation
A 21-year-old obese G1P0 woman presented to the hospital at 28 4/7 weeks of gestation with severe right-sided abdominal pain. Her abdominal pain started 1 week ago in the right upper quadrant and more recently she also developed an additional right lower quadrant pain. This was associated with nausea and emesis of brown-coloured food content. She denied fevers or diarrhoea but endorsed chills. Her medical history was significant for childhood asthma and her medications included prenatal vitamins and iron supplements. She was a non-smoker, denied history of deep venous thrombosis or pulmonary embolism, using oral contraceptives and any prior pregnancy. She did not have a family history of bleeding or clotting disorders, nor of spontaneous abortions. She worked as a primary school teacher with no recent sick contacts.
Physical examination on presentation was significant for a distended abdomen from pregnancy, with fundus palpated 7 cm above umbilicus and exquisite tenderness to palpation over the right upper and lower quadrants with a positive Murphy’s sign. Initial lab work revealed moderate anaemia, haemoglobin 0.8 g/L and haematocrit 28%, mild leucocytosis, white blood cell count 13.5 ×109/L, and mildly elevated alkaline phosphatase to 124 U/L. A right upper quadrant ultrasound revealed gallbladder sludge and a right lower quadrant ultrasound could not visualise the appendix.
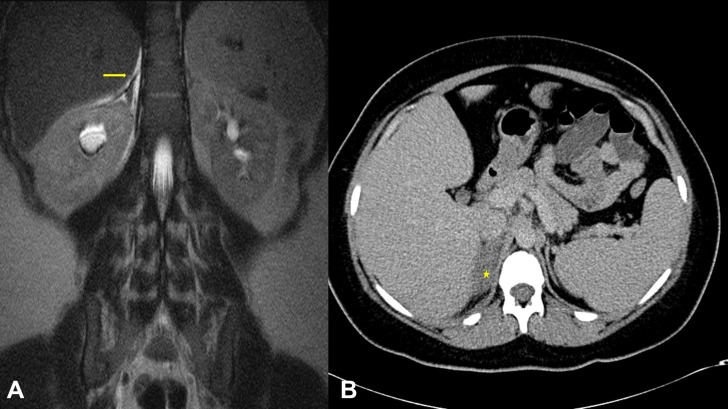
Due to concerns of acute appendicitis, she was started on intravenous piperacillin-tazobactam and a MRI abdomen/pelvis without contrast (figure 1A) was obtained to further evaluate for appendicitis. The MRI revealed a normal appendix and gallbladder but showed an abnormal right adrenal gland with increased T2 signal with surrounding oedema, concerning for non-haemorrhagic adrenal infarct. The left adrenal gland appeared normal. An urgent duplex study was obtained to look for renal and/or adrenal vein thrombosis, and it revealed patent right and left renal and adrenal veins as well as a patent inferior vena cava. After discussion with radiology team, a limited upper abdomen CT scan with intravenous contrast (figure 1B) was performed which showed a hypoenhancing enlarged right adrenal gland with mild stranding, consistent with unilateral non-haemorrhagic adrenal infarction.
Figure 1.
(A) Coronal section of a T2-weighted MRI of the abdomen showing a hyperintense right adrenal gland (yellow arrow) as compared with the left adrenal gland. (B) Transverse section of a contrast-enhanced CT scan of the abdomen showing poor enhancement of the right adrenal gland (yellow star) as compared with the left adrenal gland.
Antibiotics were discontinued, and pain was controlled with intravenous morphine. We pursued further workup for thrombophilia with possible arterial thrombus, differentials being antiphospholipid syndrome, paroxysmal nocturnal haemoglobinuria, JAK2 mutation and the rare paradoxical venous thromboembolism. Thrombophilia evaluation was unremarkable with a normal international normalized ratio (INR), factors V and VIII, prothrombin time (PT), partial thromboplastin time (PTT) levels, normal homocysteine, negative factor V Leiden or prothrombin gene 20210A mutations, negative lupus anticoagulant, negative anti-cardiolipin IgG and IgM antibodies, negative anti-beta-2 glycoprotein IgG and IgM antibodies and negative JAK2 mutation. A lower extremity Doppler ultrasound did not reveal any evidence of deep vein thrombosis. A morning cortisol level of 16.3 ug/dL was reassuring for an adequately functioning left adrenal gland. She was started on subcutaneous enoxaparin 80 mg two times per day and discharged home with plan for outpatient echocardiogram with bubble study.
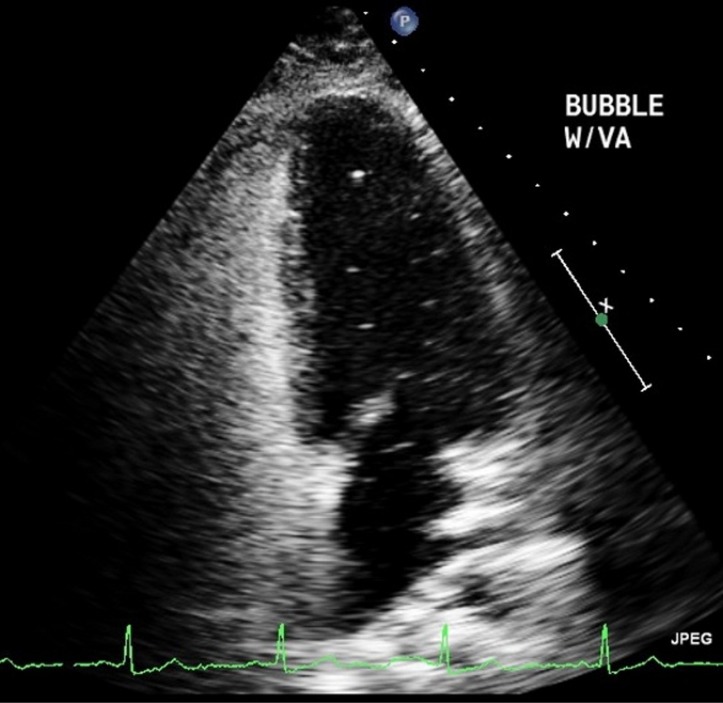
An echocardiogram with a bubble study (figure 2) demonstrated the presence of bubbles in the left-sided chambers, suggestive of a patent foramen ovale(PFO). At 40 weeks of gestation, the patient was admitted for induction of labour, at which time her enoxaparin was switched to heparin drip followed by delivery of a healthy female neonate. She was restarted on enoxaparin at discharge as a bridge to coumadin anticoagulation. The patient was advised to remain anticoagulated for at least 4–6 weeks but unfortunately, she did not comply with these recommendations. She was subsequently seen by a cardiologist who recommended lifelong aspirin therapy with expert evaluation for PFO closure in the future.
Figure 2.
Echocardiogram with agitated saline showing bubbles in the left ventricle.
Outcome and follow-up
The patient was seen in the clinic 8 weeks after discharge and had not complied with anticoagulation or aspirin recommendations as above. But fortunately, she was doing well. She did not report any recurrent abdominal pain, lower extremity pain, swelling or breathing difficulties.
Discussion
Unilateral adrenal infarct can present as an acute abdomen with sudden onset severe unilateral abdominal pain, associated with nausea and vomiting. Patients also usually have mild leucocytosis, probably reactive in nature. Since the presentation is concerning for more common surgical conditions like acute cholecystitis, acute pancreatitis, acute appendicitis, ureteric stone or even ovarian torsion, ultrasonography is usually the first radiological investigation performed in a pregnant woman. In the absence of an obvious diagnosis with ultrasound, MRI is the next preferred modality due to its safety in pregnancy. MRI findings of non-haemorrhagic adrenal infarction have only recently been defined in a 10-year single institution study.6 These consist of increased T2 signal intensity of the infarcted adrenal with surrounding oedema and without T1 intensification to suggest lack of haemorrhage, as seen in our patient. She further underwent a contrast-enhanced CT scan which confirmed a hypoenhancing infarcted adrenal gland.
The pathogenesis of adrenal infarction has been best described by Fox in his seminal paper in the Journal of Pathology.7 He reports that most cases of adrenal infarctions are venous in nature due to thrombi in the intra-adrenal veins, venous sinuses, cortical sinuses, capsular veins and the main extra-adrenal veins. The lack of adrenal haemorrhage in our patient could be explained by one of his hypotheses—either the blood is able to bypass the thrombosed vein and leave the gland through other veins. Or spasm of the capsular arteries, in response to adrenal vein thrombosis, causes cortical necrosis without haemorrhage.
Once the diagnosis of adrenal infarction is made, anticoagulation is usually started in consultation with a haematologist. This has been believed to prevent recurrent thrombosis, specially in the physiologically hypercoagulable state of pregnancy. Low-molecular weight heparin like enoxaparin is recommended as first choice for anticoagulation in pregnancy because it does not cross the placenta and is safe for the fetus. Since the risk of postpartum thromboembolic events is higher than the risk during pregnancy, anticoagulation should be continued for 6–8 weeks post partum.8 Although, one author recommends conservative management based on a single case report showing complete resolution of symptoms and uncomplicated perinatal outcome without anticoagulation.9 A more recent case series of four patients with five instances of adrenal infarctions showed that two patients who did not receive anticoagulation had uncomplicated perinatal outcome but the third patient who was discharged without anticoagulation in the 17th week of gestation presented back in her 35th week with contralateral adrenal infarction and was then anticoagulated for 6 months.6
Adrenal insufficiency is very rare with adrenal infarction but should be considered in patients with haemodynamic compromise and/or electrolyte abnormalities. According to Jung et al, a morning cortisol level less than 16.3 ug/dL should raise concerns for adrenal insufficiency in the second trimester.10 If further workup is warranted, then a basal adrenocorticotrophic hormone (ACTH) level and cosyntropin stimulation test may be undertaken in addition to evaluating mineralocorticoid deficiency.
Our patient did have a small PFO diagnosed on an echocardiogram but the significance of this finding is unclear and is most likely an incidental finding. Unfortunately, there are no guidelines for definitive management of such patients including vis-à-vis closure of PFO and/or lifelong antiplatelet or anticoagulant therapy to prevent embolic events, especially cerebrovascular accidents.
Learning points.
Adrenal infarction in pregnancy is a rare event and may be associated with a systemic clotting disorder. Although the hypercoagulable state of pregnancy may in itself be a cause.
Adrenal infarction usually presents with an acute abdomen and initial diagnostic tests like ultrasound are unrevealing.
MRI is the next preferred modality in a pregnant woman due to its safety. The infarcted adrenal gland demonstrates increased T2 signal intensity with surrounding oedema and without T1 hyperintensity.
Once diagnosed, patients should receive a haematology consultation to determine need for therapeutic anticoagulation.
Footnotes
Contributors: KAA is responsible for the design of the work, analysis and interpretation of data, drafting the work, final approval of the version published and is accountable for all aspects of the work. MHS is responsible for the design of the work, analysis and interpretation of data, drafting the work, final approval of the version published and is accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Godfrey RL, Clark J, Field B. Bilateral adrenal haemorrhagic infarction in a patient with antiphospholipid syndrome. BMJ Case Rep 2014;2014:bcr2014207050 10.1136/bcr-2014-207050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green PA, Ngai IM, Lee TT, et al. Unilateral adrenal infarction in pregnancy. BMJ Case Rep 2013;2013:bcr2013009997 10.1136/bcr-2013-009997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sormunen-Harju H, Sarvas K, Matikainen N, et al. Adrenal infarction in a healthy pregnant woman. Obstet Med 2016;9:90–2. 10.1177/1753495X15627959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aljenaee KY, Ali SA, Cheah SK, et al. Unilateral adrenal infarction in pregnancy secondary to elevated factor VIII. Saudi Med J 2017;38:654–6. 10.15537/smj.2017.6.18520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guenette JP, Tatli S. Nonhemorrhagic Adrenal Infarction With Magnetic Resonance Imaging Features During Pregnancy. Obstet Gynecol 2015;126:775–8. 10.1097/AOG.0000000000000884 [DOI] [PubMed] [Google Scholar]
- 6. Glomski SA, Guenette JP, Landman W, et al. Acute Nonhemorrhagic Adrenal Infarction in Pregnancy: 10-Year MRI Incidence and Patient Outcomes at a Single Institution. AJR Am J Roentgenol 2018;210:785–91. 10.2214/AJR.17.18739 [DOI] [PubMed] [Google Scholar]
- 7. Fox B. Venous infarction of the adrenal glands. J Pathol 1976;119:65–89. 10.1002/path.1711190202 [DOI] [PubMed] [Google Scholar]
- 8. American College of Obstetricians and Gynecologists Women’s Health Care Physicians. ACOG Practice Bulletin No. 138: Inherited thrombophilias in pregnancy. Obstet Gynecol 2013;122:706–16. 10.1097/01.AOG.0000433981.36184.4e [DOI] [PubMed] [Google Scholar]
- 9. Gavrilova-Jordan L, Edmister WB, Farrell MA, et al. Spontaneous adrenal hemorrhage during pregnancy: a review of the literature and a case report of successful conservative management. Obstet Gynecol Surv 2005;60:191–5. 10.1097/01.ogx.0000157357.15401.c3 [DOI] [PubMed] [Google Scholar]
- 10. Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab 2011;96:1533–40. 10.1210/jc.2010-2395 [DOI] [PubMed] [Google Scholar]