Abstract
Primary adrenal leiomyosarcoma, a malignant soft tissue tumour originating from the smooth muscle of the inferior vena cava and adrenal vein, is rarely described in the literature. Cases are often diagnosed at an advanced stage as the tumour is not hormone-producing. We describe a 70-year-old man who presented with lower extremity swelling and abdominal varices and was subsequently found to have a large adrenal mass on imaging. Our case is among the few reported primary adrenal leiomyosarcomas in which a CT-guided biopsy was used to aid in diagnosis.
Keywords: urological cancer, pathology, interventional radiology, urological surgery, vascular surgery
Background
Leiomyosarcomas are malignant soft tissue tumours of smooth muscle origin. Traditionally, leiomyosarcomas are most commonly associated with the uterus, but they also frequently occur in the bowel, vasculature and dermis.1 Primary adrenal leiomyosarcomas (PAL) are extremely rare variants of these soft tissue neoplasms with 35 cases reported in the western literature, including the patient presented in this report. PAL is thought to originate from the smooth muscle wall of the central adrenal vein and its branches.2 Because PAL does not produce hormones, symptoms generally do not manifest until the tumour has reached an advanced size. Diagnosis of PAL is usually made via histological and immunohistochemical evaluation after surgical resection. We herein describe a case of a 70-year-old man who presented with lower extremity swelling and was diagnosed with PAL via a CT-guided biopsy prior to surgical resection.
Case presentation
The patient is a 70-year-old man who originally presented to his primary care physician with abdominal varices and lower extremity swelling. CT demonstrated a 12.2 cm mass arising from the right adrenal gland with extension into the inferior vena cava (IVC). A CT-guided biopsy was performed, and the subsequent pathology was consistent with leiomyosarcoma. Microscopic examination revealed a highly cellular atypical smooth muscle neoplasm with 40% proliferation on ki-67 stain; the tumour also stained strongly for vimentin and desmin and negatively for S-100 further supporting the diagnosis. The patient was accordingly scheduled for surgical resection.
Investigations
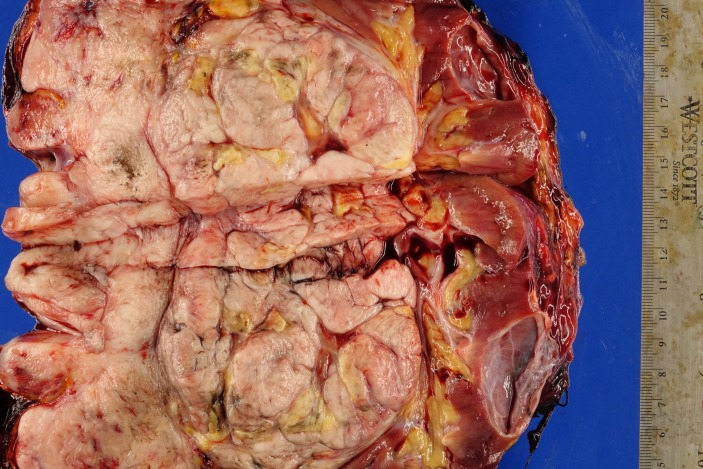
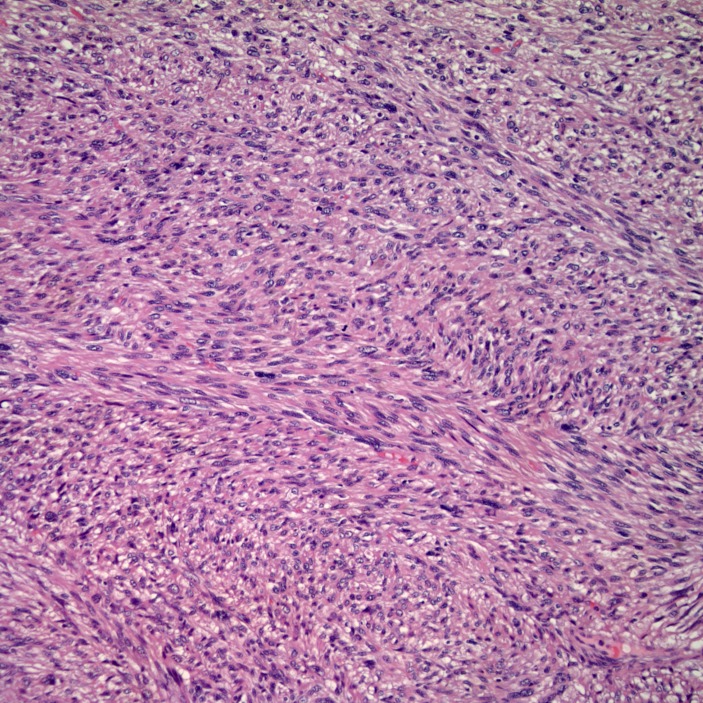
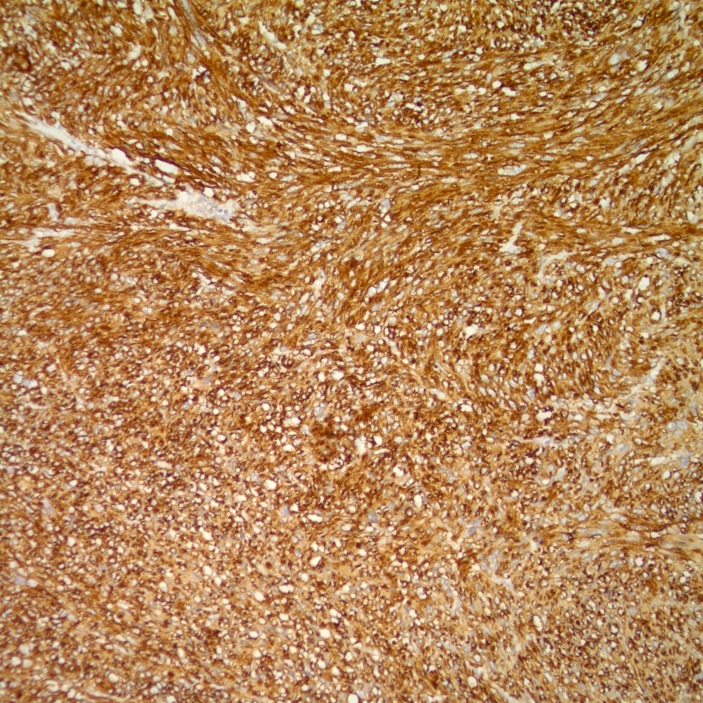
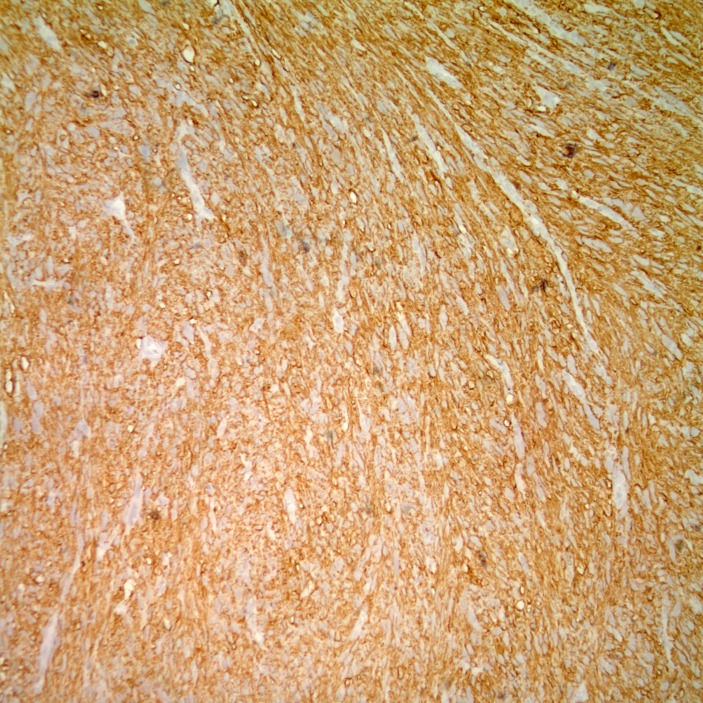
Gross examination of the surgical specimen (figure 1) demonstrated a 9.0×7.5×4.3 cm tan multilobulated mass which obliterated the adrenal gland and was adherent to the kidney; a tissue plane could not be identified. The right intracaval margin did not demonstrate tumour. Histological examination of the neoplasm demonstrated a hypercellular spindle cell neoplasm. The cells were cigar-shaped, running in parallel fascicles to one another, and demonstrated small, prominent nucleoli (figure 2). The tumour demonstrated strong, diffuse immunoreactivity to smooth muscle actin and caldesmon (figures 3 and 4), supporting the diagnosis of leiomyosarcoma. The final diagnosis rendered was that of a leiomyosarcoma, high grade (grade 3) with negative margins. There was no evidence of vascular invasion seen. Microscopic analysis of the mass divulges a stage pT2b, grade 3 leiomyosarcoma with no lymphovascular invasion and 1 cm negative surgical margins.
Figure 1.
Gross examination of the surgical specimen.
Figure 2.
The cells are cigar shaped, running in parallel fascicles to one another, and demonstrate small, prominent nucleoli.
Figure 3.
The tumour demonstrates strong, diffuse immunoreactivity to smooth muscle actin.
Figure 4.
The tumour also demonstrates strong, diffuse immunoreactivity to smooth muscle caldesmon.
Treatment
The surgery took place in April 2017 under the diagnosis of retroperitoneal leiomyosarcoma with the invasion of the IVC. A venogram was initially performed and revealed that the IVC was the only patent to the level immediately below the origin of the renal veins. The venogram also showed significant collateralisation, most notably a prominent vessel posterior to the expected course of the IVC draining directly into the right atrium. The patient was subsequently placed in the modified flank position to proceed with resection. Exposure of the right flank demonstrated significant varices in the mesentery of the hepatic flexure and an adrenal mass densely adherent to the upper pole of the right kidney, the renal hilum and the adjacent renal vasculature. It was determined that the right kidney could not be safely dissected from the mass and the decision was made to perform a total nephrectomy and adrenalectomy. Careful dissection established an appropriate plane, but the IVC was significantly thrombosed and tumour adhesions were noted on the inferior surface of the liver.
Tumour extension into the IVC and the consequent thrombosis was then addressed. Exposure and identification of the suprahepatic IVC and portal triad were first performed for prompt control of potential IVC bleeding. The intrahepatic IVC above the tumour and its accompanying draining vessels (most notably a small vein draining the caudate lobe of the liver) were identified and ligated with silastic vessel loops. The infrahepatic IVC below the level of the tumour, the adjacent renal veins and the large posterior venous collateral (previously noted on venography) were then ligated and a venotomy was made at the base of the tumour. The IVC was then transected just above the posterior collateral drainage and the tumour and kidney were subsequently removed en bloc as a specimen. Chronic adherent thrombus noted on the margin of the vessel was removed via thromboendarterectomy techniques, and the resulting specimen was sent to pathology to document sufficient margins.
Outcome and follow-up
Following recovery from surgery, the patient had the resolution of his symptomatic lower extremity swelling and abdominal varices. However, at 1-year follow-up, pulmonary metastases were discovered, and the patient passed in June 2018. The patient mortality occurred approximately 14 months after diagnosis.
Discussion
PAL is an extremely rare neoplasm of mesenchymal origin that is believed to originate from the smooth muscle wall of the IVC, central adrenal vein and its tributaries.2 To our knowledge, there are 35 cases reported in the English literature, including the patient presented in this report. Below, we describe the clinical and pathological characteristics of PAL by providing a thorough analysis of known cases.
PAL appears to be most prevalent after the fifth decade with an average age of presentation of 55.6±15 years. The one outlier found was a 14-year-old girl with AIDS who presented with bilateral tumours.3 While the precise aetiology of PAL is not known, it has been reported that incidence may be associated with positive HIV/EBV serology.2 4–7 This may explain the especially early presentation seen in this one patient. The most common symptom at presentation is ipsilateral flank pain occurring in roughly half of those afflicted. The incidence of PAL does not exhibit gender predilection with a roughly even distribution of cases noted between males and females. In addition, there does not appear to be any laterality preference, as right-sided and left-sided tumours occur with similar frequency (table 1).
Table 1.
Summary of patient/tumour characteristics, treatments and outcomes of known cases of PAL in the literature2–6 9 10 13 15 17–41
| Study | Age (years) | Gender | Location | Size (cm) | Extension | Presentation | Treatment | Outcome |
| Choi and Liu | 50 | F | L | 16 | No | Flank pain | Adx+partial Nx | 12 months DFS |
| Lack et al | 49 | M | R | 11 | No | Flank pain | Adx+partial Nx | 9 months, metastasis |
| Zetler et al | 30 | M | L | 11 | N/D | N/D | Adx | 20 months DFS |
| Boman et al | 48 | M | R | 2 | N/D | Cachexia | None | N/D |
| Boman et al | 29 | M | L | 0.8 | N/D | Cachexia | None | N/D |
| Etten et al | 73 | F | R | 27 | IVC | IVC syndrome | N/D | 3 weeks, deceased |
| Matsui et al | 61 | F | R | N/D | IVC+right atrium | Flank pain | Adx+Nx+thrombectomy | 1 month, deceased |
| Lujan and Hoang | 63 | M | R | 25 | AO+PM | Enlarging RUQ mass | CT+Adx+ Nx+hepaticlobectomy+ cholecystectomy |
Deceased shortly after surgery |
| Thamboo et al | 68 | F | R | 13 | No | Flank pain | Adx+Nx | 12 months DFS |
| Linos et al | 14 | F | B | 3.5 | No | N/D | N/D | N/D |
| 4 | N/D | N/D | N/D | |||||
| Kato et al | 59 | M | L | 10 | IVC | Flank pain | Adx+Nx+thrombectomy | 6 months, deceased |
| Wong et al | 57 | M | L | N/D | IVC+common iliac arteries | Groin pain, cold feet | Adx+Nx+thrombectomy | 6 months, recurrence |
| Candanedo-Gonzalez et al | 59 | F | L | 16 | AO | Flank pain | Adx+CT+RT | 36 months, metastasis |
| Lee et al | 49 | M | L | 3 | No | Flank pain | Adx | 10 months DFS |
| Mohanty et al | 47 | F | L | 10 | No | Abdominal pain | Adx+Nx+RT | 9 months, metastasis |
| Wang et al | 64 | F | R | 14 | IVC+right atrium | B/I LEE, productive cough | Adx+thrombectomy | 10 months DFS |
| Goto et al | 73 | F | R | 8 | AO | Flank pain | Adx+Nx | 10 months DFS |
| Mencoboni et al | 75 | F | R | 8 | No | Flank pain+abdominal pain | Adx | 12 months DFS |
| Van Laarhoven et al | 78 | M | L | N/D | Multiple metastases | N/D | RT | 11 days, deceased |
| Hamada et al | 62 | F | B | 8 | No | Flank pain | Bil Adx+CT+RFA+RT | 16 months, deceased |
| 4 | No | |||||||
| Karaosmanoglu and Gee | 63 | M | R | N/D | IVC | B/l LEE+abdominal pain | CT | 3 months, deceased |
| Shao et al | 66 | M | L | 10 | Renal vein | N/D | Adx | 18 months DFS |
| Kanthan et al | 28 | F | L | 16 | No | Abdominal pain | Adx+Nx+partial Nx | N/D |
| Deshmuku et al | 60 | F | L | 5 | No | Flank pain | Adx | 21 months DFS |
| Gulpinar et al | 48 | M | R | 11 | No | Lower Urinary Tract Symptoms |
Adx | 8 months DFS |
| Ozturk | 70 | F | R | 8 | IVC | Flank pain | Adx+cavatomy+CT | 6 months, metastasis |
| Lee et al | 28 | M | R | 15 | No | Flank pain+weight loss | Adx | 18 months DFS |
| Bhalla et al | 45 | M | R | 11 | Multiple metastases | Flank pain | CT | 9 months, metastasis |
| Wei et al | 57 | F | L | 8 | No | None | Adx | 29 months DFS |
| Zhou et al | 49 | F | L | 6 | No | Flank pain+abdominal pain | Adx | 6 months DFS |
| Onishi et al | 34 | M | R | 5.2 | IVC | Flank pain | Adx+Ldx | No recurrence |
| Nagaraj et al | 61 | M | L | 16 | No | Flank pain | Adx | N/D |
| Alam et al | 35 | F | L | 8.5 | No | Flank pain | Adx | N/D |
| Aoki et al | 81 | F | R | 7 | No | Abdominal pain | Adx | 12 months, deceased |
| Doppalapudi | 70 | M | R | 9 | IVC | B/l LEE+abdominal varices | Adx+Nx cavatomy+thrombectomy | 12 months, deceased |
Adx, adrenalectomy; AO, adjacent organ; B, bilateral; B/l LEE, bilateral lower extremity oedema; CT, chemotherapy; IVC, inferior vena cava; N/D, not disclosed; Nx, nephrectomy; PAL, primary adrenal leiomyosarcoma; PM, pulmonary metastases; RFA, radiofrequency ablation; RT, radiation therapy; RUQ, right upper quadrant.
Symptoms associated with PAL appear to be primarily attributed to mass effect and local invasion. For instance, those patients with invasion or malignant thrombosis into the IVC often presented with lower extremity oedema, spider angiomata and/or abdominal varices. In fact, vascular invasion at presentation is common, occurring in 26.5% of reported cases. Early vascular invasion seen with PAL may be attributed to its smooth muscle wall origins in the IVC and central adrenal vein. Additionally, because symptoms of PAL are presumably due to mass effect, tumour size at presentation is often large with an average size of 10±5.9 cm.
Diagnosis of PAL is generally made via surgical pathology. Preoperative diagnosis is difficult because there is a lack of identifiable biomarkers or specific endocrinological changes associated with this neoplasm. Consequently, advances in urine steroid metabolomics and steroid profiling may not have a great impact in detecting PAL; the value of this diagnostic tool appears to be greater in differentiating adrenocortical carcinoma (ACC) from adrenocortical adenoma (ACA).8 Neuron-specific enolase has been suggested as a potential biomarker for early detection of PAL, but lack of replicable results has hampered its value as a suitable tumour marker.2 9 In addition, there are no radiological features on CT or MRI that distinguish PAL from other adrenal malignancies. However, radiological studies are still useful for assessing resectability and metastasis. Imaging may also be valuable for distinguishing between potentially benign and malignant lesions by evaluating growth rate (via serial studies) and tumour size. The current guidelines for the management of adrenal tumours recommend against further imaging for incidentalomas under 4 cm of size with benign characteristics, although this is a weak recommendation. In fact, this cut-off of 4 cm to delineate potential malignancy (34%–61% specific) is challenged by Iniguez et al, in which only 31% of adrenal tumours above 4 cm were malignant. However, two notable risk factors for malignancy in this study were larger tumour size (7 cm) and non-incidental mode of discovery, suggesting that imaging still serves an integral role in the evaluation of adrenal tumours.10–12 Additionally, we utilised radiological guidance in the form of a CT-guided biopsy to ascertain a tissue diagnosis for our patient prior to surgical resection. A core biopsy prior to surgical diagnosis has been reported in only two previous PAL cases, and we believe that it should be undertaken whenever feasible as an accurate preoperative diagnosis can be useful in surgical planning.3 13 It must be stressed, however, that prior to biopsy, a thorough initial evaluation should include clinical, hormonal and radiological studies. This is especially true in cases of pheochromocytoma, in which improper manipulation of the tumour can lead to catecholamine-induced hypertensive crisis and mortality. In addition, while a CT-guided biopsy is generally discouraged in the evaluation of ACC and pheochromocytomas, it has shown potential value in the diagnosis of malignant adrenal tumours in which surgery may not be the first step for treatment (lymphomas).14 Due to the non-hormone-producing nature of our reported tumour and the low index of suspicion of ACC and pheochromocytoma on imaging, a CT-guided biopsy was deemed a logical step prior to further intervention.
Ultimately, histopathological diagnosis is needed to confirm the diagnosis. Histopathological evaluation of PAL reveals highly cellular spindle cells with eosinophilic cytoplasm and elongated, centrally located nuclei. Immunohistochemical staining shows reactivity to various smooth muscle markers including desmin, SMA (smooth muscle actin), vimentin and smooth muscle heavy chains. Leiomyosarcomas are also characteristically negative for S-100, alfa-inhibin and CD117.15 Our analysis of current literature shows that SMA (73.5%), desmin (50%) and vimentin (47%) are the most common markers associated with PAL (table 2). Pleiomorphic subtypes are rare with five known cases (14.7%) and may show variable expression of these markers. As previously mentioned, a core biopsy of the tumour was sufficient in making the diagnosis in our patient before surgical resection was performed. Pathology analysis revealed a highly cellular atypical smooth muscle neoplasm that stained strongly for desmin and vimentin and negatively for S-100.
Table 2.
Pathologic characteristics of known cases of PAL in the literature2–6 9 10 13 15 17–41
| Study | Pathology |
| Choi and Liu | N/D |
| Lack et al | Vimentin/actin/SMA+ |
| Zetler et al | SMA+ |
| Boman et al | SMA/HHF35/vimentin/desmin+ |
| SMA/HHF35+ | |
| Etten et al | SMA+ |
| Matsui et al | SMA+ |
| Lujan and Hoang | Pleomorphic |
| Thamboo et al | SMA/vimentin/actin/desmin+ |
| Linos et al | SMA/viminten/actin/HHF+ |
| Kato et al | Pleomorphic+SMA/desmin/vimentin+ |
| Wong et al | N/D |
| Candanedo-Gonzalez et al | Pleomorphic+SMA/desmin/vimentin+ |
| Lee et al | Desmin+ |
| Mohanty et al | Pleomorphic+desmin/calpinin/actin+ |
| Wang et al | SMA/desmin+ |
| Goto et al | SMA/NSE+ |
| Mencoboni et al | SMA/desmin/actin+ |
| Van Laarhoven et al | SMA/actin/vimentin+ |
| Hamada et al | SMA+ |
| Karaosmanoglu and Gee | Actin/vimentin/desmin/keratin+ |
| Shao et al | SMA/desmiin+ |
| Kanthan et al | Pleomorphic+SMA/vimentin+ |
| Deshmuku et al | SMA/vimentin/desmin+ |
| Gulpinar et al | SMA/vimentin+ |
| Ozturk et al | SMA/desmin+ |
| Lee et al | SMA/desmin+ |
| Bhalla et al | Desmin/actin+ |
| Wei et al | SMA/vimentin/actin/desmin+ |
| Zhou et al | SMA/desmin/vimentin+ |
| Onishi et al | SMA+ |
| Nagaraj et al | Desmin/vimentin+ |
| Alam et al | N/D |
| Aoki et al | SMA/vimentin+ |
| Doppalapudi | Vimentin/desmin+ |
PAL, primary adrenal leiomyosarcoma; SMA, smooth muscle actin.
The mainstay of PAL treatment is radical surgical excision of the mass. In fact, the most important prognostic factor for survival is the ability to achieve microscopically negative margins during resection.15 Other important prognostic factors include tumour size, grade, location, presence of venous thrombosis and distant metastasis. Our analysis of the 35 reported cases of PAL particularly highlights the potential importance of patient age and tumour size on clinical outcomes. For instance, the average age of patients experiencing recurrence or mortality (61.9±10.9 years) was older than those experiencing disease-free survival (53.1±15.7 years). Similarly, the average tumour size at presentation was also larger in deceased individuals and those experiencing recurrence (13.3±6.8 cm) when compared with those who are disease free (9.5±4 cm). While noteworthy, these findings are not statistically significant (p=0.095 and 0.0938, respectively).
The definitive role of chemotherapy and radiotherapy is unclear. There seems to be a potential role for radiotherapy in the treatment of PAL as radiation is often utilised as adjuvant therapy in many soft tissue sarcomas of the extremities.16 In one case reported by Mohanty et al, radiotherapy was justified due to the extreme degree of pleomorphism and high mitotic activity seen in the index tumour.9 Neoadjuvant chemotherapy seems to warrant lesser consideration. Paclitaxel was utilised preoperatively in Lujan and Hoang but continued growth of the adrenal mass was noted prior to resection.3 Chemotherapy should be considered as a palliative measure in metastatic disease, however. Although leiomyosarcomas are known to be slow growing with late metastasis, the prognosis is generally poor and local recurrence is common.
Learning points.
Primary adrenal leiomyosarcoma (PAL) is an extremely rare neoplasm of mesenchymal origin that arises from the smooth muscle wall of the inferior vena cava (IVC), central adrenal vein or its tributaries.
Patients most commonly present with ipsilateral flank pain and symptoms generally develop due to mass effect and local invasion. There is no gender or laterality predilection.
Discovery of a mass is generally made via cross-sectional imaging (CT or MRI) and treatment generally consists of surgical resection of the adrenal gland and the remaining tumour burden with special consideration for consolidation radiation in pleomorphic cases.
Diagnosis is made via histopathological evaluation and immunohistochemistry. Histopathological evaluation of PAL reveals highly cellular spindle cells with eosinophilic cytoplasm and elongated, centrally located nuclei. Immunohistochemical staining shows reactivity to various smooth muscle markers including desmin, smooth muscle actin, vimentin and smooth muscle heavy chains.
Although PAL grows slowly with late metastasis, prognosis is poor and recurrence is common.
Acknowledgments
Jessica Connor, a medical student from Rutgers-New Jersey Medical School, was a valuable contributor to this case report and was an essential member in editing this manuscript. The primary reason she is not an author is because she was the last to enter the authorship group and only four authors are allowed when submitting to BMJ Case Reports.
Footnotes
Contributors: SD: wrote the vast majority of the manuscript and performed a thorough literature regarding the topic, embedding a synthesis of current data in the discussion. TS: edited the manuscript and wrote the abstract for the paper. VAF: wrote the pathology-related portions of the manuscript. VB: performed the surgery, acquired next of kin consent for this case report and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Mankin HJ, Casas-Ganem J, Kim JI, et al. Leiomyosarcoma of somatic soft tissues. Clin Orthop Relat Res 2004;421:225–31. 10.1097/01.blo.0000119250.08614.82 [DOI] [PubMed] [Google Scholar]
- 2. Zhou Y, Tang Y, Tang J, et al. Primary adrenal leiomyosarcoma: a case report and review of literature. Int J Clin Exp Pathol 2015;8:4258–63. [PMC free article] [PubMed] [Google Scholar]
- 3. Lujan MG, Hoang MP. Pleomorphic leiomyosarcoma of the adrenal gland. Arch Pathol Lab Med 2003;127:e32–5. [DOI] [PubMed] [Google Scholar]
- 4. Choi SH, Liu K. Leiomyosarcoma of the adrenal gland and its angiographic features: a case report. J Surg Oncol 1981;16:145–8. 10.1002/jso.2930160205 [DOI] [PubMed] [Google Scholar]
- 5. Lack EE, Graham CW, Azumi N, et al. Primary leiomyosarcoma of adrenal gland. Case report with immunohistochemical and ultrastructural study. Am J Surg Pathol 1991;15:899–905. [DOI] [PubMed] [Google Scholar]
- 6. Zetler PJ, Filipenko JD, Bilbey JH, et al. Primary adrenal leiomyosarcoma in a man with acquired immunodeficiency syndrome (AIDS). Further evidence for an increase in smooth muscle tumors related to Epstein-Barr infection in AIDS. Arch Pathol Lab Med 1995;119:1164–7. [PubMed] [Google Scholar]
- 7. Suankratay C, Shuangshoti S, Mutirangura A, et al. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis 2005;40:1521–8. 10.1086/429830 [DOI] [PubMed] [Google Scholar]
- 8. Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab 2011;96:3775–84. 10.1210/jc.2011-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohanty SK, Balani JP, Parwani AV. Pleomorphic leiomyosarcoma of the adrenal gland: case report and review of the literature. Urology 2007;70:591.e5–7. 10.1016/j.urology.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 10. Yamakita N, Saitoh M, Mercado-Asis LB, et al. Asymptomatic adrenal tumor; 386 cases in Japan including our 7 cases. Endocrinol Jpn 1990;37:671–84. 10.1507/endocrj1954.37.671 [DOI] [PubMed] [Google Scholar]
- 11. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1–G34. 10.1530/EJE-16-0467 [DOI] [PubMed] [Google Scholar]
- 12. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes 2018;2:30–9. 10.1016/j.mayocpiqo.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhalla A, Sandhu F, Sieber S. Primary adrenal leiomyosarcoma: a case report and review of the literature. Conn Med 2014;78:403–7. [PubMed] [Google Scholar]
- 14. Sharma KV, Venkatesan AM, Swerdlow D, et al. Image-guided adrenal and renal biopsy. Tech Vasc Interv Radiol 2010;13:100–9. 10.1053/j.tvir.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Onishi T, Yanagihara Y, Kikugawa T, et al. Primary adrenal leiomyosarcoma with lymph node metastasis: a case report. World J Surg Oncol 2016;14:176 10.1186/s12957-016-0936-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strander H, Turesson I, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol 2003;42:516–31. 10.1080/02841860310014732 [DOI] [PubMed] [Google Scholar]
- 17. Boman F, Gultekin H, Dickman PS. Latent Epstein-Barr virus infection demonstrated in low-grade leiomyosarcomas of adults with acquired immunodeficiency syndrome, but not in adjacent Kaposi’s lesion or smooth muscle tumors in immunocompetent patients. Arch Pathol Lab Med 1997;121:834–8. [PubMed] [Google Scholar]
- 18. Etten B, van Ijken MG, Mooi WJ, et al. Primary leiomyosarcoma of the adrenal gland. Sarcoma 2001;5:95–9. 10.1155/S1357714X01000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsui Y, Fujikawa K, Oka H, et al. Adrenal leiomyosarcoma extending into the right atrium. Int J Urol 2002;9:54–6. 10.1046/j.1442-2042.2002.00413.x [DOI] [PubMed] [Google Scholar]
- 20. Thamboo TP, Liew LC, Raju GC. Adrenal leiomyosarcoma: a case report and literature review. Pathology 2003;35:47–9. [PubMed] [Google Scholar]
- 21. Linos D, Kiriakopoulos AC, Tsakayannis DE, et al. Laparoscopic excision of bilateral primary adrenal leiomyosarcomas in a 14-year-old girl with acquired immunodeficiency syndrome (AIDS). Surgery 2004;136:1098–100. 10.1016/j.surg.2003.07.007 [DOI] [PubMed] [Google Scholar]
- 22. Kato T, Kato T, Sakamoto S, et al. Primary adrenal leiomyosarcoma with inferior vena cava thrombosis. Int J Clin Oncol 2004;9:189–92. 10.1007/s10147-004-0383-7 [DOI] [PubMed] [Google Scholar]
- 23. Wong C, Von Oppell UO, Scott-Coombes D. Cold feet from adrenal leiomyosarcoma. J R Soc Med 2005;98:418–20. 10.1177/014107680509800910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Candanedo-González FA, Vela Chávez T, Cérbulo-Vázquez A. Pleomorphic leiomyosarcoma of the adrenal gland with osteoclast-like giant cells. Endocr Pathol 2005;16:075–82. 10.1385/EP:16:1:075 [DOI] [PubMed] [Google Scholar]
- 25. Lee CW, Tsang YM, Liu KL. Primary adrenal leiomyosarcoma. Abdom Imaging 2006;31:123–4. 10.1007/s00261-005-0343-3 [DOI] [PubMed] [Google Scholar]
- 26. Wang TS, Ocal IT, Salem RR, et al. Leiomyosarcoma of the adrenal vein: a novel approach to surgical resection. World J Surg Oncol 2007;5:109 10.1186/1477-7819-5-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto J, Otsuka F, Kodera R, et al. A rare tumor in the adrenal region: neuron-specific enolase (NSE)-producing leiomyosarcoma in an elderly hypertensive patient. Endocr J 2008;55:175–81. 10.1507/endocrj.K07E-020 [DOI] [PubMed] [Google Scholar]
- 28. M M, M B, M T, et al. Primary adrenal leiomyosarcoma: a case report and literature review. Clin Med Oncol 2008;2:CMO.S627–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Laarhoven HW, Vinken M, Mus R, et al. The diagnostic hurdle of an elderly male with bone pain: how 18F-FDG-PET led to diagnosis of a leiomyosarcoma of the adrenal gland. Anticancer Res 2009;29:469–72. [PubMed] [Google Scholar]
- 30. Hamada S, Ito K, Tobe M, et al. Bilateral adrenal leiomyosarcoma treated with multiple local therapies. Int J Clin Oncol 2009;14:356–60. 10.1007/s10147-008-0844-5 [DOI] [PubMed] [Google Scholar]
- 31. Karaosmanoglu AD, Gee MS. Sonographic findings of an adrenal leiomyosarcoma. J Ultrasound Med 2010;29:1369–73. 10.7863/jum.2010.29.9.1369 [DOI] [PubMed] [Google Scholar]
- 32. Shao IH, Lee WC, Chen TD, et al. Leiomyosarcoma of the adrenal vein. Chang Gung Med J 2012;35:428–31. [DOI] [PubMed] [Google Scholar]
- 33. Kanthan R, Senger JL, Kanthan S. Three uncommon adrenal incidentalomas: a 13-year surgical pathology review. World J Surg Oncol 2012;10:64 10.1186/1477-7819-10-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deshmukh SD, Babanagare SV, Anand M, et al. Primary adrenal leiomyosarcoma: a case report with immunohistochemical study and review of literature. J Cancer Res Ther 2013;9:114–6. 10.4103/0973-1482.110394 [DOI] [PubMed] [Google Scholar]
- 35. Gulpinar MT, Yildirim A, Gucluer B, et al. Primary leiomyosarcoma of the adrenal gland: a case report with immunohistochemical study and literature review. Case Rep Urol 2014;2014:1–4. 10.1155/2014/489630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oztürk H. Vena cava ınvasion by adrenal leiomyosarcoma. Rare Tumors 2014;6:5275:55–6. 10.4081/rt.2014.5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee S, Tanawit GD, Lopez RA, et al. Primary leiomyosarcoma of adrenal gland with tissue eosinophilic infiltration. Korean J Pathol 2014;48:423–5. 10.4132/KoreanJPathol.2014.48.6.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei J, Sun A, Tao J, et al. Primary adrenal leiomyosarcoma: case report and review of the literature. Int J Surg Pathol 2014;22:722–6. 10.1177/1066896914526777 [DOI] [PubMed] [Google Scholar]
- 39. Nagaraj V, Mustafa M, Amin E, et al. Primary adrenal leiomyosarcoma in an Arab male: a rare case report with immunohistochemistry study. Case Rep Surg 2015;2015:1–4. 10.1155/2015/702541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alam MM, Naser MF, Islam MF, et al. Primary adrenal leiomyosarcoma in an adult female. Mymensingh Med J 2014;23:380–3. [PubMed] [Google Scholar]
- 41. Aoki C, Tanaka S, Suzuki K, et al. Primary adrenal leiomyosarcoma in an aged japanese woman: a rare case report. J Clin Case Rep 2017;07:935 10.4172/2165-7920.1000935 [DOI] [Google Scholar]