Abstract
Mucoepidermoid carcinoma (MEC) can be rarely found as a primarily intraosseous lesion and mistaken for other intraosseous or odontogenic pathology. A 65-year-old man had a poorly defined radiolucency distal to the left mandibular second molar root. Periapical radiographs demonstrated a minor radiolucency from 2.5 years prior. An oral and maxillofacial surgeon felt the radiolucency represented periodontal disease, extracting tooth #18. The differential diagnosis of mixed radiolucent/radio-opaque mandibular lesions includes: (1) fibro-osseous lesion, (2) odontogenic and non-odontogenic cyst, (3) infection and inflammatory lesion, or (4) benign or malignant neoplasm (odontogenic, non-odontogenic, or metastatic). Histological analysis revealed low-grade MEC. A composite resection was performed with a 1 cm margin from first molar to ascending ramus. A buccal fat pad advancement flap covered the defect with an iliac crest bone graft placed later for a resulting osseous defect. Careful examination and diagnostic work-up for odontogenic cysts should be provided as they may harbour malignant tumours.
Keywords: otolaryngology / ENT, oral and maxillofacial surgery, dentistry and oral medicine, head and neck cancer
Background
Mucoepidermoid carcinoma (MEC) is the most common salivary gland malignancy and comprises 5%–10% of all malignant salivary tumours.1 Salivary gland neoplasms trend toward malignancy as their size decreases. MEC is not exclusive to the major and minor salivary glands, and can be seen in other areas such as the breast.2 Additionally, MEC can be found as a primarily intraosseous lesion, making up 2%–3% of head and neck MEC, with less than 200 cases reported since 1939.3 The mandibular premolar–molar region is the most common site with up to 50% associated with dental cysts and/or impacted teeth.4 The association with cysts or impacted teeth give credibility to the theory that odontogenic epithelium can give rise to mucous secretory cells which undergo malignant transformation.1 They can be mistaken for other common intraosseous or odontogenic pathology. We report a case of slow-growing, low-grade primary intraosseous MEC thought to initially represent periodontal disease.
Case presentation
A 65-year-old man with unremarkable medical and social history had an incidental finding of a poorly defined radiolucency at the distal root of a fractured left mandibular second molar (tooth #18) during a routine dental visit. On referral to an outside oral and maxillofacial surgeon (OMS), the patient was noted to have pain with palpation of the left retromolar pad as well as with periodontal probing of the distal aspect of #18. The patient denied change in occlusion, trismus, dysphagia, odynophagia, fever, chills, night sweats, paraesthesia of the lip, chin or tongue or weight loss but did endorse an 8-month history of fatigue. He had had an impacted adjacent third molar removed more than 40 years prior. Periapical radiographs from 2.5 years prior to presentation were obtained for comparison, with evidence of a minor radiolucency posterior to the second molar (figure 1A). In comparison, 6 months prior to presentation there is a clear radiolucent lesion with ill-defined borders, with a sclerotic border inferiorly extending to the second molar without inferior alveolar canal displacement (figure 1B). An outside OMS felt the radiolucency represented a periodontal lesion and extracted tooth #18 under local anaesthesia in the clinic. The curetted socket and along with an excisional biopsy of the adjacent radiolucent lesion specimen were sent for pathological evaluation. A postextraction panoramic radiograph redemonstrated the area of radiolucency with tooth #18 now removed (figure 1C).
Figure 1.
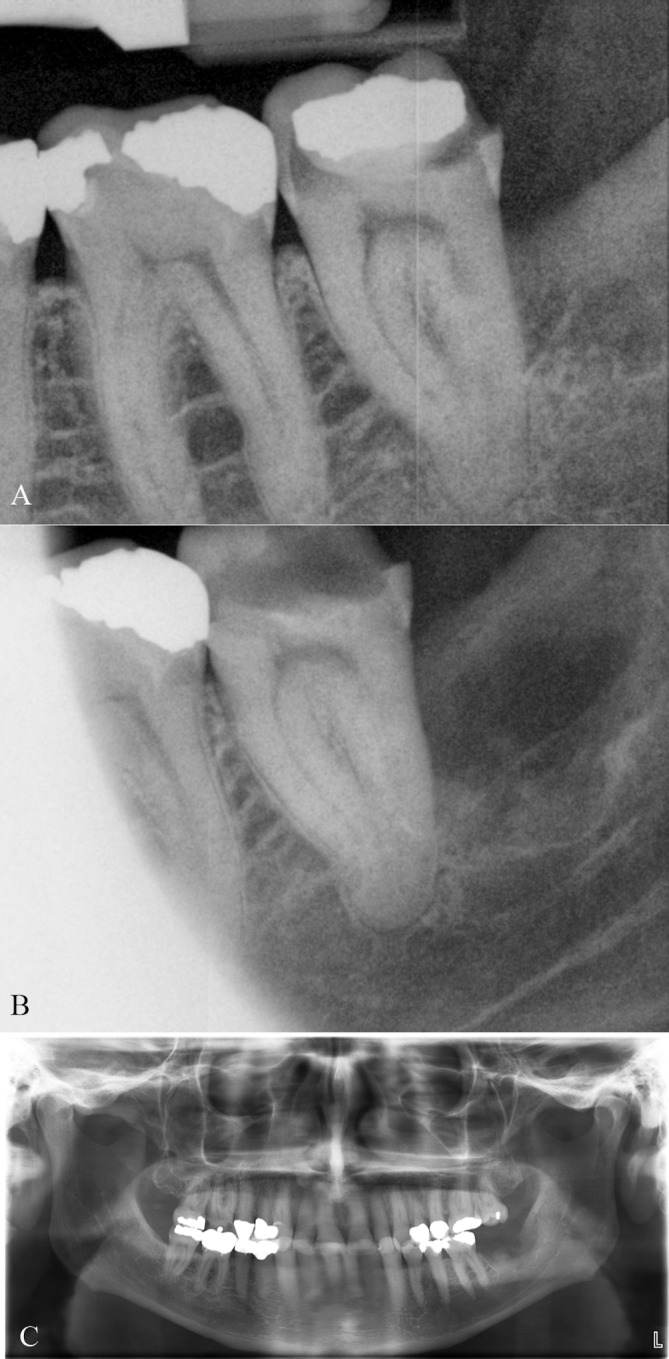
(A) Periapical radiograph 2 years prior to presentation demonstrating minor radiolucency posterior to the second molar. (B) Periapical radiograph 6 months prior to presentation demonstrating a radiolucent lesion with ill-defined borders, with a sclerotic border inferiorly extending to the second molar. (C) Postextraction panoramic radiograph showing the area of radiolucency with tooth #18 now removed.
Investigations
Histological analysis of the excisional biopsy revealed low-grade MEC, clear cell type (figure 2A–D). He was referred to OMS for further management. On examination, the dentition was heavily restored and in fair repair. In the left posterior mandible, there was an extraction site of tooth #18 with lingual mucosal dehiscence (figure 2E). There was no erythema, drainage, or discharge. The floor of mouth was soft, neck had no lymphadenopathy, and the evaluation of the oropharynx was unremarkable. CT with iodinated intravenous contrast and MRI with intravenous gadolinium were obtained showing enhancement of the left posterior lingual mandibular surface submucosa with lingual cortical perforation and marrow space enhancement, concerning for residual tumour (figure 3A-F). There was no cervical lymphadenopathy.
Figure 2.
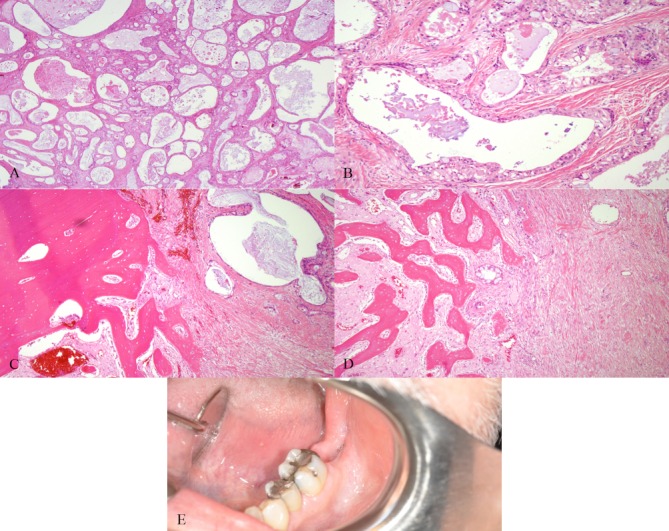
Low-grade, clear cell type mucoepidermoid carcinoma is displayed. Mucicarmine stain is positive in tumour-related mucocytes. H&E stains demonstrating: (A) prominent macrocysts and microcysts, (B) abundant mucus cells with excess cytoplasm and large mucin vacuoles, (C) cystic areas invading cancellous bone and (D) cancellous bone between sheets of neoplastic cells representing sclerotic septae. (E) Heavily restored dentition with extraction site of tooth #18 with lingual mucosa dehiscence.
Figure 3.
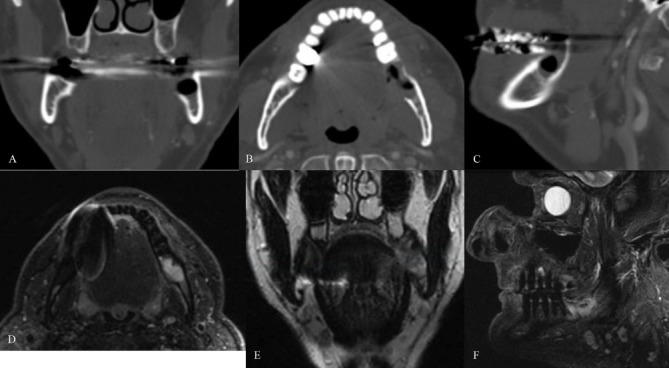
(A–C) CT coronal, axial and sagittal images demonstrating postsurgical excisional bony biopsy changes involving the left posterior mandible with contrast enhancing soft tissue extending along the lingual surface of the mandible concerning for residual tumour. There was no evidence of cervical lymphadenopathy. (D) Short TI Inversion Recovery (STIR) and (E) T2 weighted magnetic resonance images showing an hyperintense lesion involving the left posterior mandible at the extraction site of teeth #17 and #18 without evidence of cortical erosion. (F) Sagittal STIR magnetic resonance image of hyperintense mandibular lesion.
Differential diagnosis
The clinical differential diagnosis for a mixed radiolucent/radio-opaque mandibular lesion in tooth-bearing areas includes: (1) fibro-osseous lesion, (2) odontogenic and non-odontogenic cyst, (3) infections and inflammatory lesion, or (4) benign or malignant neoplasm (odontogenic, non-odontogenic, or metastatic). Cemento-osseous dysplasia is a relatively common fibro-osseous lesion. Odontogenic cysts include dentigerous and radicular cysts while aneurysmal bone cyst is an example of a non-odontogenic cyst. Sclerosing osteomyelitis and periapical granulomas are examples of infections and inflammatory lesions. Benign odontogenic neoplasms include keratocystic odontogenic tumour, ameloblastoma and myxoma, while benign non-odontogenic neoplasms include ossifying fibroma, giant cell lesions, and schwannomas. Malignancies can include squamous cell carcinoma, osteosarcoma, haematopoietic neoplasms as well as metastatic disease such as lung or prostate cancer. Ill-defined lesions are more likely to be malignant due to tissue infiltration and destruction of normal tissue. Benign processes typically displace normal anatomy leading to distinct borders on imaging and pathology. The histological differential diagnosis of MEC can include necrotising sialometaplasia, pleomorphic adenoma, cystadenoma, squamous cell carcinoma and clear cell tumours. Typically cords, sheets and clusters of mucous, squamous, intermediate and clear cells are seen with MEC, all partial features of the above differential. A head and neck surgical pathologist should be consulted if initial pathology is unclear.
Treatment
He was taken to the operating room for composite resection. A marginal mandibulectomy was performed with a 1 cm margin from the middle of the first molar to the ascending ramus (figure 4A–C). The lingual nerve ran within the 1 cm margin on the mandibular lingual surface where there was a small area of cortical perforation and so was sacrificed. The inferior alveolar nerve was also within the margin and was taken with the en-bloc specimen. The remainder of tooth #17 was extracted and frozen margins were negative. A left lingual nerve neurorrhaphy was performed using a microscope and 8–0 nylon sutures (figure 4D). It was additionally wrapped in a collagen tube for protection. A pathological fracture of the mandible was noted at the time of resection and fixated with a six-hole, 2 mm thickness plate with return of premorbid occlusion (figure 4E). A buccal fat pad advancement flap was used to cover the defect over the neurorrhaphy and mandibular hardware. The resulting bony defect was subsequently treated with an iliac crest bone graft 6 months after the initial resection (figure 4F).
Figure 4.
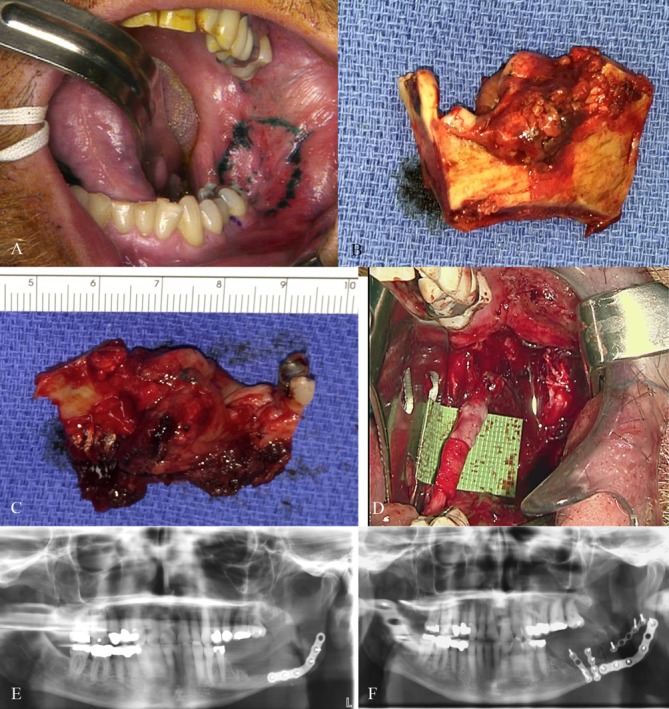
(A–C) Marginal mandibulectomy was performed with a 1 cm margin from the middle of the first molar to the ascending ramus. (D) A left lingual nerve neurorrhaphy was performed using a microscope, 8–0 nylon sutures and wrapped in a collagen sheath for protection. (E) Postoperative panoramic radiographic of the pathological fracture open reduction internal fixation with return of premorbid occlusion. (F) Postoperative panoramic radiographic of the iliac crest bone graft fixed to the mandibular body for the continuity defect.
Outcome and follow-up
The patient resumed a normal diet following speech and swallowing therapy 3 months after surgery. He has done well without functional deficits. Oncologically, he has remained disease free until this submission 3 years after surgery. They underwent routine surveillance with neck and chest CT scans every 3 months for the first 2 years and then biannually thereafter. They will continue biannual scans to the 5-year mark and then annually thereafter.
Discussion
This case demonstrates the rare entity of primary intraosseous MEC with prediagnostic radiographic evolution, intraoperative photographs and key histological features needed for pathological diagnosis. Since 1974, there have been six criteria for diagnosing intraosseous MEC.5 Diagnostic criteria include: (1) an absence of salivary gland primary lesions or pathology, (2) the absence of any odontogenic tumours, (3) radiographic evidence of bony destruction, (4) intact cortices on radiographic and pathological analysis, (5) positive mucin staining on histology and (6) microscopic confirmation of the diagnosis. While there was a small perforation of thin lingual cortex adjacent to the lesion, there was no evidence of mucosal involvement and the perforation is believed to have occurred during the curettage of the lesion by the outside clinician. We therefore submit that these criteria were all fulfilled in our case.
The aetiology of intraosseous MEC is still unknown, but there are three theories on its aetiology.4 6–10 One possibility is the mucous-secreting cells from a dentigerous cyst pluripotent epithelial lining of impacted third molars can undergo malignant degeneration. The second theory proposes retromolar mucous glands can become entrapped within the mandible during development leaving mucous-type secretory cell nests to undergo neoplastic transformation. The third theory involves ectopic salivary gland tissue being included within the mandible during development, which has been described to occur inferior to the mandibular canal. Finally, puberty can influence the above possibilities allowing malignant transformation of mucous-secreting cell nests considering growth factor influence on neoplastic degeneration.11
Intraosseous MEC is seen in women twice as much as men, and involves the mandible three times as often as the maxilla.1 There is no distinct region of either bone where the tumours present most commonly as the minor salivary tissue is present diffusely over both structures. These tumours have been seen from years one through 78, but the overwhelming majority are in the 30s and 40s.4 The symptomatic features of intraosseous MEC are mainly pain and swelling, with trismus, paresthesias and tooth mobility being less common. Radiographically, lesions are usually well-circumscribed, uniocular or multilocular, radiolucent areas. They are sometimes difficult to identify and may be confused with benign or malignant odontogenic tumours. Our differential was broad, including both odontogenic cysts/tumours and non-odontogenic tumours, as noted above.
With unclear bony lesions of the mandible, excisional biopsy is recommended to reduce sampling error and lead to a proper diagnosis. The histopathological criteria for diagnosing MEC include the presence of mucin-producing cells, intermediate cells and cysts.8 Low grade includes a highly differentiated neoplastic lesion with predominantly microcysts and macrocysts and mucus more than epidermoid cells. Intermediate grade lesions have mucus cells as abundant as epidermoid cells, fewer and smaller cysts, and increasing pleomorphism with mitotic figures. High-grade lesions are poorly differentiated, with the predominance of intermediate and epidermoid cells in solid sheets over mucus cells. The above findings of this case were consistent with a low-grade malignancy. Additionally, intraosseous MEC harbours a unique genetic profile, which can help establish the diagnosis via fluorescence in situ hybridisation (FISH) analysis, as seen by Bell et al.1 The t(11;19) fusion gene transcript CRTC1-MAML2 was seen in 9 of 18 intraosseous MECs at MD-Anderson Cancer Centre. More than 50% of intraosseous MECs demonstrate the CRTC1-MAML2 transcript and can be readily identified by FISH.
Low-grade MEC is treated surgically with wide en-bloc resection. High-grade lesions require wide en-bloc resection with neck dissection. The MD-Anderson Cancer Centre experience demonstrated lesions less than 2×2 cm or with negative surgical margins could be treated with partial mandibulectomy or subtotal maxillectomy without neck dissection or adjuvant treatment.1 Low-grade tumours greater than the 2×2 cm threshold or with positive margins should undergo selective neck dissection and adjuvant treatment. Positive margins should be addressed with re-resection, although it has been shown initial oral cavity positive margins are a marker of poor prognosis due to the aggressive nature of the disease.12 High-grade MEC requires wide resection with upfront elective neck dissection followed by adjuvant treatment, as this carries a risk of occult metastasis of 30%–50%.13 The survival rates for low-grade lesions approaches 95% at 5 years, while high-grade tumours drop to 40% survival at 5 years.14 15
Learning points.
Primary intraosseous mucoepidermoid carcinoma (MEC) is extremely rare and is often found mimicking benign odontogenic cysts or tumours.
Misdiagnosis is common due to indolent symptoms and subtle radiographic presentation.
Intraosseous MECs are usually low-grade and less aggressive than the non-intraosseous MECs.
Careful examination and diagnostic work-up should be provided for odontogenic cysts as they may harbour malignant tumours.
Definitive oncological surgery, including neck dissection and adjuvant therapy based on tumour size and grade, should be pursued to minimise locoregional and distant recurrence.
Footnotes
Contributors: NBA, MEL, JZ and ETL: Substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data. Drafting the work or revising it critically for important intellectual content. Final approval of the version published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Bell D, Lewis C, El-Naggar AK, et al. Primary intraosseous mucoepidermoid carcinoma of the jaw: Reappraisal of The MD Anderson Cancer Center experience. Head Neck 2016;38(Suppl 1):E1312–E1317. 10.1002/hed.24219 [DOI] [PubMed] [Google Scholar]
- 2. Fujino M, Mori D, Akashi M, et al. Mucoepidermoid Carcinoma of the Breast Found during Treatment of Lymphoma. Case Rep Oncol 2016;9:806–14. 10.1159/000452792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pires FR, Paes de Almeida O, Lopes MA, et al. Central mucoepidermoid carcinoma of the mandible: report of four cases with long-term follow-up. Int J Oral Maxillofac Surg 2003;32:378–82. 10.1054/ijom.2002.0374 [DOI] [PubMed] [Google Scholar]
- 4. Eversole LR, Sabes WR, Rovin S. Aggressive growth and neoplastic potential of odontogenic cysts: with special reference to central epidermoid and mucoepidermoid carcinomas. Cancer 1975;35:270–82. [DOI] [PubMed] [Google Scholar]
- 5. Alexander RW, Dupuis RH, Holton H. Central mucoepidermoid tumor (carcinoma) of the mandible. J Oral Surg 1974;32:541–7. [PubMed] [Google Scholar]
- 6. Brookstone MS, Huvos AG. Central salivary gland tumors of the maxilla and mandible: a clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg 1992;50:229–36. 10.1016/0278-2391(92)90317-S [DOI] [PubMed] [Google Scholar]
- 7. Maremonti P, Califano L, Mangone GM, et al. Intraosseous mucoepidermoid carcinoma. Report of a long-term evolution case. Oral Oncol 2001;37:110–3. 10.1016/S1368-8375(00)00050-6 [DOI] [PubMed] [Google Scholar]
- 8. Melrose RJ, Abrams AM, Howell FV. Mucoepidermoid tumors of the intraoral minor salivary glands: a clinicopathologic study of 54 cases. J Oral Pathol 1973;2:314–25. 10.1111/j.1600-0714.1973.tb01849.x [DOI] [PubMed] [Google Scholar]
- 9. Pierri LK, Schneider KL, Super S, et al. Intraosseous high-grade mucopidermoid carcinoma with four potential microscopic diagnoses. J Oral Med 1986;41:47–50. [PubMed] [Google Scholar]
- 10. Stoch RB, Smith I. Mucoepidermoid carcinoma in the mandible: report of case. J Oral Surg 1980;38:56–8. [PubMed] [Google Scholar]
- 11. Baj A, Bertolini F, Ferrari S, et al. Central mucoepidermoid carcinoma of the jaw in a teenager: a case report. J Oral Maxillofac Surg 2002;60:207–11. 10.1053/joms.2002.29827 [DOI] [PubMed] [Google Scholar]
- 12. Szewczyk M, Golusinski W, Pazdrowski J, et al. Positive fresh frozen section margins as an adverse independent prognostic factor for local recurrence in oral cancer patients. Laryngoscope 2018;128:1093–8. 10.1002/lary.26890 [DOI] [PubMed] [Google Scholar]
- 13. Moss WJ, Coffey CS, Brumund KT, et al. What is the role of elective neck dissection in low-, intermediate-, and high-grade mucoepidermoid carcinoma? Laryngoscope 2016;126:11–13. 10.1002/lary.25588 [DOI] [PubMed] [Google Scholar]
- 14. Nallamilli SM, Tatapudi R, Reddy RS, et al. Primary intraosseous mucoepidermoid carcinoma of the maxilla. Ghana Med J 2015;49:120–3. 10.4314/gmj.v49i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee RJ, Tan AP, Tong EL, et al. Epidemiology, prognostic factors, and treatment of malignant submandibular gland tumors: a population-based cohort analysis. JAMA Otolaryngol Head Neck Surg 2015;141:905–12. 10.1001/jamaoto.2015.1745 [DOI] [PubMed] [Google Scholar]


