Abstract
Acute transverse myelopathy in a young person may be due to infection, postinfective or inflammatory demyelination, or vascular causes. Rarely, a completely reversible cause of acute transverse myelopathy may be seen, as described here in our case of transverse myelopathy due to extramedullary haematopoiesis (EMH). An 18-year-old man who had a history of a lone blood transfusion at age of 7 years presented with paraplegia. MRI showed multiple epidural space masses of EMH compressing the spinal cord. He was detected to have thalassaemia intermedia and was treated with blood transfusions, steroids and radiotherapy to the involved paraspinal areas. He recovered fully over 15 days and remained symptom free at 6 months.
Keywords: spinal cord, haematology (incl blood transfusion)
Background
Extramedullary haematopoiesis (EMH), occurring due to a variety of causes like haemolytic anaemias, leukaemias, lymphomas and myeloproliferative disorders, usually involves liver, spleen, lymph nodes and other sites like thymus, paravertebral areas, presacral area, retroperitoneum and intrathoracic areas.1 Rarely, EMH can occur in epidural space and compress the spinal cord causing neurological deficits.2 Acute transverse myelopathy in a young person may be due to infection, postinfective or inflammatory demyelination, or vascular causes. We describe a case of acute reversible transverse myelopathy in an 18-year-old man due to epidural space masses of EMH causing thoracic cord compression and presenting as paraplegia, which resolved clinically within 2 weeks on treatment. This rare cause of cord compression should be considered while evaluating paraplegia, especially at a young age.
Case presentation
An 18-year-old man, right handed, presented with progressive weakness of both lower limbs over 15 days, beginning proximally and gradually affecting distal areas too. He had the symptom of a band-like sensation over the trunk below the level of umbilicus. He had developed urinary hesitancy 3 days prior to admission, without any urgency or retention. There was no history of upper limb weakness or visual disturbances. There was no history of recent loose motions, fever, vaccination or trauma. Patient gave history of jaundice 1 month ago which was symptomatically treated but not investigated. There was history of a single blood transfusion received at the age of 7 years, with no further details.
Patient had pallor and icteric tinge to the bulbar conjunctiva, moderate hepatomegaly and massive splenomegaly. All peripheral pulsations were well felt. Neurological examination revealed an awake and alert patient with Mini Mental Scale Examination score of 30/30. Cranial nerves were normal. There was hypertonia in both lower limbs. Power was grade 2/5 in both lower extremities at all joints and 5/5 in upper limbs. Ankle clonus and brisk knee reflexes were seen bilaterally, with extensor plantar responses. A zone of hyperaesthesia was elicited at and below the umbilicus for two segments. Perineal sensations were preserved. Patient had been catheterised.
Investigations
Investigations revealed a haemoglobin of 81 g/L (normal range 135–175 g/L), WBC count 7 x 109/L (normal range 4 –10 x 109/L), platelet count of 128 x 109/L (normal range 150 –450 x 109/L), mean corpuscular volume of 67.3 fL (normal range 78–94 fL), mean corpuscular haemoglobin—18.9 pg (range 27–32 pg), mean corpuscular haemoglobin concentration—28.1 g/dL (normal range 32–36 g/dL) and reticulocyte count of 4.32% (range 0.5%–2.5%). Peripheral smear showed microcytic, hypochromic red blood cells (RBCs). Biochemical parameters were: serum total bilirubin 2.1 mg/dL (normal range 0.2–1.2 mg/dL), direct bilirubin 0.9 mg/dL (normal range <0.3 mg/dL), serum lactate dehydrogenase level 550 U/L (normal range 115–221 U/L), serum Alanine transaminase (ALT) 62 U/L (normal range 7–41 U/L), serum Aspartate transaminase (AST) 53 U/L (normal range 12–38 U/L). Vitamin B12 level was 1507 pg/mL (normal range 279–996 pg/mL). Cerebrospinal fluid study was done, pending MRI, which showed 1 lymphocyte/cmm, 350 mg/dL of proteins (normal range 15–45 mg/dL) and 64.2 mg/dL sugars (normal range 40–80 mg/dL), which was suggestive of Froin’s syndrome. MRI spine (figures 1–3) showed large extradural, intraspinal lobulated soft tissue lesions in dorsal spine from D3 to D9 level causing spinal cord compression and cord oedema along with paravertebral soft tissue masses. Similar intraspinal extradural soft tissue was present in sacral spinal canal compressing the cauda equina (figure 4). Large soft tissue lesions were seen along the posterior ends of ribs bilaterally with diffuse rib widening (figure 5). Diffuse heterogeneity of the marrow within these ribs was also seen. These features were suggestive of EMH. Haemoglobin high-performance liquid chromatography (HPLC) study done to investigate extramedullary haematopoiesis showed HbF 93.6%, HbA 3.7%, HbA2 2.7% which was suggestive of beta thalassaemia intermedia. Bone marrow aspiration showed increased cellularity with an M:E ratio of 1:6, normoblastic hyperplasia of erythroid series and normal myeloid series. The marrow study was consistent with a diagnosis of haemolytic anaemia.
Figure 1.
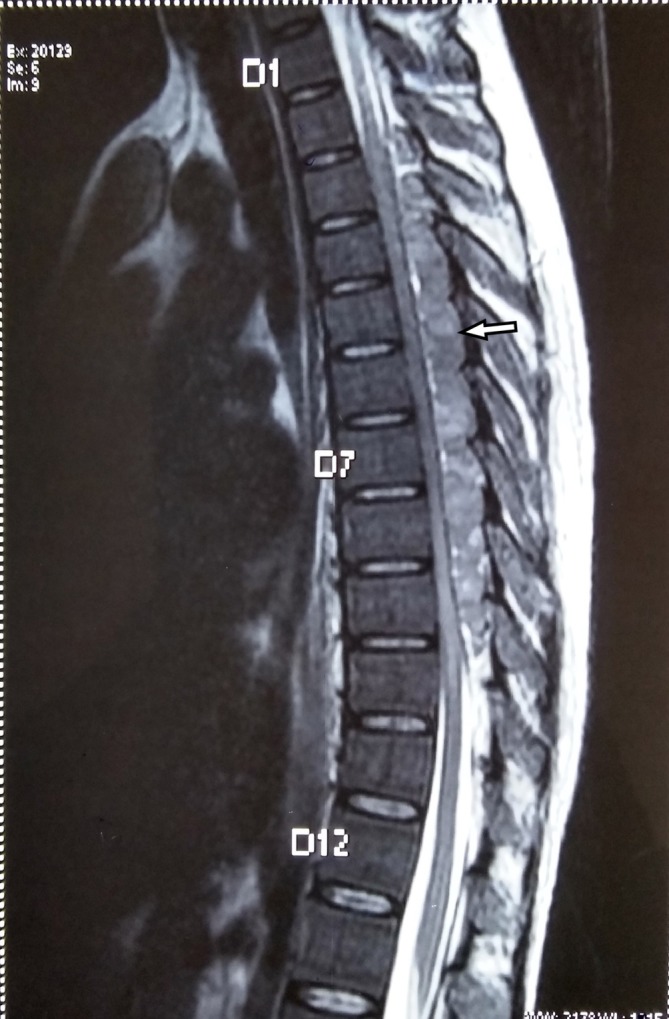
MRI T2W (T2-weighted) sagittal section showing intraspinal extradural soft tissue masses (white arrow) in thoracic spine region with compression of spinal cord.
Figure 2.
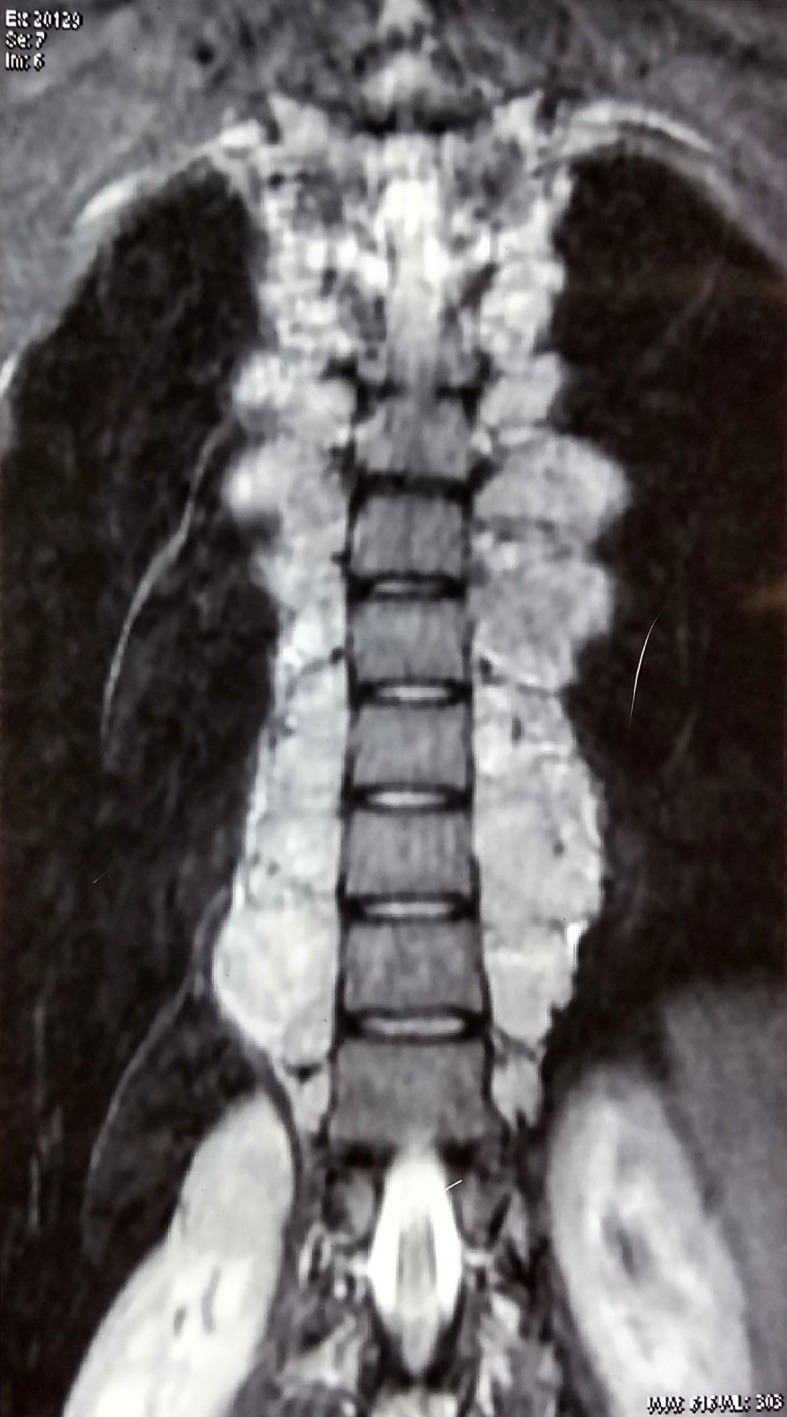
MRI spine coronal section Short-T1 Inversion Recovery (STIR) image showing paraspinal masses in thoracic area.
Figure 3.
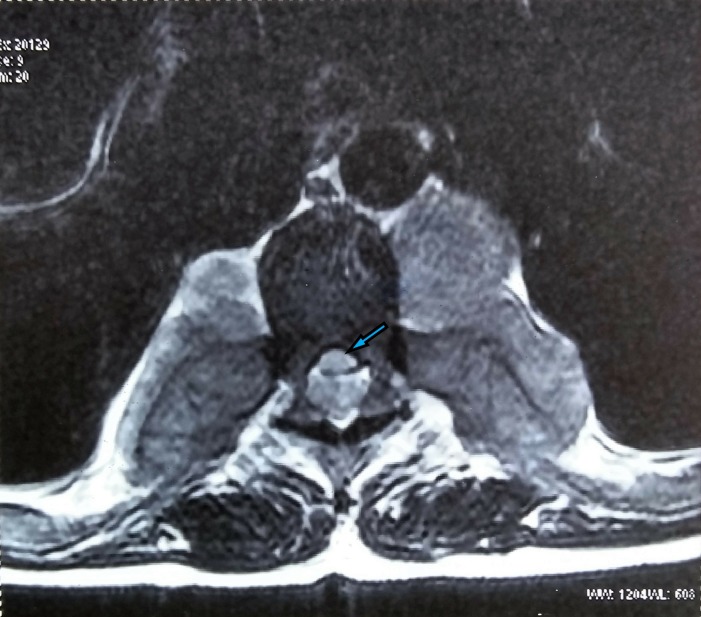
T2 axial image (pretreatment) at D7 level showing compression of the spinal cord (blue arrow) by the intraspinal soft tissue.
Figure 4.
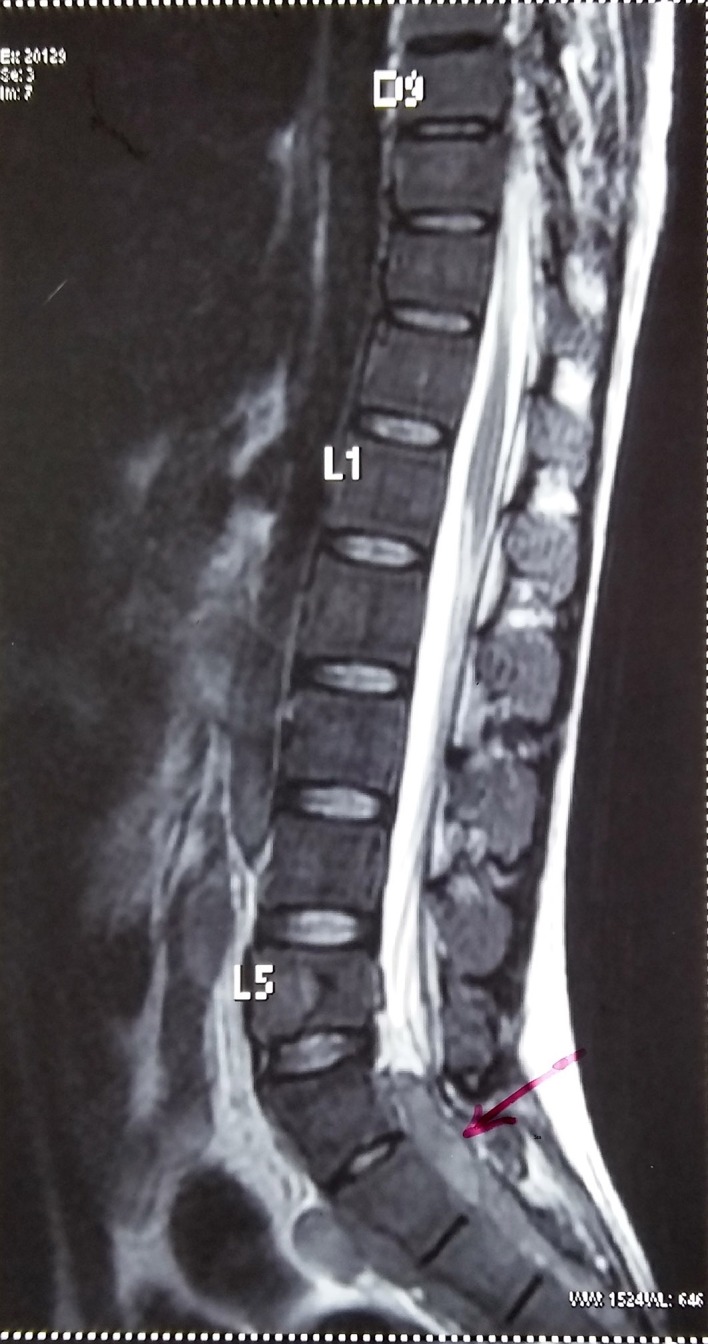
MRI sagittal section of spine showing intraspinal extradural soft tissue masses (pink arrow) in sacral canal.
Figure 5.
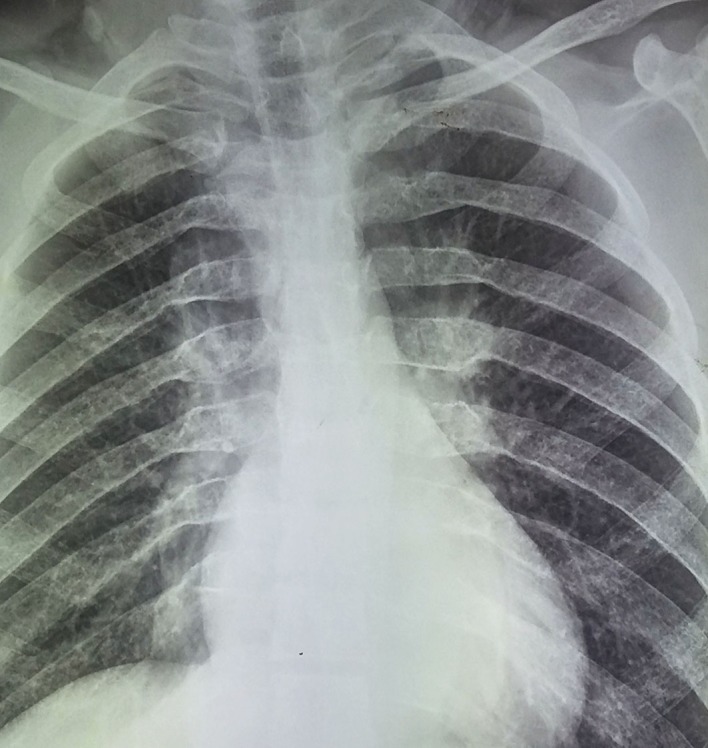
X-ray chest showing widening of posterior ends of ribs.
Differential diagnosis
Acute transverse myelopathy, as in this case, could be due to infective or postinfective acute transverse myelitis. Rarely, rapidly enlarging extramedullary tumours or epidural abscess could cause this presentation.
Treatment
Considering that the patient had evidence of spinal cord compression, injection methylprednisolone was immediately started, and given at a dose of 1 g intravenously for 3 days. He was also transfused three bags of packed RBCs to bring up his haemoglobin above 100 g/L. On the seventh day after admission, haemoglobin HPLC study results were available. At this point, we decided to give external beam radiation therapy (EBRT) to the patient. Patient received EBRT to D3–D12 spine using Cobalt-60 gamma radiation. A total dose of 25.2 Gy divided in 14 cycles was administered over a 21-day period.
Outcome and follow-up
After the third cycle, power in both lower limbs increased to grade 3/5 and he was able to walk by the 15th day, with complete resolution of all neurological deficits. A repeat MRI at 7 days post radiotherapy (figures 6 and 7) showed significant resolution of the paraspinal masses in the thoracic and lumbosacral areas and relief of cord compression. Patient was discharged on hydroxyurea and supplemental folic acid tablets.
Figure 6.
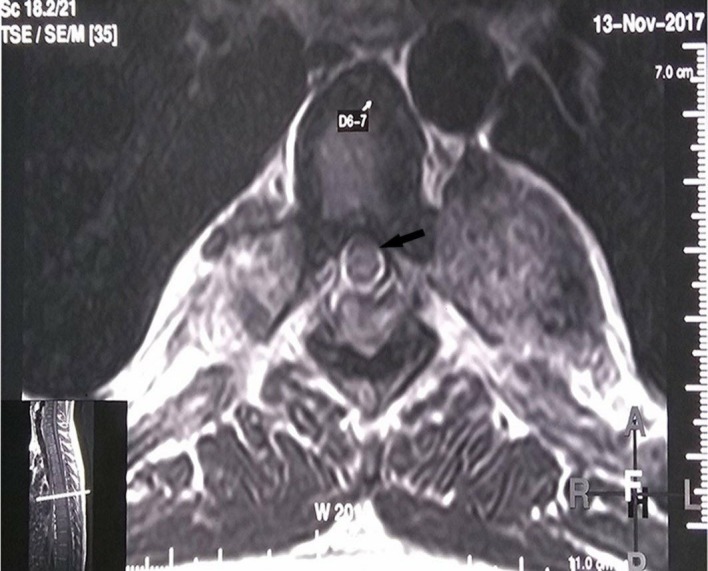
Post-radiotherapy MRI, T2 axial image at D6-7 level showing substantial reduction in the cord compression (black arrow). The inner image depicts the level of the spine at which this axial section is taken.
Figure 7.
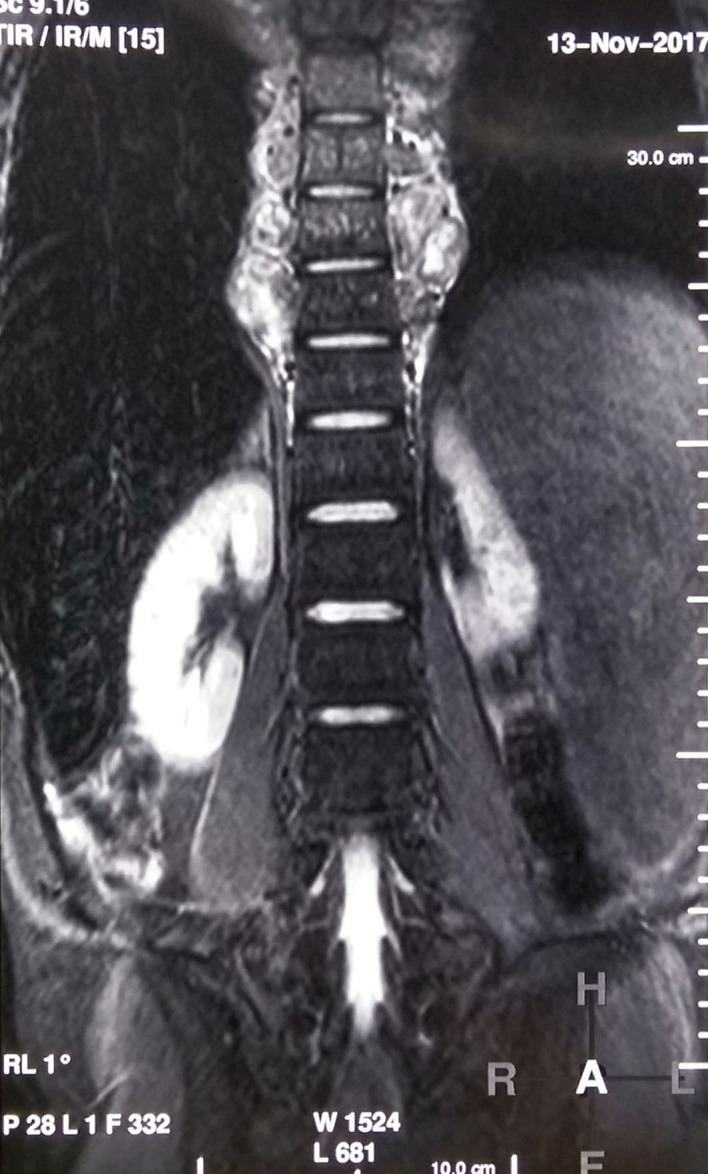
Postradiotherapy MRI spine STIR coronal section showing reduction in the paraspinal masses in thoracic area.
At 3 and 6 months follow-up, the patient remained symptom free without any residual neurological deficit and did not require any further transfusions.
Discussion
Here we have reported a haematological cause for acute transverse myelopathy in a young boy which completely reversed clinically, on treatment over 2 weeks. Beta thalassaemia is an inheritable disorder of autosomal recessive type. It involves defective synthesis of beta globin chains and compensatory synthesis of gamma globin, leading to increased synthesis of HbF.3 Thalassaemia patients inheriting two mutant beta alleles can have severe illness needing blood transfusions from infancy and regularly thereafter, known as thalassaemia major. But many patients with the same genotype can have a milder illness or a transfusion independent state. This group of patients who present in later childhood and remain largely transfusion-independent are said to have thalassaemia intermedia.3 4 This transfusion independence is a result of compensatory expansion of the haematopoietic tissue outside the marrow medulla and leads to formation of haematopoietic masses in other regions of the body. This phenomenon is termed extramedullary haematopoiesis (EMH).3 4
In 1954, the first case of spinal cord compression secondary to EMH was reported by Gatto et al.5 The incidence of intraspinally located haematopoietic masses among the documented cases of thalassaemia-associated EMH is 11%–15%.6 7 EMH usually forms a soft deep red mass which resembles a haematoma on its cut surface. Histologically, these tissues are composed of all haematopoietic elements, that is, myeloid, erythroid and megakaryocytic. Active lesions are rich in vasculature whereas inactive older lesions show fatty tissue and iron deposits.4 8
The pathophysiology of EMH within the intraspinal space could be explained by the theories which include the possibility of direct extension of haematopoietic material from the medulla of the vertebral body or thinned trabeculated posterior ends of ribs and costovertebral junctions,8 9 embolism of haematopoietic tissue into the intraspinal space,1 as well as anomalous activation of embryonic stem cells that reside in the epidural space.10 Within the spine, the most common site is the thoracic region followed by the lumbar region. This may be due to the fact that the subarachnoid space and the spinal canal are narrow in the thoracic region and small intraspinal haematopoietic tissue may readily cause compression of the spinal cord at this level bringing it to early attention.10 11
Due to its rarity, there are no existing evidence-based guidelines for the treatment of paraspinal pseudotumours caused by EMH.4 Intervention options to relieve the cord compression include multiple blood transfusions to downgrade erythropoietin production, radiation therapy to halt the production of overgrown marrow tissue, and surgical decompression, or a combination of these.11 Blood transfusion corrects the anaemia, and therefore decreases the need for EMH. These EMH tissues undergo shrinkage probably due to a reduction in blood flow rather than atrophy.12 13 Hypertransfusion to maintain Hb over 120 g/L to completely suppress haematopoiesis has been described.11–14 However, improvement is usually incomplete and short lived.12 Currently, it can be recommended only in cases of mild spinal cord compression or in pregnant patients to obviate the need for surgery or radiotherapy.14–16 Low dose radiotherapy has been reported to yield excellent results in up to 50% patients as haematopoietic tissue is extremely radiosensitive.17 Various anecdotal case reports have shown benefit with these modalities.18–22 We elected to transfuse our patient till his Hb rose to 100 g/L, administer steroids and irradiate locally. There was complete resolution clinically, verified on imaging at 1-week postradiotherapy, and patient showed sustained improvement at 6 months’ review.
Patient’s perspective.
‘I was not sure if the paralysis of my legs would get alright, but I was diagnosed accurately and treated very well’.
Learning points.
Extramedullary haematopoiesis can occur in unusual sites like paraspinal and intraspinal areas.
Thalassaemia intermedia should be thought of when haemolytic anaemia is suspected without regular requirement of blood transfusions.
The extramedullary haematopoietic tissues are radiosensitive so when there is significant cord compression with severe neurological deficits, radiotherapy should be started as early as possible.
Footnotes
Contributors: PD identified the clinical signs, did background literature search, was primary author in writing the case and discussion. US assisted in writing the case report, discussion and referencing. US did critical review of the article. NK helped in preparing the manuscript and researching reference articles. AK helped in researching reference articles and gathering patient data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Amirjamshidi A, Abbassioun K, Ketabchi SE. Spinal extradural hematopoiesis in adolescents with thalassemia. Report of two cases and a review of the literature. Childs Nerv Syst 1991;7:223–5. [DOI] [PubMed] [Google Scholar]
- 2. Monti L, Romano DG, Gozzetti A, et al. Myelodysplasia presenting as thoracic spinal epidural extramedullary hematopoiesis: a rare treatable cause of spinal cord myelopathy. Skeletal Radiol 2012;41:611–4. 10.1007/s00256-011-1268-2 [DOI] [PubMed] [Google Scholar]
- 3. Weatherall DJ, Clegg JB. The thalassemia syndromes. 4th edn Oxford: Blackwell Scientific Publications, 2001. [Google Scholar]
- 4. Haidar R, Mhaidli H, Taher AT. Paraspinal extramedullary hematopoiesis in patients with thalassemia intermedia. Eur Spine J 2010;19:871–8. 10.1007/s00586-010-1357-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gatto I, Terrana V, Biondi L. [Compression of the spinal cord due to proliferation of bone marrow in epidural space in a splenectomized person with Cooley’s disease]. Haematologica 1954;38:61–76. [PubMed] [Google Scholar]
- 6. Munn RK, Kramer CA, Arnold SM. Spinal cord compression due to extramedullary hematopoiesis in beta-thalassemia intermedia. Int J Radiat Oncol Biol Phys 1998;42:607–9. 10.1016/S0360-3016(98)00245-4 [DOI] [PubMed] [Google Scholar]
- 7. Dore F, Cianciulli P, Rovasio S, et al. Incidence and clinical study of ectopic erythropoiesis in adult patients with thalassemia intermedia. Ann Ital Med Int 1992;7:137–40. [PubMed] [Google Scholar]
- 8. Pantongrag-Brown L, Suwanwela N. Case report: chronic spinal cord compression from extramedullary haematopoiesis in thalassaemia-MRI findings. Clin Radiol 1992;46:281–3. 10.1016/S0009-9260(05)80172-2 [DOI] [PubMed] [Google Scholar]
- 9. Da Costa JL, Loh YS, Hanam E. Extramedullary hemopoiesis with multiple tumor-simulating mediastinal masses in hemoglobin E-thalassemia disease. Chest 1974;65:210–2. 10.1378/chest.65.2.210 [DOI] [PubMed] [Google Scholar]
- 10. Abbassioun K, Amir-Jamshidi A. Curable paraplegia due to extradural hematopoietic tissue in thalassemia. Neurosurgery 1982;11:804–7. 10.1227/00006123-198212000-00017 [DOI] [PubMed] [Google Scholar]
- 11. Chehal A, Aoun E, Koussa S, et al. Hypertransfusion: a successful method of treatment in thalassemia intermedia patients with spinal cord compression secondary to extramedullary hematopoiesis. Spine 2003;28:E245–9. 10.1097/01.BRS.0000067282.47308.4D [DOI] [PubMed] [Google Scholar]
- 12. Coşkun E, Keskin A, Süzer T, et al. Spinal cord compression secondary to extramedullary hematopoiesis in thalassemia intermedia. Eur Spine J 1998;7:501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parsa K, Oreizy A. Nonsurgical approach to paraparesis due to extramedullary hematopoiesis. Report of two cases. J Neurosurg 1995;82:657–60. 10.3171/jns.1995.82.4.0657 [DOI] [PubMed] [Google Scholar]
- 14. Lee AC, Chiu W, Tai KS, et al. Hypertransfusion for spinal cord compression secondary to extramedullary hematopoiesis. Pediatr Hematol Oncol 1996;13:89–94. 10.3109/08880019609033375 [DOI] [PubMed] [Google Scholar]
- 15. Phupong V, Uerpairojkij B, Limpongsanurak S. Spinal cord compression: a rareness in pregnant thalassemic woman. J Obstet Gynaecol Res 2000;26:117–20. 10.1111/j.1447-0756.2000.tb01293.x [DOI] [PubMed] [Google Scholar]
- 16. Malik M, Pillai LS, Gogia N, et al. Paraplegia due to extramedullary hematopoiesis in thalassemia treated successfully with radiation therapy. Haematologica 2007;92:e28–30. 10.3324/haematol.10199 [DOI] [PubMed] [Google Scholar]
- 17. Singhal S, Sharma S, Dixit S, et al. The role of radiation therapy in the management of spinal cord compression due to extramedullary haematopoiesis in thalassaemia. J Neurol Neurosurg Psychiatry 1992;55:310–2. 10.1136/jnnp.55.4.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evrard C, Pichereau D, Alsweis S, et al. [Extramedullary hematopoiesis of thoracic and vertebral intraductal localization. Apropos of a case. Review of the literature]. Ann Chir 1994;48:284–93. [PubMed] [Google Scholar]
- 19. Bukhari SS, Junaid M, Rashid MU. Thalassemia, extramedullary hematopoiesis, and spinal cord compression: A case report. Surg Neurol Int 2016;7:148 10.4103/2152-7806.177891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martínez-Rodrigo MA, Sanjuanbenito L, Rodríguez del Barrio E, et al. [Spinal cord compression secondary to epidural extramedullary hematopoiesis in thalassemia: a clinical case and review of literature]. Rev Neurol 1998;27:998–1004. [PubMed] [Google Scholar]
- 21. Cianciulli P, Sorrentino F, Morino L, et al. Radiotherapy combined with erythropoietin for the treatment of extramedullary hematopoiesis in an alloimmunized patient with thalassemia intermedia. Ann Hematol 1996;72:379–81. 10.1007/s002770050190 [DOI] [PubMed] [Google Scholar]
- 22. Wang A, Carberry N, Solli E, et al. Spinal cord compression secondary to extramedullary hematopoiesis: case report and review of the literature. Case Rep Oncol 2016;9:290–7. 10.1159/000446473 [DOI] [PMC free article] [PubMed] [Google Scholar]