Abstract
Tranexamic acid (TXA) is an antifibrinolytic which minimises bleeding and transfusions, with thrombotic risk. Our patient had known coronary artery disease with post-TXA acute ST-elevation myocardial infarction (STEMI) due to in-stent thrombosis. He had five drug-eluting stents (DES): two overlapping DES in mid-LAD (3 years ago), and two overlapping DES in distal right coronary artery and one DES in obtuse-marginal (1.5 years ago). After TXA, both overlapping stent locations thrombosed. Of nine reports of post-TXA acute MI, only one had complex stent anatomy (bifurcation stent to left circumflex/first obtuse-marginal) with other single stents, and only the complex stent thrombosed. Post-TXA MI was more often STEMI caused by arterial thrombosis, rather than non-STEMI caused by blood loss, hypotension or demand ischaemia. Overlapping and bifurcation stents thrombosed; single stents remained patent. In conclusion, overlapping stents, bifurcation stents, excessive stent length and previous in-stent restenosis/thrombosis may increase thrombotic risk. TXA should be administered cautiously with complex stent anatomy.
Keywords: unwanted effects/adverse reactions, cardiovascular medicine, ischaemic heart disease, perioperative care, orthopaedics
Background
Tranexamic acid (TXA) is an antifibrinolytic agent used to minimise bleeding in multiple clinical settings. It reversibly binds plasminogen receptors, inhibiting the proteolytic activity of plasmin and subsequent breakdown of fibrin. Food and Drug Administration approval is for menorrhagia, and bleeding prophylaxis in haemophilia and von Willebrand disease.1 2 TXA is widely used in multiple surgical settings to minimise blood loss and need for blood transfusions.
Several randomised-controlled trials, meta-analyses, systematic literature reviews and retrospective database analyses within the last 10 years have reinforced the safety and efficacy of perioperative TXA to minimise blood transfusions.1 3–7 TXA significantly reduced perioperative blood loss and rate of blood transfusions compared with placebo or no treatment, without significantly increasing postprocedural incidence of thromboembolic events such as myocardial infarction, stroke, deep vein thrombosis or pulmonary embolism, and without increasing postoperative mortality. These articles report TXA use in a variety of surgical settings, including trauma surgery in which TXA significantly reduced all-cause mortality and death due to bleeding,7 orthopaedic surgery in particular total hip and knee arthroplasty,1 4–6 cardiac surgery including coronary artery bypass grafting (CABG) and combined CABG with valve replacement,3 6 and other surgeries including cranial and orthognathic, gynaecological, hepatic, urological and vascular surgeries.6 These trials included very few patients at high risk for arterial or venous thromboembolic complications, since TXA is considered high risk in these populations.2 4 8 9 This included patients with current or prior arterial or venous thromboembolic disorders such as deep vein thrombosis, pulmonary embolism, myocardial infarction, cerebrovascular disease or stroke, transient ischaemic attack, vascular or arterial stents and chronic hypercoagulable states.1 In women undergoing treatment with TXA for menorrhagia, this may also include those taking hormonal contraceptives, those with obesity, and smokers.10
For these reasons, there is a paucity of data regarding safety of TXA in high-thromboembolic-risk populations, due to exclusion of patients with known risk factors from trials. In particular, arterial thromboembolic events following total hip or knee arthroplasty are infrequent and are rarely reported.1 We present a patient with prior known coronary artery disease with complex stents who received intraoperative intravenous TXA during a total hip arthroplasty, and subsequently suffered an ST-elevation myocardial infarction (STEMI) from in-stent thrombosis requiring emergent intervention.
Case presentation
Our patient is a 59-year-old man with total right hip arthroplasty 4 years prior, with revision for Propionibacterium acnes infection <2 years prior, admitted for repeat total hip arthroplasty revision due to recurrent prosthetic joint infection. He had erythema, swelling, induration and pain in his thigh, and a right thigh aspirate from 1 month prior grew Propionibacterium acnes.
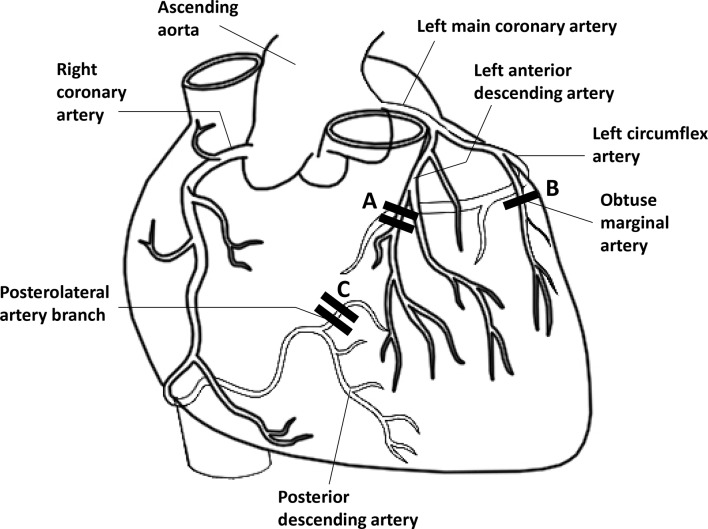
He had known coronary artery disease with prior placement of five drug-eluting stents (DES) (figure 1).11 Three years prior to admission, he suffered an anterior wall STEMI with total occlusion of the mid-left anterior descending (mLAD) artery, and two overlapping DES were placed. Left ventricular ejection fraction was 55%. Eighteen months prior to admission, he had chest pain with a positive stress test, and angiography revealed 80% stenosis of the marginal branch (obtuse marginal [OM]) of the left circumflex, and 70% stenosis of the distal right coronary artery (dRCA). One DES was placed in the 80% OM lesion, and two overlapping DES were placed in the 70% dRCA lesion. Prior LAD stents were widely patent. He was prescribed dual antiplatelet therapy with aspirin and prasugrel for both instances, and had no further cardiac symptoms between his most recent stent placement and the present surgery. He also had hypertension, hyperlipidaemia, chronic kidney disease stage II-III, former tobacco use (1/3 pack/day for 20 years, quit 5 years prior), rare alcohol use, obesity (body mass index 40 kg/m2), obstructive sleep apnoea on continuous positive airway pressure, Gilbert’s syndrome and ankylosing spondylitis.
Figure 1.
Anatomy of coronary artery disease and stents (adapted from Erb and Seeberger).11 Drug-eluting stents: Stents A. Three years prior: total occlusion of mLAD → two overlapping DES (2.75×24 mm Xience DES, 2.75×14 mm Promus DES); Stent B. Eighteen months prior: 80% occlusion of proximal OM of LCX → 1 DES to OM (2.5×23 mm Xience); Stents C. Eighteen months prior: 70% occlusion of dRCA/posterolateral branch → two overlapping DES (2.5×28 mm Xience DES, 2.5×12 mm Xience DES). Stents A and C: Total occlusion due to in-stent thrombosis after tranexamic acid. DES, drug eluting stent; dRCA, distal right coronary artery; LCX, left circumflex artery; mLAD, mid-left anterior descending coronary artery; OM, obtuse marginal (coronary artery).
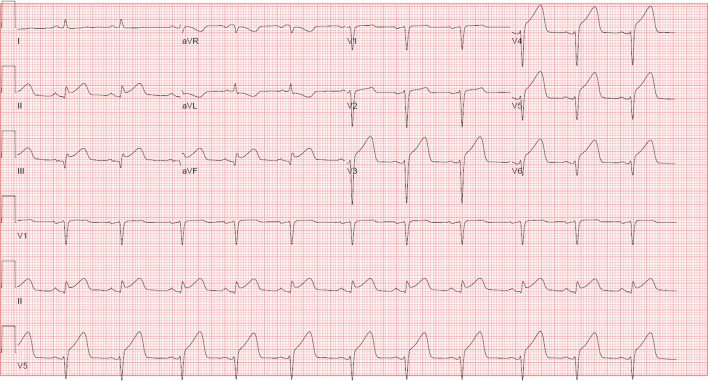
During his preoperative evaluation, he was instructed to continue aspirin 81 mg daily, and stop prasugrel 5 days prior to his procedure. He was subsequently admitted for his revision total hip arthroplasty. An initial intraoperative ECG was normal. Twenty minutes after the start of the procedure TXA was administered as a 500 mg intravenous bolus, followed by another 500 mg intravenous bolus 10 min later. Three hours after TXA, ST elevations were noted in leads II and V5 on the cardiac monitor, without prior documented ST segment depressions. Due to patient position, an ECG could not be obtained immediately. Given the patient was haemodynamically stable, surgery was continued. An ECG obtained >1 hour later showed an acute inferolateral STEMI, with ST elevations in leads II, III, aVF and V3-V6 (figure 2). The patient was immediately transported to the cardiac catheterisation laboratory. Troponin T levels before and after the ECG were initially negative.
Figure 2.
ST-elevation myocardial infarction, with ST elevation in leads II, III, aVF and V3-V6.
At the start of spinal anaesthesia, the patient’s non-invasive blood pressure (BP) was 158/90 mm Hg (mean arterial pressure [MAP] 113), with a heart rate (HR) of 58 beats/min. His non-invasive BPs fluctuated and decreased to BP 95/54 mm Hg (MAP 68) with HR 58 beats/min prior to incision, BP 90/60 mm Hg (MAP 70) and HR 54 beats/min at the time of TXA administration and BP 80/50 mm Hg (MAP 60) with HR 72 beats/min just after switching from spinal anaesthesia and monitored anaesthesia care to general anaesthesia and noticing ST elevations on the monitor. The surgical case was converted from spinal anaesthesia to general anaesthesia as the procedure required more time than anticipated. An arterial line was placed. The low non-invasive MAPs were treated with phenylephrine boluses and PlasmaLyte infusions. After placing an arterial line, a phenylephrine drip was titrated to control BP. A nitroglycerin infusion was started after noting ST elevations. Hypotension was suspected to be sedation-related, as propofol was initiated at the start time of surgery. The patient did not receive inotropic support. He continued to have intermittent hypotension, as low as arterial line BP 78/48 mm Hg (MAP 58) with HR 70 beats/min during the left heart catheterisation.
Investigations
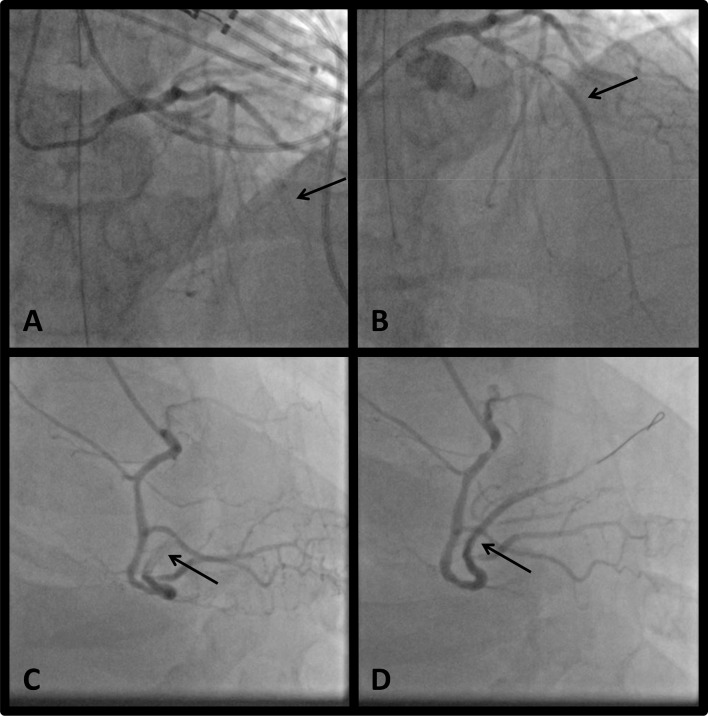
Coronary angiography showed acute in-stent thrombosis with total occlusion of the mLAD (at the site of overlapping DES ×2 placed 3 years prior), and acute in-stent thrombosis with total occlusion of the dRCA (at the site of overlapping DES ×2 placed 18 months prior) (figure 3), with no stenosis or occlusion at the prior stent to the OM (DES ×1 placed 18 months prior).
Figure 3.
Coronary angiography (A): mLAD with total occlusion due to in-stent thrombosis at site of prior overlapping stents, as shown by the arrow. (B) mLAD after balloon angioplasty performed on totally occluded artery, with no residual stenosis, as shown by the arrow. (C) dRCA/right posterolateral branch with total occlusion due to in-stent thrombosis at site of prior overlapping stents, as shown by the arrow. (D) dRCA/right posterolateral branch after balloon angioplasty performed on totally occluded artery, with no residual stenosis, as shown by the arrow. dRCA, distal right coronary artery; mLAD, mid-left anterior descending coronary artery.
Treatment
The patient underwent balloon angioplasty of the mLAD, the embolised thrombus in the distal LAD, and the dRCA. There were no residual occlusions and no new stents were placed (figure 3). He received aspirin 81 mg, ticagrelor 180 mg and a cangrelor bolus followed by infusion.
Outcome and follow-up
Troponin T level obtained the next morning was 7.070 ng/mL. Aspirin 81 mg was continued, and ticagrelor 90 mg two times per day was initiated. The patient was on high-dose atorvastatin 80 mg and metoprolol succinate for prior coronary artery disease. Captopril was added. Transthoracic echocardiogram at the time of the left heart catheterisation showed moderate systolic dysfunction with a left ventricular ejection fraction of 36%, and hypokinesis to akinesis of the mid- to distal anteroseptum, anterior wall, anterolateral wall and the apex. A repeat echocardiogram 3 days later showed ejection fraction of 52%, with persistent severe hypokinesis to akinesis of the entire left ventricular apex. The patient was discharged home on postoperative day 7.
Discussion
To our knowledge, our patient is only the second reported case of a STEMI due to in-stent thrombosis after TXA administration in the setting of known coronary artery disease.
Although there have been several recent high-quality studies discussing the safety and efficacy of systemic TXA use in several surgical settings,1 3–7 high-risk patients such as ours were typically not included. For example the Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2 (CRASH-2) trial7 included young (average age 35 years) trauma patients who likely did not have cardiovascular comorbidities. In addition, there was bias in the selection of trial candidates: patients with clear indications for TXA received TXA, and those with clear contraindications did not, therefore potential trial candidates were not all randomly assigned to treatment groups. Wind et al 5 reported a retrospective review of patients undergoing primary total hip arthroplasty in a single institution, whose policy was to give topical rather than intravenous TXA to patients at elevated risk (ie, myocardial infarction during the prior 6 months, stent placement within the last 1 year or prior embolic event), which was found to be ineffective compared with intravenous TXA at reducing blood transfusions. Of note, our patient’s most recent stent was >1 year prior, which may have suggested that intravenous TXA would be relatively safe. In the Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial,3 the authors concede that few patients were included who were at the highest risk for bleeding or thrombosis; only 2.5% of these patients had a previous angioplasty or stenting, and 1.2%–1.6% had previous cardiac surgery.
There are nine case reports of patients who suffered acute myocardial infarction hours to days after TXA, though most of these patients did not have previously known coronary artery disease or other vascular comorbidities indicating elevated risk (table 1).2 8–10 12–16 Seven patients had no known coronary artery or vascular disease, whereas two had a significant cardiac history. In six of these cases, the mechanism of acute myocardial infarction (MI) was thrombosis or acute plaque rupture requiring coronary intervention (stent placement in five of six, and only balloon angioplasty in one case), and of these five out of six had STEMIs with one non-ST-elevation MI (NSTEMI). The remaining three MIs were more likely due to demand ischaemia in the setting of blood loss and hypotension, two of three were NSTEMIs with one STEMI, and angiography showed no specific lesions therefore no intervention was performed.
Table 1.
Comparison of our case to prior case studies reporting acute myocardial infarction after tranexamic acid administration
| Case reports | Age/ gender | TXA Indication | TXA dose/duration | Type of infarction (STEMI/NSTEMI) |
Underlying mechanism | Angiography findings | Intervention | Prior history of CAD | Cardiovascular risk factors | Thrombotic risk factors |
| No history of CAD | ||||||||||
| Mekontso‐ Dessap et al 12 | 77/F | Haemoptysis due to pulmonary tuberculosis | 500 mg PO two times per day/5 days, then 1 g PO two times per day/2 days | NSTEMI: 2 days after dose increase to 1 g two times per day/1 week after initiating TXA | Demand ischaemia: blood loss* | 30% mLAD stenosis |
|
No |
|
|
| Iacobellis and Iacobellis8 | 42/F | Menorrhagia associated with a uterine leiomyoma | 3 g IM in daily divided doses+combined OCP/2 months | NSTEMI: two months after starting TXA+OCP |
Possible demand ischaemia* | Ulcerated plaque on pLAD without significant narrowing |
|
No |
|
|
| Sirker et al 13 | 28/F | Shoulder arthroscopy and decompression, with known bleeding diathesis (desmopressin-responsive platelet dysfunction) | DDAVP 0.3 μg/kg intravenous prior to procedure, then TXA 1 g PO four times a day/1 week | Inferior STEMI: 3 days after completing therapy (10 days postoperative) | Thrombosis | dRCA occlusion |
|
No |
|
|
| Gupta et al 10 | 41/F | Menorrhagia and dysmenorrhoea due to uterine fibroid | TXA 500 mg PO+mefenamic acid 250 mg PO three times a day (NSAID)/2 years, for 5-day period with each menses |
Inferior/RV STEMI: 14 days after last dose | Thrombosis | 99% pRCA occlusion with thrombus |
|
No |
|
|
| Garg et al 2 | 56/F | Right hip arthroplasty | 10 mg/kg intravenous/x1 dose 1 hour prior to surgery | Inferior STEMI (II, III, aVF): immediately postoperative | Thrombosis | 100% dRCA occlusion |
|
No |
|
|
| Ngo‐Thai et al 14 | 41/F | Menorrhagia | 1 g PO three times a day from day 1 to completion of menses | NSTEMI: 7 days after completing course | Thrombosis on pre-existing plaque | 70% stenosis of proximal/mid D1 of LAD |
|
No |
|
|
| Gerstein et al 9 | 66/M | Revision spinal fusion surgery | 1 g intravenous bolus followed by 1 mg/kg/hour infusion / intraoperative |
Anterolateral STEMI: within 12 hours postoperative | Thrombosis/?plaque rupture | Severe pLAD lesion, complete occlusion of diagonal branch. TTE: large apical LV thrombus |
|
No |
|
|
| History of CAD | ||||||||||
| Mandal and Missouris15 | 60/M | Severe lower GI bleed, presumed angiodysplasia, required 11 units pRBCs | 1 g PO prior to emergency laparotomy | Anteroseptal STEMI (V1‐V4): 1 hour after TXA | ?Demand ischaemia: hypotension and acute blood loss (?TXA-induced)* | Occluded RCA (?from prior MI), patent LAD |
|
Yes: inferior MI 2 weeks prior to presentation (no further details) |
|
|
| Bridges and Wilson16 | 71/M | Shoulder arthroplasty | 20 mg/kg intravenous, 30 min after start of surgery | Inferior wall STEMI, with complete heart block: 4.5 hours after surgery complete | Acute coronary thrombus | Acute in‐stent thrombus with total occlusion at LCX / OM1 bifurcation stent |
|
Yes: 4 DES ≥5 years prior. (1)Bifurcation DES to LCX/OM1, restenosis of OM1 (balloon angioplasty), (2)in‐stent thrombosis of OM1 (repeat DES), (3)DES x1 to RCA, (4)DES x1 to D1. |
|
|
| Kaptein | 59/M | Right hip arthroplasty | 500 mg intravenous x2 doses 10 min apart/intraoperatively | Inferolateral STEMI (II, III, aVF, V3‐V6): 3 hours after TXA |
Acute in‐stent thrombosis of overlapping stents | Acute in‐stent thrombus with total occlusion at sites of overlapping DES at mLAD and dRCA; no stenosis or occlusion at single DES to OM |
|
Yes: prior anterior STEMI with overlapping DES x2 to mLAD, DES x1 to OM, and overlapping DES x2 to dRCA. Placed ≥18 months prior |
|
|
Age in years.
*Data provided suggest demand ischaemia mechanism (no intervenable lesion on angiography).
ASA, aspirin; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; D1, first diagonal branch (coronary artery); DAPT, dual antiplatelet therapy; DDAVP, desmopressin; DES, drug eluting stent; dLAD, distal left anterior descending coronary artery; dRCA, distal right coronary artery; DVT, deep vein thrombosis; F, female; GI, gastrointestinal; HAART, highly active antiretroviral therapy; HLD, hyperlipidaemia; HTN, hypertension; ICU, intensive care unit; IM, intramuscular; IUD, intrauterine device; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LDL, low density lipoprotein; LV, left ventricle; M, male; MI, myocardial infarction; mLAD, mid-left anterior descending coronary artery; NSAID, non-steroidal anti-inflammatory drug; NSTEMI, non ST-elevation myocardial infarction; OCP, oral contraceptive pills; OM1, first obtuse marginal (coronary artery); PCI, percutaneous coronary intervention; pLAD, proximal left anterior descending coronary artery; PO, orally; ppd, packs per day; pRBCs, packed red blood cells; pRCA, proximal right coronary artery; RCA, right coronary artery; RV, right ventricle; STEMI, ST elevation myocardial infarction; TIA, transient ischaemic attack; TTE, transthoracic echocardiogram; TXA, tranexamic acid.
These observations warrant consideration about the mechanism by which TXA may predispose to acute myocardial infarction in susceptible patients. As an antifibrinolytic and haemostatic agent, the primary concern is acute MI due to thrombosis and arterial occlusion. In the meta-analysis by Fillingham et al,1 arterial thromboembolic events were rare complications following primary hip or knee arthroplasty, and are rarely reported in randomised clinical trials due to the exclusion of patients with known risk factors. Devereaux and Eikelboom17 report that myocardial infarction is the most common major vascular complication following noncardiac surgery, proposing that the major mechanism is demand ischaemia from operative blood loss rather than a thromboembolic event. In support of this argument, Devereaux cites that most postoperative myocardial infarctions are NSTEMIs suggesting a supply-demand mismatch mechanism though many of these NSTEMI patients also had coronary artery thrombus on intracoronary optical coherence tomography (OCT), and MI was more frequent with greater intraoperative blood loss and transfusion requirements. Devereaux also references the CRASH-2 trial that showed TXA significantly reduced bleeding, and patients receiving TXA had a significantly lower incidence of MI, presumably due to less demand ischaemia7 but also probably due to patient selection.
Taking this into account, those three case reports that described patients who suffered acute MI most likely due to demand ischaemia (two of three NSTEMI, one STEMI) fit the pathophysiology discussed by Devereaux and Eikelboom,17 and TXA administration may not have caused the subsequent MI. The remaining six cases that suffered acute MI due to plaque rupture or acute thrombosis were more likely to be STEMI, all required coronary intervention, and are more likely to have been precipitated by the administration of TXA, as with our patient.
We consider our case report important in that it is one of the few cases published of post-TXA acute MI in a patient with known coronary artery disease. Acute in-stent thrombosis only occurred at the sites of overlapping DES, one site stented almost 3 years prior, and the other site 18 months prior (the single DES placed 18 months prior remained patent). The anatomy of the stents may have played a greater role in the risk of late in-stent thrombosis than the age or type of stent (drug-eluting vs bare metal), and this increased risk may be extended to other patients who have highly thrombogenic stent anatomy such as overlapping stents, bifurcation stents and excessive stent length.16 18 In addition, patients who have had a history of in-stent restenosis or thrombosis may be at elevated risk.16
In support of this argument, our case and that reported by Bridges and Wilson16 are the only two patients with a history of high-risk stent anatomy in addition to other single stents, who received TXA and suffered a subsequent MI due to in-stent thrombosis only of the high-risk stents. Their case16 was a 71-year-old man with a history of coronary artery disease with four DES placed ≥5 years prior, including a bifurcation DES to the left circumflex artery/first obtuse marginal branch (LCX/OM1) with a history of restenosis requiring balloon angioplasty, and later in-stent thrombosis requiring a repeat DES placement. He also had a single DES to the RCA, and a single DES to the first diagonal artery. This patient received intravenous TXA during a shoulder arthroplasty, and 4.5 hours later suffered an inferior wall STEMI with complete heart block due to in-stent thrombosis with total occlusion at the LCX/OM1 bifurcation stent, requiring another DES placement, in addition to inotropes and diuresis for cardiogenic shock. The two single DES remained patent. This patient was at additional risk for perioperative MI, as both antiplatelet agents were held 7 days prior to surgery, rather than continuing aspirin and only holding the prasugrel as in our case.
In conclusion, these findings suggest that for patients with known history of coronary artery disease who may be candidates for TXA, stent anatomy should be carefully evaluated. Overlapping stents, excessive stent length and stents at bifurcations, or history of previous in-stent restenosis or thrombosis, may indicate a high risk for thrombosis.16 18 Complex stent anatomy may be a more important risk factor for MI caused by arterial thrombus than just coronary artery disease or prior stents alone. For patients with complex stent anatomy, perioperative management of dual antiplatelet agents should be carefully assessed. These considerations will likely not affect the incidence of postoperative MI caused by blood loss or demand ischaemia, and as mentioned before, some literature supports the use of TXA to minimise blood loss and the risk of demand-ischaemia type NSTEMI postoperatively. Protocols on the administration of TXA and preoperative evaluation of high-cardiac-risk patients should take these considerations into account when weighing the risks (thrombotic STEMI) and benefits (minimising blood loss and demand-NSTEMI) of TXA administration. Randomised trials assessing the risks and benefits of TXA in high-cardiac-risk patients are unlikely to be performed in the future.
Learning points.
Patients with known history of coronary artery disease who may receive tranexamic acid (TXA) should first be carefully evaluated for stent anatomy.
Overlapping stents, excessive stent length and stents at bifurcations, or history of previous in-stent restenosis or thrombosis may place patients at high risk for post-TXA thrombosis.
Complex stent anatomy may be a more important risk factor for post-TXA myocardial infarction caused by arterial thrombus, than just the presence of coronary artery disease or single stents.
TXA should be administered cautiously in patients with complex stent anatomy.
Footnotes
Contributors: YEK is the sole author of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Fillingham YA, Ramkumar DB, Jevsevar DS, et al. Tranexamic Acid Use in Total Joint Arthroplasty: The Clinical Practice Guidelines Endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty 2018;33:3065–9. 10.1016/j.arth.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 2. Garg J, Pinnamaneni S, Aronow WS, et al. ST elevation myocardial infarction after tranexamic acid: first reported case in the United States. Am J Ther 2014;21:e221–4. 10.1097/MJT.0b013e31828fdb06 [DOI] [PubMed] [Google Scholar]
- 3. Myles PS, Smith JA, Forbes A, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med 2017;376:136–48. 10.1056/NEJMoa1606424 [DOI] [PubMed] [Google Scholar]
- 4. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:g4829 10.1136/bmj.g4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty 2014;29:387–9. 10.1016/j.arth.2013.05.026 [DOI] [PubMed] [Google Scholar]
- 6. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054 10.1136/bmj.e3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 8. Iacobellis G, Iacobellis G. Combined treatment with tranexamic acid and oral contraceptive pill causes coronary ulcerated plaque and acute myocardial infarction. Cardiovasc Drugs Ther 2004;18:239–40. 10.1023/B:CARD.0000033646.21346.e4 [DOI] [PubMed] [Google Scholar]
- 9. Gerstein NS, Brierley JK, Culling MD. Left ventricle thrombus after tranexamic acid for spine surgery in an HIV-positive patient. Spine J 2016;16:e77–82. 10.1016/j.spinee.2015.10.039 [DOI] [PubMed] [Google Scholar]
- 10. Gupta PN, Mullamalla UR, Sabin P, et al. Acute MI in a young hypertensive woman: could it be due to tranexamic acid? BMJ Case Rep 2013:pii: bcr2013009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erb JM, Seeberger MD. Radiolology key: fastest radiology insight engine. Diagnosis of myocardial ischemia: anatomy and physiology of the myocardial blood supply. 2016. https://radiologykey.com/diagnosis-of-myocardial-ischemia/ (accessed 26 Sep 2018).
- 12. Mekontso-Dessap A, Collet JP, Lebrun-Vignes B, et al. Acute myocardial infarction after oral tranexamic acid treatment initiation. Int J Cardiol 2002;83:267–8. [DOI] [PubMed] [Google Scholar]
- 13. Sirker A, Malik N, Bellamy M, et al. Acute myocardial infarction following tranexamic acid use in a low cardiovascular risk setting. Br J Haematol 2008;141:907–8. 10.1111/j.1365-2141.2008.07128.x [DOI] [PubMed] [Google Scholar]
- 14. Ngo-Thai LL, Gellatly R, Nanayakkara S. Tranexamic acid precipitating onset of acute myocardial infarction. J Pharm Pract Res 2015;45:46–8. 10.1002/jppr.1050 [DOI] [Google Scholar]
- 15. Mandal AKJ, Missouris CG. Tranexamic acid and acute myocardial infarction. Br J Cardiol 2005;12:306–7. [Google Scholar]
- 16. Bridges KH, Wilson SH. Acute Coronary Artery Thrombus After Tranexamic Acid During Total Shoulder Arthroplasty in a Patient With Coronary Stents: A Case Report. A A Pract 2018;10:212–4. 10.1213/XAA.0000000000000667 [DOI] [PubMed] [Google Scholar]
- 17. Devereaux PJ, Eikelboom J. Insights into myocardial infarction after noncardiac surgery in patients with a prior coronary artery stent. Br J Anaesth 2016;116:584–6. 10.1093/bja/aew111 [DOI] [PubMed] [Google Scholar]
- 18. Cutlip D, Abbott JD. Coronary artery stent thrombosis: incidence and risk factors : Cannon CP, Windecker S, UpToDate. Alphen aan den Rijn. The Netherlands: Wolters Kluwer Health, 2018. [Google Scholar]