Abstract
May-Thurner syndrome (MTS) is a clinical condition where the left common iliac vein gets compressed by the overlying right common iliac artery anterior to the fifth lumbar vertebra and the sacral promontory. It results in vessel wall injury and predisposition to thrombosis. We present a case of a 21-year-old African-American man with no significant past medical history who came to the emergency department with left lower limb swelling associated with shortness of breath, and was eventually diagnosed to have extensive left lower extremity deep vein thrombosis (DVT) along with acute bilateral extensive pulmonary embolism (PE) as a consequence to MTS. MTS should be considered in the differential when young patients present with unprovoked or recurrent left-sided DVT. Diagnosis of this anatomical variant is critical as it may need long-term anticoagulation and consideration of pharmaco-mechanical intervention such as mechanical thrombectomy and venoplasty with or without stenting.
Keywords: venous thromboembolism, pulmonary embolism
Background
May-Thurner syndrome (MTS) presenting with acute bilateral pulmonary embolism (PE) is not common, and only a few cases have been reported in the literature. A more common presentation of this syndrome is deep vein thrombosis (DVT) of the left lower extremity characterised by swelling and pain, while chronic leg pain, skin pigmentation changes, recurrent skin ulcers may be present in some cases. A vast majority of the population with this anatomy may remain undetected throughout life.
MTS is more of an underdiagnosed clinical entity rather than a less prevalent one. When detected early, this syndrome can be treated effectively. The management options include anticoagulation which may be lifelong in some cases, endovascular stenting to correct the anatomical condition or catheter-guided thrombolysis.
Case presentation
We present a case of a 21-year-old African-American man with no significant past medical history presented to the emergency department with severe left leg pain and swelling of 1-month duration and non-productive cough with some exertional shortness of breath for the past couple of days. He has a sedentary lifestyle, a history of blood clot in one of his uncles, active cigarette smoker (one pack a day) and a remote history of intravenous drug abuse. Admission vitals were remarkable for mild tachycardia 111 bpm. Left calf was swollen and tender compared with the right calf. ECG showed sinus tachycardia. Patient’s D-dimer was >5000 ng/mL. A duplex scan was positive for acute DVT in left common femoral, left proximal femoral, left popliteal and posterior tibial veins. It was noted in the emergency department that his tachycardia worsened during ambulation. With the aetiology of such extensive DVT not evident, acute PE was suspected, and a protocol-driven CT pulmonary angiography study was done immediately. It showed bilateral extensive pulmonary emboli, a central occlusive embolus in a segmental branch of the left lower lobe and multiple peripheral non-occlusive emboli in the left lower lobar pulmonary artery, right middle lobar pulmonary artery, and right lower lobar pulmonary artery (figures 1 and 2). CT of the abdomen and pelvis with oral and intravenous contrast was performed to reveal any possible proximal extension of the clot into the iliac veins, to evaluate the clot burden and also to identify any underlying condition that might be responsible for the extensive unprovoked thrombogenesis, like a hitherto undetected malignancy or a chronic inflammatory state. Suspicions were based on the findings of anaemia (around 9 g%, with evidence of iron deficiency), thrombocytosis (~600 000/cumm) and raised pro-inflammatory markers, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). CT showed severe compression of the left common iliac vein by the right common iliac artery (figures 3 and 4) and extension of the thrombus in the external and common iliac vein on the left side. Portal vein and infrarenal inferior vena cava (IVC) were patent, and no thrombus was identified. Echocardiogram did not show any evidence of right ventricular strain or pulmonary hypertension. There was no significant haemodynamic instability. Intravenous heparin drip was started and was subsequently converted to rivaroxaban. The patient’s symptoms improved and he was discharged after a week of hospitalisation with a plan of long-term anticoagulation.
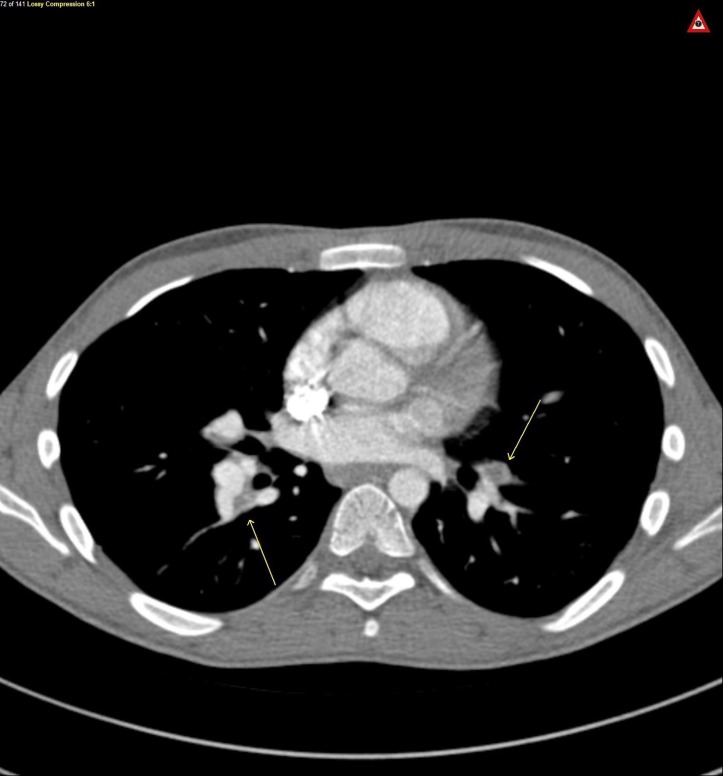
Figure 1.
Axial view CT chest, arrows on bilateral pulmonary embolism.
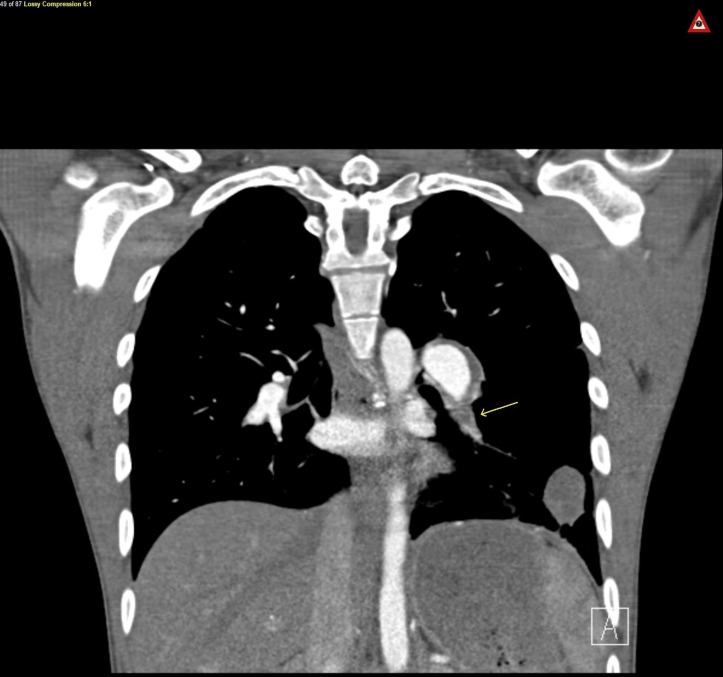
Figure 2.
Coronal view CT chest, arrow on left lower lobe pulmonary embolism.
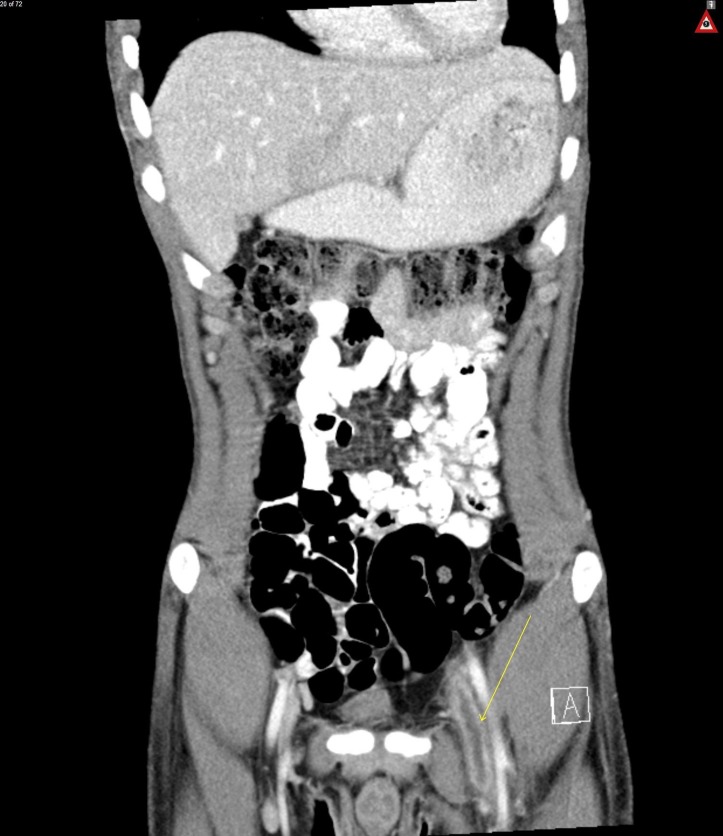
Figure 3.
Coronal view CT abdomen/pelvis, arrow on left deep vein thrombosis.
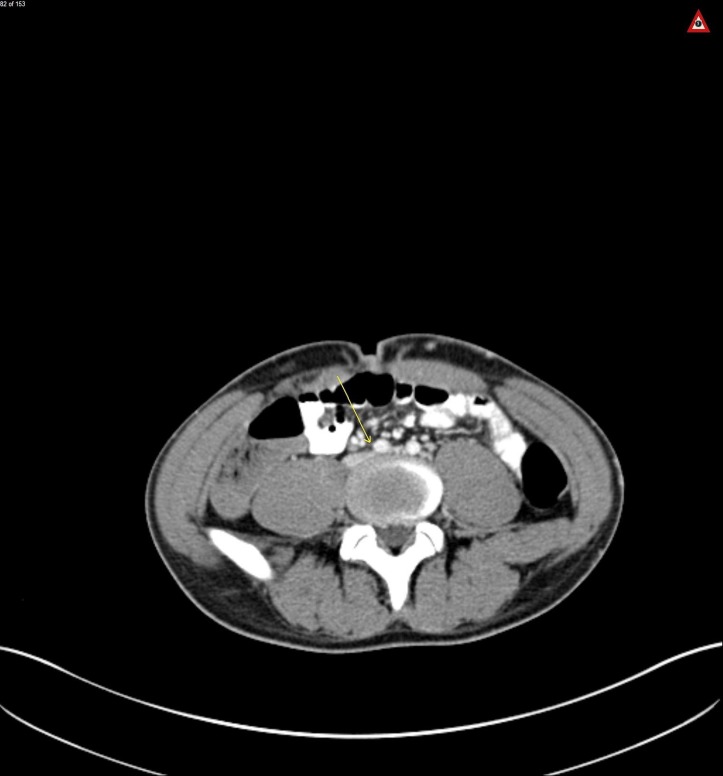
Figure 4.
Axial view CT abdomen/pelvis, arrow on compression of left common iliac vein by right common iliac artery, also known as May-Thurner.
Thrombophilia workup done as an outpatient was negative for anti- nuclear antibody (ANA), anticardiolipin antibodies including beta-2 GP1 Ab (glycoprotein 1Ab), protein C deficiency (both qualitative and functional activity), protein S deficiency, factor V Leiden mutation as well as antithrombin III deficiency.
Whereas routine thrombophilia workup is not recommended in unselected patients with a diagnosis of venous thromboembolism (VTE), consensus guidelines recommend thrombophilia work up only in one or more selected clinical scenarios like recurrent thromboembolism, VTE at a young age <45 years (as in our case), strong family history of thromboembolism, unusual site thrombosis and/or patients with arterial thrombosis.1–3
In our patient, with no risk factors of inherited thrombophilia, correction of the anatomical abnormality through endovascular approach can prevent future episodes of recurrent thrombosis and VTE. Termination of the anticoagulation can be contemplated in such a scenario, which can be of significant benefit to the patient, considering his very young age and the morbidity of long term anticoagulation.
Investigations
Duplex ultrasound of the left lower extremity venous system, CT abdomen with contrast, CT pulmonary angiography, echocardiography and 12-lead ECG.
Differential diagnosis
Young African-American man presents with an unprovoked DVT and extensive bilateral PE with a history of smoking and family history of a clot; inherited thrombophilia was the top differential. Any occult malignancy also needed to be excluded. An underlying chronic systemic inflammation required to be ruled out as the pro-inflammatory markers were elevated. CT was done also to evaluate clot burden, which revealed the classic MTS.
Treatment
The patient was initially treated with intravenous heparin for the acute PE and the DVT. He was subsequently discharged with rivaroxaban. Endovascular stenting may be contemplated to prevent recurrence of VTE or its long-term sequelae, for example, post-thrombotic syndrome or chronic venous insufficiency.
Outcome and follow-up
The patient is stable and currently following up with the department of internal medicine as well as pulmonary and critical care medicine.
Discussion
We report an interesting case of MTS presenting as bilateral PE and left-sided DVT. Although cases of DVT have been frequently associated with this syndrome in the past, only a few cases have presented with acute bilateral pulmonary emboli.4–6
This vascular variant should be considered with high suspicion in left lower extremity DVT in young patients with no other aetiology to justify thrombosis. Prolonged anticoagulation, thrombectomy or stent placement for the relief of mechanical obstruction have been used in various clinical settings.6
First described in 1957 in cadavers during autopsy,7 MTS is an anatomical variant where there is an overriding right common iliac artery, compressing the left common iliac vein against the spine. There is intimal hyperplasia causing potential for venous stasis and resultant thrombosis. The anatomical variation is not infrequent among asymptomatic individuals.8 Autopsy studies7 indicate a probable incidence rate of the MTS to be 22%–32%, but the incidence of MTS related lower limb DVT is only 2%–3%.4 Left-sided DVT is five times more prevalent in the population compared with the right side.9 This seems to validate the fact that an associated MTS is likely an underdiagnosed clinical entity and therefore should be considered in recurrent or unprovoked DVT. Although rare, small retrospective studies have shown a possible association of MTS with pregnancy and obesity, probably due to alteration in the anatomic alignment of blood vessels due to increased soft tissue deposition.5 10 11 Doppler ultrasound can detect characteristics of proximal obstruction even if extremity venous study may be normal, for example, a loss of collapsibility of common femoral vein, lack of respiratory variations and an absence of response to Valsalva manoeuvre.5
The presence of known risk factors possibly masks the diagnosis of this anatomical entity as investigations are likely to be halted if risk factors for thrombogenesis or thrombophilia are present.10–12 It is important to know that only medical management and failure to correct the anatomical aspect may lead to the development of recurrent VTE, chronic venous stasis, and in some instances, even iliac vein rupture.13 14
Testing for thrombophilia can alter the duration of the anticoagulation treatment (recommendation level C) and is therefore important in selected cases of VTE.1–3 Further, documentation of biological risk factors is necessary in order to decide future genetic testing and counselling of first degree relatives, management of conditions that increase the risk of the future thrombotic or thromboembolic events (eg, need for aggressive thromboprophylaxis in the setting of major surgery when there is thrombophilia, avoidance of hormonal contraception in women of childbearing age or thromboprophylaxis of pregnant patients with documented antithrombin deficiency). This approach is consistent with guidelines published by the British Committee for Standards in Haematology and the International Consensus statement on the prevention and treatment of VTE.1–3 A diagnosis of MTS should not preclude a thrombophilia workup in a majority of cases.12 It is worth mentioning in this context that our patient had no risk factors for thrombophilia and his coagulation workup was negative.
Vascular intervention in MTS may require MR/CT venography, or intravascular ultrasound to assess the degree of the stenosis and the aftermath of iliac vein compression.15–18 Rivaroxaban (trade name Xarelto) has been United States Food and Drug Administration (US FDA) approved for the treatment of DVT or PE17 and was prescribed in this case. However, long-term anticoagulation alone may not always be sufficient for prevention of future sequelae.12 A combination strategy for medical management along with surgical and endovascular intervention has been preferred over corrective surgical repair of the damaged vein alone.18 Surgical thrombectomy followed by endovascular reconstruction of the iliofemoral venous system was shown to achieve good long-term iliofemoral patency rates in one study (primary and secondary patency rates; 74% [14/19] and 84% [16/19], respectively).18 Catheter-directed thrombolysis with percutaneous mechanical thrombectomy12 has been suggested. Placement of a retrievable IVC filter has been suggested before thrombolysis in individuals with large clot burden, to prevent further embolisation during the intervention.12 Continuing thrombolytic infusion for an additional 24–48 hours has also been suggested for the prevention of clot embolism.12 16 One case series mentions the use of catheter guided thrombolysis with tissue plasminogen activator, mechanical thrombectomy, IVC filter placement, the use of intravascular stent deployment with or without balloon venoplasty.5 Endovascular therapy is directed to restore inline patency to the left deep venous system using catheter-directed thrombolytic treatment and treatment of the compression with a stent or angioplasty alone. Stent placement results have been shown to be excellent in one study (a primary patency rate of 95% with cumulative patency of 90%– 100% over 1 year).12 In one study, a 2-year follow-up evaluation has suggested primary and secondary post-venous stenting patency rates like 78% and 95%, respectively.19 Poststent placement, anticoagulation for at least 6 months has been suggested.9 Retrospective studies indicate that patients who have concurrent pre-existing thrombophilia may have a higher probability of stent thrombosis and therefore should be managed with long-term anticoagulation.9 19
Learning points.
To have a low threshold for suspicion of May-Thurner syndrome (MTS) when there is unilateral left leg deep vein thrombosis (DVT) in the young population.
To have a suspicion of MTS as the potential cause of recurrent unprovoked DVT/pulmonary embolism in an otherwise unsuspecting young patient with no clear risk factors for thrombogenesis.
Management options to be sorted out, depending on what suits the patient the most; this may vary on a case-to-case basis. Iliofemoral venous stenting have shown excellent success rates.
Acknowledgments
Dr Roli Iwere participated in patient care.
Footnotes
Contributors: RB was involved in planning, conduct, reporting, conception and design, acquisition of data and interpretation of data. He was also directly involved in patient care. MS was the primary attending under whose care the patient was admitted. He was involved in overall foreseeing the data and recommendations as needed. DVI was the pulmonary and critical care consultant involving in caring for the patient. Overall the literature review and managing the final texture of the article can be attributed to his credit. KR has been involved in the overall review, incorporating valuable input as regards patient care experience and final drafting the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis 2016;41:154–64. 10.1007/s11239-015-1316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010;149:209–20. 10.1111/j.1365-2141.2009.08022.x [DOI] [PubMed] [Google Scholar]
- 3. Cardiovascular Disease Educational and Research Trust Cyprus Cardiovascular Disease Educational and Research Trust European Venous Forum International Surgical Thrombosis Forum International Union of Angiology Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006;25:101. [PubMed] [Google Scholar]
- 4. Kalu S, Shah P, Natarajan A, et al. May-thurner syndrome: a case report and review of the literature. Case Rep Vasc Med 2013;2013:1–5. 10.1155/2013/740182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sedhai YR, Golamari R, Salei A, et al. May-Thurner Syndrome. Am J Med Sci 2018;355:510–4. 10.1016/j.amjms.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 6. Fasanya AA, LaCapra G. May-thurner syndrome with pulmonary embolism as the first presentation rather than deep vein thrombosis. Cureus 2016;8:e509 10.7759/cureus.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419–27. 10.1177/000331975700800505 [DOI] [PubMed] [Google Scholar]
- 8. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg 2004;39:937–43. 10.1016/j.jvs.2003.12.032 [DOI] [PubMed] [Google Scholar]
- 9. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 10. O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2000;11:823–36. 10.1016/S1051-0443(07)61796-5 [DOI] [PubMed] [Google Scholar]
- 11. Thijs W, Rabe KF, Rosendaal FR, et al. Predominance of left-sided deep vein thrombosis and body weight. J Thromb Haemost 2010;8:2083–4. 10.1111/j.1538-7836.2010.03967.x [DOI] [PubMed] [Google Scholar]
- 12. Moudgill N, Hager E, Gonsalves C, et al. May-Thurner syndrome: case report and review of the literature involving modern endovascular therapy. Vascular 2009;17:330–5. 10.2310/6670.2009.00027 [DOI] [PubMed] [Google Scholar]
- 13. Kim JY, Choi D, Guk Ko Y, et al. Percutaneous treatment of deep vein thrombosis in May-Thurner syndrome. Cardiovasc Intervent Radiol 2006;29:571–5. 10.1007/s00270-004-0165-7 [DOI] [PubMed] [Google Scholar]
- 14. Jiang J, Ding X, Zhang G, et al. Spontaneous retroperitoneal hematoma associated with iliac vein rupture. J Vasc Surg 2010;52:1278–82. 10.1016/j.jvs.2010.06.102 [DOI] [PubMed] [Google Scholar]
- 15. Kölbel T, Lindh M, Akesson M, et al. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther 2009;16:483–91. 10.1583/09-2719.1 [DOI] [PubMed] [Google Scholar]
- 16. Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010;22:223–30. 10.1177/1531003511400426 [DOI] [PubMed] [Google Scholar]
- 17. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 18. Hölper P, Kotelis D, Attigah N, et al. Longterm results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg 2010;39:349–55. 10.1016/j.ejvs.2009.09.028 [DOI] [PubMed] [Google Scholar]
- 19. Titus JM, Moise MA, Bena J, et al. Iliofemoral stenting for venous occlusive disease. J Vasc Surg 2011;53:706–12. 10.1016/j.jvs.2010.09.011 [DOI] [PubMed] [Google Scholar]