Abstract
Headache is a common presenting complaint in the paediatric population, with often migraine being a clinical diagnosis. Hemiplegic migraine is characterised by aura, sudden onset weakness of one side of the body which usually recovers without any residual neurological deficit. We report a child with a history of seizure disorder, well controlled and off medication for 3 years, who presented with a headache, aura and transient hemiplegia. Similar history in the patient’s mother suggests the diagnosis of familial hemiplegic migraine. We would like to emphasise the importance of detailed history as an important aid in the diagnosis of neurological disorders in children.
Keywords: paediatrics (drugs and medicines)
Background
Headache is a common presenting symptom in a paediatric outpatient clinic with a variety of differentials, ranging from benign to sinister conditions. With no specific diagnostic modality available, the clinical history and examination remain critical to the diagnosis. Migraine is a common differential diagnosis of adolescents with a headache. Hemiplegic migraines are rare, can be familial or sporadic and present with a wide variety of motor, visual and speech auras in association with headache.1 We report this case to emphasise the importance of clinical history and examination in diagnosing aetiology of paediatric neurological disorders, especially of paediatric headache.
Case presentation
A 13-year-old boy, who stayed in a boarding school, presented with episodic frontotemporal headache, blurred vision and vomiting associated with weakness of the left upper limb and lower limb and aphasia. The weakness lasted for 7 hours, and no residual neurological deficit was identified after that. The patient narrated the history and he had no history of loss of consciousness. School performance was reported as normal and he had no stressors. On examination, he is well nourished, normotensive and had no neurocutaneous skin markers. Central nervous system (CNS) examination during the episode revealed decreased power and tone of the left upper limb and lower limb, with intact sensations and no other cranial nerve deficits elicited. He was also found to have a history of head trauma at the age of 2 years. He presented with afebrile seizures at the age of 5 years and was started on antiepileptic drugs, which were stopped 3 years back as he was seizure free. The patient’s mother reported having had similar complaints, characterised by episodic headache associated with transient left upper limb and lower limb weakness, during her adolescent period; however, she was asymptomatic at the time of her sons presentation.
Investigations
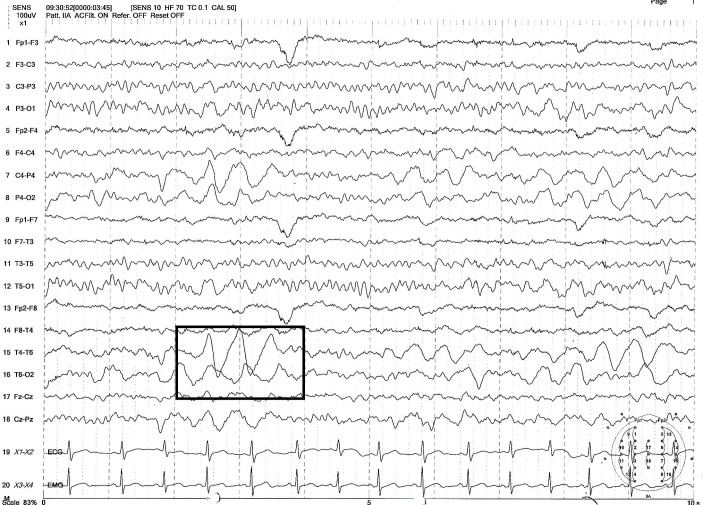
He was evaluated for the cause of the headache. He had normal blood counts, electrolytes and liver enzymes. Sleep deprived electroencephalogram (EEG) done showed moderate to severe degree of focal disturbance of electrical function over the right hemisphere and maximum over the right posterior head region, with no epileptiform abnormalities (figure 1). MRI of the brain was normal. An expert clinical psychology evaluation is done; however, no stressors were identified.
Figure 1.
Electroencephalogram showing slow delta activity over the right hemisphere and maximum over the right posterior head region, no epileptiform abnormalities.
Differential diagnosis
Transient ischaemic attack, generalised seizures with Todd’s palsy can present with a severe headache associated with brief neurological weakness. Functional disorders mimicking neurological disorders are rare but can present with neurological symptoms and signs, with no underlying identifiable CNS lesion.
Treatment
The acute episode subsided with rest and analgesics, with no residual weakness. He was started on 5 mg of flunarizine once daily dose for migraine prophylaxis. He was counselled to avoid factors that can pose a risk for further attack. The family was counselled about the possibility of recurrence and the need for regular medication. As he had daytime drowsiness even after dose adjustment, he was started on propranolol prophylaxis instead of flunarizine
Outcome and follow-up
Treatment with flunarizine improved his episodes in a follow-up period of 3 months. He had no further episodes of headache nor neurological weakness. At follow-up after 9 months of treatment, he reported improvement in symptoms and improvement in school performance.
Discussion
Familial hemiplegic migraine (FHM) is characterised by migraine with aura, associated with transient neurological signs and symptoms.2 3 The clinical spectrum of hemiplegic migraines ranges from attacks with temporary hemiparesis to severe forms with recurrent coma, prolonged hemiparesis, epilepsy, transient blindness or decreased mentation. FHM can further be divided into a pure hemiplegic migraine, affecting 80% of the families and those with permanent cerebellar signs, affecting 20% of the families.4 The motor aura manifests as unilateral weakness. Additional aura symptoms were visual, sensory and aphasic. A population-based study found FHM patients having all four symptoms coexisting and at least two aura symptoms during each attack. A hemiplegic migraine can be sporadic with no family history of similar symptoms.5
An epidemiological study of hemiplegic migraines in the entire Danish population showed an incidence of 0.01%, with familial and sporadic forms being equally present.1 Familial hemiplegic migraine is observed to be of autosomal dominant inheritance. Genetic studies show evidence of mutations in protein-encoding genes involved in ion transportation. However, up to 25% of families and almost all sporadic cases do not have the mutation.6 The genetic variants of familial hemiplegic migraine are associated with different monogenic mutations ranging across different chromosomes: FHM1 (CACNA1A), FHM2 (ATP1A2) and FHM3 (SCN1A) vary in clinical presentation and phenotype.3 7
A careful elicitation of history considering the International Classification of Headache Disorders, 3rd edition (ICHD-3) diagnostic criteria is critical in diagnosing hemiplegic migraine (1.2.3.1: Familial Hemiplegic Migraine).8 In our case, the child had two attacks following the ICHD-3 criteria, with fully reversible motor weakness in the left upper limb and bilateral lower limbs along with aphasia and unilateral visual disturbance. The paralysis lasted for 7 hours, and visual disturbance was unilateral. Aura was accompanied by the headache within 10 min of onset.
Neuroimaging is not ideal as a diagnostic modality in patients with FHM. Many authors reported electroencephalogram changes; however, normalisation of electrical activity was observed at a period of 3–7 days postictus.9–11 Certain reports have shown noticeable changes in MRI brain. In a few cases reported, an MRI showed normal diffusion tensor imaging, but perfusion-weighted imaging showed an area of hypoperfusion and the susceptibility weighted imaging showed areas of prominent hypointense draining sulcal veins.12 13 It is imperative to understand that these imaging modalities are not feasible in the paediatric population. Additionally, there are not adequate correlations between the symptoms and imaging. However, imaging can be used to rule out other neurological lesions. Some small studies have shown that susceptibility weighted imaging has greater diagnostic value in an acute setting of paresis when combined with MRI to characterise the ictal cerebral blood flow changes, but the prognostic significance of the same has not been established.12
Learning points.
Clinical history and examination are essential in the diagnosis of paediatric neurological disorders. like familial hemiplegic migraine, especially in children with co-existing seizure disorder.
Children with headache disorders need careful history about nature of symptom and associated complaints to identify rare syndromes like hemiplegic migraine.
Training of medical students and early career doctors should emphasise the importance of history taking and physical examination skills.
Acknowledgments
The authors would like to thank the patient and family for their co-operation during the process of manuscript preparation.
Footnotes
Contributors: PB and PKK have been involved in the preparation of the manuscript. APH has been active in giving her expert inputs for manuscript editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Parental/guardian consent obtained.
References
- 1. Gupta SN, Gupta VS, Borad N. Spectrum of migraine variants and beyond: the individual syndromes in children. Brain Dev 2016;38:10–26. 10.1016/j.braindev.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 2. Pelzer N, Stam AH, Haan J, et al. . Familial and sporadic hemiplegic migraine: diagnosis and treatment. Curr Treat Options Neurol 2013;15:13–27. 10.1007/s11940-012-0208-3 [DOI] [PubMed] [Google Scholar]
- 3. Black DF. Sporadic and familial hemiplegic migraine: diagnosis and treatment. Semin Neurol 2006;26:208–16. 10.1055/s-2006-939921 [DOI] [PubMed] [Google Scholar]
- 4. Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol 2011;10:457–70. 10.1016/S1474-4422(11)70048-5 [DOI] [PubMed] [Google Scholar]
- 5. Black DF. Sporadic hemiplegic migraine. Curr Pain Headache Rep 2004;8:223–8. 10.1007/s11916-004-0056-2 [DOI] [PubMed] [Google Scholar]
- 6. Lykke Thomsen L, Kirchmann Eriksen M, Faerch Romer S, et al. . An epidemiological survey of hemiplegic migraine. Cephalalgia 2002;22:361–75. 10.1046/j.1468-2982.2002.00371.x [DOI] [PubMed] [Google Scholar]
- 7. Hiekkala ME, Vuola P, Artto V, et al. . The contribution of CACNA1A, ATP1A2 and SCN1A mutations in hemiplegic migraine: A clinical and genetic study in Finnish migraine families. Cephalalgia 2018;38:1849–63. 10.1177/0333102418761041 [DOI] [PubMed] [Google Scholar]
- 8. Ashina S, Olesen J, Lipton RB. How Well Does the ICHD 3 (Beta) Help in Real-Life Migraine Diagnosis and Management? Curr Pain Headache Rep 2016;20:66 10.1007/s11916-016-0599-z [DOI] [PubMed] [Google Scholar]
- 9. Degen R, Degen HE, Palm D, et al. . [The EEG during the hemiplegic migraine attack of children]. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb 1980;11:128–34. [PubMed] [Google Scholar]
- 10. Kashiwagi M, Tanabe T, Hara K, et al. . {[Changes} of electroencephalographic findings in a case of migraine with various neurological symptoms]. Hattatsu Brain Dev 2006;38:214–8. [PubMed] [Google Scholar]
- 11. Murphy OC, Merwick A, OʼMahony O, et al. . Familial hemiplegic migraine with asymmetric encephalopathy secondary to ATP1A2 Mutation: a case series. J Clin Neurophysiol 2018;35:e3–e7. 10.1097/WNP.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 12. Altinok D, Agarwal A, Ascadi G, et al. . Pediatric hemiplegic migraine: susceptibility weighted and MR perfusion imaging abnormality. Pediatr Radiol 2010;40:1958–61. 10.1007/s00247-010-1826-0 [DOI] [PubMed] [Google Scholar]
- 13. Bosemani T, Burton VJ, Felling RJ, et al. . Pediatric hemiplegic migraine: role of multiple MRI techniques in evaluation of reversible hypoperfusion. Cephalalgia 2014;34:311–5. 10.1177/0333102413509432 [DOI] [PubMed] [Google Scholar]