Abstract
Immunoglobulin A nephropathy (IgAN) is the most commonly diagnosed glomerulonephritis worldwide. It is usually idiopathic and may be associated with many other diseases. Recently, biological agents including tumour necrosis factor alpha (TNFα) inhibitors have been identified as a potential cause for IgAN. We report the case of a 39-year-old woman who presented with renal dysfunction and visible haematuria. She had a background of Crohn’s disease (CD) and had been on adalimumab for 4 years following a right hemicolectomy. Subsequently, she underwent a renal biopsy that demonstrated IgAN and adalimumab was ceased. Following a flare in her CD, she was commenced on infliximab, which led to remission of the IgAN and CD. This is the first case to demonstrate the occurrence of IgAN as a complication of a TNFα inhibitor (adalimumab) that remained in remission despite the commencement of a second TNFα inhibitor (infliximab).
Keywords: drug interactions, drugs: gastrointestinal system, inflammatory bowel disease, crohn’s disease, unwanted effects/adverse reactions
Background
Immunoglobulin A nephropathy (IgAN) (also known as Berger’s disease) is a mesangial proliferative glomerulonephritis (GN) characterised by diffuse deposits of IgA within the kidney mesangium.1 It is the most common form of GN globally and has a higher prevalence among younger males and patients with East Asian or Caucasian ethnicity.2 3 While IgAN is an idiopathic condition, its onset has been associated with a number of inflammatory diseases including liver cirrhosis, rheumatoid arthritis, ankylosing spondylitis, HIV, hepatitis B, celiac disease, ulcerative colitis and rarely Crohn’s disease (CD).4 5
The development of IgAN is thought to be due to individual genetic factors combined with a precipitating inflammatory event, usually an upper respiratory tract infection, resulting in IgG binding to abnormally glycosylated IgA and forming deposits of immune complexes in the mesangium of the kidney.5
Adalimumab (a fully humanised) and infliximab (chimeric) are monoclonal antitumour necrosis factor alpha (TNFα) antibodies that are approved for the management of several chronic inflammatory diseases including CD.6 These agents are widely used and their effectiveness has been demonstrated for both the induction and maintenance of remission in CD by multiple clinical trials.7 These biological agents are usually reserved for patients with complex, fistulising or refractory disease who have failed standard therapy with 5-aminosalicylate, corticosteroids and non-biological immunosuppressants.6
TNFα inhibitors are potent immunomodulators and have been associated with the development of autoimmunity. Both these agents have been reported to cause IgAN among patients with CD and other autoimmune conditions. However, infliximab has also been documented to successfully treat a patient with IgAN secondary to CD.8–10 We describe the first case of IgAN as a complication of a TNFα inhibitor (adalimumab) that remained in remission despite the commencement of a second TNFα inhibitor (infliximab).
Case presentation
A 39-year-old woman was referred to the renal clinic with a decline in her renal function detected on routine follow-up. She has a history of CD, which was diagnosed 7 years ago. Two years after this diagnosis, she developed an acute large bowel obstruction secondary to CD and a right hemicolectomy was performed. She was then started on azathioprine. However, a year after this surgery, the patient had a recurrence at the anastomotic site. Adalimumab was commenced in addition to her azathioprine, and this combined therapy led to a sustained remission of her CD.
On review in the clinic, the patient reported a 1-month history of increasing lethargy and malaise. She denied a history of a recent upper respiratory tract infection, diarrhoea or abdominal pain, and had no extraintestinal manifestations of her CD.
There was no additional past medical nor a personal or a family history of kidney disease. She had undergone tonsillectomy during childhood and was adherent to her medications, which were adalimumab and azathioprine. She denied the use of complementary or over the counter medications, was a lifelong non-smoker and did not regularly consume alcohol.
Clinical examination revealed a blood pressure of 130/74 mm Hg, heart rate of 74 beats per minute and stable vital signs. She was euvolaemic with no added breath sounds and nil pitting pedal oedema. There was no vasculitic rash. Abdominal examination was unremarkable.
Investigations
Investigations following the renal clinic appointment revealed an elevated serum creatinine of 151 µmol/L (reference range 45–90 µmol/L) and an estimated glomerular filtration rate (eGFR) of 37 mL/min/1.73 m2 (reference range>90 mL/min/1.73 m2).
Urine microscopy showed haematuria with 300×106/L erythrocytes (reference range <10×106/L) and 30×106/L leucocytes (reference range<10×106/L). Her urine protein:creatinine ratio was elevated at 63 mg/mmol creatinine (reference range<15 mg/mmol creatinine). Urinary casts or eosinophils were not present. The renal function prior to commencing adalimumab had been normal with an eGFR of >90 mL/min/1.73 m2, serum creatinine of 55 µmol/L and unremarkable urine microscopy. After commencement of adalimumab, her renal function started to decline and at the time of referral to the renal team, her eGFR was 55 mL/min/1.73 m2.
Further investigations included a full blood count, complement levels (C3 and C4), C-reactive protein, antinuclear antibodies, hepatitis B, C and HIV serology, which were all normal. The antistreptolysin O titre was normal (135; reference range<200); however, the anti-DNase B titre was mildly elevated (410; reference range<200). An ultrasound of the kidneys revealed normal size and echotexture. There was no evidence of renal artery stenosis, hydronephrosis or renal calculi.
A renal biopsy demonstrated seven glomeruli with one showing global sclerosis. There was no tubulitis or tubular atrophy and scant red cells were seen in the tubules (figure 1). Moderate chronic inflammatory cell infiltrates were seen within the interstitium. There was no interstitial fibrosis or vascular changes. Immunofluorescence demonstrated 3+ staining for both IgA and C3 within the mesangium as well as trace staining for mesangial IgM (figure 2). Electron microscopy revealed the presence of moderate volume mesangial and electron dense deposits (figure 3).
Figure 1.
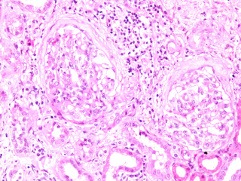
Light microscopy showing two glomeruli with periglomerular fibrosis (H&E ×20).
Figure 2.
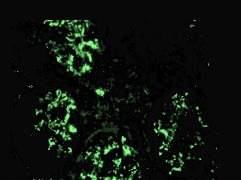
Immunofluorescence showing positive staining for immunoglobulin A stained with periodic acid-schiff (PAS) methenamine silver (original magnification ×20).
Figure 3.
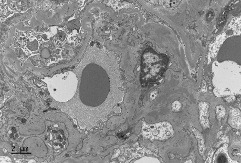
Electron microscopy of the glomerulus reveals the presence of moderate volume mesangial and electron dense deposit. (Stain: uranyl acetate and lead citrate).
Differential diagnosis
We postulated three possible aetiologies for the development of IgAN in this patient.
IgAN has been recognised as a rare complication of CD. However, this usually occurs among patients with poorly controlled CD.11 12 Thus, this was felt to be less likely in our patient, who had stable disease since commencing adalimumab.
De novo development of IgAN. The absence of preceding upper respiratory tract symptoms and serological proof of infection suggest that this was a less likely cause for her presentation.
Lastly, IgAN has been reported in the literature to be a rare side effect of adalimumab, which can occur years after the commencement of therapy.13 As the decrease in eGFR in this patient was relatively subacute, a provisional diagnosis of adalimumab-associated IgAN was made.
Treatment
Adalimumab was discontinued after the renal biopsy and she was commenced on 300 mg of irbesartan. The eGFR and creatinine remained stable post cessation of adalimumab. The protein:creatinine ratio started to decrease once the adalimumab was ceased (figure 4). The proteinuria improved slightly on commencement of irbesartan, although it did not decrease significantly. Unfortunately, a month after discontinuing adalimumab, she presented with worsening diarrhoea, which was diagnosed as a relapse of her CD. This was monitored and 3 months after discontinuation of adalimumab, in consultation with her treating gastroenterologist, an 8 weekly infliximab course was started to manage her relapse. With the commencement of infliximab, the patient’s proteinuria continued to drop and renal function improved.
Figure 4.
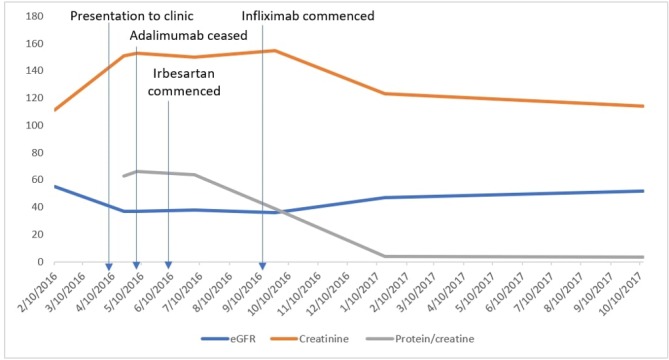
The patient’s trend in renal function on cessation of adalimumab and commencement of irbesartan and infliximab. eGFR, estimated glomerular filtration rate.
Outcome and follow-up
At the first month review following the commencement of infliximab, the patient’s diarrhoea had improved. The renal function remained stable with a creatinine of 153 µmol/L, eGFR of 38 mL/min/1.73 m2 and a protein:creatinine ratio of 64 mg/mmol. At the 4 month review, her CD was well controlled with a serum creatinine 155 µmol/L and eGFR 36 mL/min/1.73 m2. The protein:creatinine ratio had decreased to 32 mg/mmol creatinine (reference range<15 mg/mmol creatinine), although microhaematuria persisted. In view of this improvement, the decision was taken to continue infliximab.
Over a year after her initial diagnosis of IgAN, her CD was quiescent and her renal function recovered significantly; eGFR of 52 mL/min/1.73 m2, creatinine of 114 µmol/L and urine protein:creatinine ratio of 5 mg/mmol creatinine (reference range<15 g/mol creatinine; no active sediments in urine). The cessation of adalimumab and the addition of irbesartan prevented the patient’s renal function from declining further. Despite being commenced on infliximab, her IgAN remained in remission (figure 4). The patient continues to be followed up on a 6 monthly basis in the renal outpatient clinic.
Discussion
We report the first case of adalimumab-induced IgAN that resolved following cessation of adalimumab and remained in remission despite the initiation of another TNFα inhibitor (infliximab). Adalimumab-induced IgAN has been described in a patient with psoriasis and CD.8 13 Of note, in all previous case reports, except in one patient who developed crescentic GN, an improvement in renal function was observed once adalimumab was ceased and alternate immunosuppressive therapy was initiated.13 In our case, adalimumab-induced IgAN was thought to be the most likely cause as the patient’s protein to creatinine ratio and her renal function improved significantly on cessation of adalimumab. While it is possible that our patient could have developed primary IgAN, this is unlikely as primary IgAN typically has progressive renal dysfunction and rarely remits over time in contrast to the significant renal recovery seen in our patient.
CD is an inflammatory disorder that involves the entire gastrointestinal tract and is associated with numerous extraintestinal complications that typically manifest in clinically active intestinal disease.14 Despite strong association between IgAN and CD, the underlying mechanism contributing to this link has yet to be determined. Multiple case reports have been published documenting IgAN as a complication of CD and, in each of these reports, IgAN only occurred in the context of active bowel disease and is, therefore, unlikely to be the cause in our patient.12 15
Infliximab has previously been reported to both cause IgAN, as well as induce remission in patients with IgAN secondary to autoimmune conditions.9 10 Why one TNFα inhibitor caused IgAN, and another did not is unclear. Adalimumab is a fully humanised monoclonal antibody whereas infliximab is a chimeric monoclonal antibody that is made up of human as well as murine sequences.16 It may be that the difference in the monoclonal structures resulted in autoantibody formation only against adalimumab. Alternatively, it could also represent an idiosyncratic drug reaction.
Unlike other glomerular diseases, IgAN is defined by the presence of an immune reactant rather than specific morphological features, with the pathognomonic finding of mesangial IgA deposits on immunofluorescence.5 IgAN has also been associated with a range of inflammatory diseases. It is likely that IgAN is not a single entity but a common immune response to various inflammatory mechanisms.5 With their immunomodulatory effect, anti-TNFα blockers may modulate this response and, both induce and aid in the treatment of IgAN as seen in this patient.
The mainstay of treatment in primary IgAN is optimised supportive care with renin-angiotensin aldosterone blockade and blood pressure control.17 The role of immunosuppressive therapy in primary IgAN is controversial with recent clinical trials showing limited benefit and significant harm associated with steroid therapy.18 This is in contrast to case reports of adalimumab-induced IgAN where renal recovery was observed following withdrawal of adalimumab and commencement of an alternate immunosuppressive regimen.8 13 15 This finding would suggest that patients with adalimumab-induced IgAN may be a distinct subset of IgAN and these patients may have a markedly different response to immunosuppression compared with idiopathic IgAN.11 12
We describe the first known patient with ongoing remission of TNFα inhibitor (adalimumab)-induced IgAN despite the commencement of a second TNFα inhibitor (infliximab). This case demonstrates that while TNFα inhibitors can induce IgAN, this is not always a class effect and the use of an alternate TNFα inhibitor can be considered in patients with TNFα inhibitor-induced IgAN and refractory autoimmune diseases with close surveillance.
Learning points.
Biological agents, such as adalimumab, can induce immunoglobulin A nephropathy (IgAN) due to their potent immunomodulatory effects and this usually resolves following cessation of adalimumab.
Kidney biopsy is essential in diagnosing IgAN and in predicting the prognosis of the disease.
Commencement of an alternative TNFα inhibitor for refractory Crohn’s disease following TNFα-induced IgAN can be considered with close monitoring.
Acknowledgments
The authors would like to thank Dr Ramey Bajwa, RB (Renal Registrar, RBWH, Queensland), for his contribution in providing assistance to edit the case report, and Dr Anne Stewart (Pathologist, RBWH, Queensland) for providing the pathology images, figures 1 to 3.
Footnotes
Contributors: AKBS and ASJ contributed equally to this paper by acquiring results and writing this case report. GJW contributed by discussing the template and editing this paper. DR assisted in providing the idea for the case report, doing final edits and overlooking the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Galla JH. IgA nephropathy. Kidney Int 1995;47:377–87. 10.1038/ki.1995.50 [DOI] [PubMed] [Google Scholar]
- 2. Simon P, Ramee MP, Boulahrouz R, et al. . Epidemiologic data of primary glomerular diseases in western France. Kidney Int 2004;66:905–8. 10.1111/j.1523-1755.2004.00834.x [DOI] [PubMed] [Google Scholar]
- 3. Jegatheesan D, Nath K, Reyaldeen R, et al. . Epidemiology of biopsy-proven glomerulonephritis in Queensland adults. Nephrology 2016;21:28–34. 10.1111/nep.12559 [DOI] [PubMed] [Google Scholar]
- 4. Terasaka T, Uchida HA, Umebayashi R, et al. . The possible involvement of intestine-derived IgA1: a case of IgA nephropathy associated with Crohn’s disease. BMC Nephrol 2016;17:122 10.1186/s12882-016-0344-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med 2013;368:2402–14. 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 6. (GESA) GSoA. Australian Guideline for General practitioners and physicians inflammatory Bowel Disease (IBD). Melbourne: Digestive Health Foundation, 2013. [Google Scholar]
- 7. Lichtenstein GR, Panaccione R, Mallarkey G. Efficacy and safety of adalimumab in crohn’s disease. Therap Adv Gastroenterol 2008;1:43–50. 10.1177/1756283X08092548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruzzese V, Lorenzetti R, Rosa M, et al. . IgA nephropathy onset in a Crohn’s disease patient treated with Adalimumab. Minerva Gastroenterol Dietol 2016;62:223–4. [PubMed] [Google Scholar]
- 9. Kluger N, Du-Thanh A, Bessis D, et al. . Psoriasis-associated IgA nephropathy under infliximab therapy. Int J Dermatol 2015;54:e79–e80. 10.1111/ijd.12622 [DOI] [PubMed] [Google Scholar]
- 10. Ueno Y, Tanaka S, Onitake T, et al. . Infliximab treatment for crohn’s disease in a patient with IgA nephropathy. Clin J Gastroenterol 2009;2:380–3. 10.1007/s12328-009-0112-x [DOI] [PubMed] [Google Scholar]
- 11. Choi JY, Yu CH, Jung HY, et al. . A case of rapidly progressive IgA nephropathy in a patient with exacerbation of Crohn’s disease. BMC Nephrol 2012;13:84 10.1186/1471-2369-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filiopoulos V, Trompouki S, Hadjiyannakos D, et al. . IgA nephropathy in association with Crohn’s disease: a case report and brief review of the literature. Ren Fail 2010;32:523–7. 10.3109/08860221003710554 [DOI] [PubMed] [Google Scholar]
- 13. Wei SS, Sinniah R. Adalimumab (TNF α Inhibitor) therapy exacerbates iga glomerulonephritis acute renal injury and induces lupus autoantibodies in a psoriasis patient. Case Rep Nephrol 2013;2013:812781 10.1155/2013/812781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol 2006;12:4819–31. 10.3748/wjg.v12.i30.4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forshaw MJ, Guirguis O, Hennigan TW. IgA nephropathy in association with Crohn’s disease. Int J Colorectal Dis 2005;20:463–5. 10.1007/s00384-004-0696-z [DOI] [PubMed] [Google Scholar]
- 16. Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. . Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011;305:1460–8. 10.1001/jama.2011.406 [DOI] [PubMed] [Google Scholar]
- 17. KDIGO. KDIGO clinical practice guidelines for Glomerulonephritis, 2012. [Google Scholar]
- 18. Schena FP, Manno C. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2016;374:992 10.1056/NEJMc1600141 [DOI] [PubMed] [Google Scholar]


