ABSTRACT
Zika virus (ZIKV) clinical presentation and frequency/duration of shedding need further clarification. Symptomatic ZIKV-infected individuals identified in two hospitals in Sao Paulo State, Brazil, were investigated regarding clinical characteristics, shedding in body fluids, and serodynamics. Ninety-four of 235 symptomatic patients (Site A: 58%; Site B: 16%) had Real-Time PCR-confirmed ZIKV infection; fever, headache and gastrointestinal symptoms were less frequent, and rash was more frequent compared to ZIKV-negative patients. Real-Time PCR in serum had worse performance compared to plasma, while urine had the highest sensitivity. Shedding in genital fluids and saliva was rare. IgM positivity was the highest <14 days after the symptoms onset (86%), decreasing >28 days (24%); IgG positivity increased >14 days (96%) remaining positive in 94% of patients >28 days. ZIKV prevalence varied importantly in two neighboring cities during the same transmission season. Urine Real-Time PCR can improve diagnostic sensitivity; serum testing is less useful. Accurate serological tests are needed to improve diagnosis and surveillance.
KEYWORDS: Zika virus, Differential diagnosis, Kinetics, Body fluids, Polymerase chain reaction, Real-Time Polymerase Chain Reaction, Serology, Brazil
INTRODUCTION
In 2016, the World Health Organization (WHO) declared Zika virus (ZIKV) infection-related microcephaly and other neurological disorders a Public Health Emergency of international concern1. Since early 2015, the geographic spread of ZIKV has been reported to increase in Brazil, with the potential for broader dissemination due to the lack of pre-existing exposure of populations, absence of effective preventive methods and widespread distribution of the transmitting Aedes mosquito vector2–6. Many reports have also shown that the infection can be transmitted from person to person through sexual contact, which could further increase the potential for ZIKV spreading7–13. However, while some studies have explored the frequency, further characterization of viral load and duration of viral shedding in urine, saliva and genital fluid of ZIKV-infected patients, could improve the understanding of potential transmission dynamics14,15.
Infection with ZIKV is commonly asymptomatic or associated with mild febrile symptoms and pruritic skin eruption16. Rare neurologic complications of acute ZIKV infection in adults, such as the Guillain-Barre syndrome, myelitis and encephalitis have also been described17–19. Further characterization of ZIKV clinical case definition is still necessary in the context of co-circulating Dengue (DENV) and Chikungunya (CHIKV) infections, frequently observed in Central and South America.
Ribeirao Preto and Araraquara are medium-sized cities in the countryside of Sao Paulo State, Brazil, with an estimated population of 682,302 and 230,770 inhabitants respectively20. Both municipalities have documented an alarmingly high incidence of DENV in 2016, accumulating almost 60,000 reported DENV cases21. During the same period, 1,376 ZIKV infections and 195 CHIKV infections were reported22,23.
In this study, we identified individuals with ZIKV infection in a cohort of patients who sought medical care with acute arboviral symptoms in Araraquara and Ribeirao Preto in 2016. We described the clinical characteristics and results from the etiologic investigation at the time of enrollment, and prospective measurements of ZIKV serodynamics and ZIKV Real-Time polymerase chain reaction (RT-PCR) in blood, urine, saliva and genital fluid samples for participants with confirmed ZIKV infection.
METHODS
Study population and settings
We enrolled consecutive patients aged 16 and older attending the emergency department at Serviço Especial de Saude in Araraquara, Brazil (Site A) and Sao Francisco Hospital in Ribeirao Preto, Brazil (Site B) between January and September 2016. Patients were considered eligible when presenting with acute (<14 days) symptoms including at least two of the following: fever (>37.8 °C axillary temperature), maculopapular rash, headache, myalgia, arthralgia, fatigue, bleeding or conjunctivitis. We excluded participants with febrile symptoms considered to be related to other infections.
Study procedures
Eligible participants were interviewed for the assessment of demographic characteristics, current symptoms and medical comorbidities. Possible past DENV exposure was ascertained through self-reporting of participants. A peripheral blood sample was collected for ZIKV testing by RT-PCR for all participants. For participants allocated in Site B, baseline investigation also included DENV and CHIKV testing by RT-PCR. For participants allocated in Site A, ZIKV was also tested by RT-PCR in urine samples. Results were communicated to healthcare providers and participants. Participants with confirmed ZIKV underwent three additional visits (<14, 15-28 and >28 days following symptoms onset) during which trained nurses collected a blood sample, while urine, saliva and cervicovaginal fluid samples were self-collected.. Whole saliva samples were self-collected by spontaneous salivation. Cervicovaginal fluid was self-collected using a cotton swab. One nursing mother provided breast milk samples at 20 and 23 days after symptoms initiation. We were also able to collect late cervicovaginal fluid samples using a brush (Evalyn Brush – Rovers Medical Devices BV, Oss, The Netherlands) one year after the baseline for ZIKV RT-PCR. Semen samples were obtained by masturbation from six participants, one year after the baseline, with complete analysis reported in a separated manuscript24.
Laboratory methods
All samples were maintained under refrigeration (4-8 °C) up to 72 h until delivery in a local laboratory. The genital swab was plunged in 500 µL of DNAse free water and slowly homogenized for 30 s. Then, they were immediately stored in aliquots of 500 µLat −80 °C until analysis. Nucleic acid extraction was performed using EasyMag automated system (bioMérieux®, Paris, France) according to the manufacturer's instructions. The one-step multiplex RT-PCR assay for CHIKV/DENV1-4 and ZIKV was performed according to the protocols and primer sets described previously25–28 using the TaqMan® Fast Virus 1-Step Master Mix (Life Technologies®, Austin, USA). Detection of anti-ZIKV IgM and IgG was performed by a commercial ELISA kit (Anti-Zika Virus ELISA, EUROIMMUN, Lübeck, Germany)29, according to the manufacturer's instructions.
Statistical analysis
Baseline characteristics and the presence of each symptom at the time of enrollment and follow-up visits were described by using counts, proportions, medians and interquartile ranges (IQR), as appropriate. We used the Wilcoxon rank-sum test, the Chi-square or the Fisher's exact test for comparisons between ZIKV-positive and -negative groups (categorical variables) and the Student's t-test for continuous quantitative variables. All analyses were performed by the Stata software, version 13.1 (StataCorp. College Station, TX: StataCorp LP).
Ethical aspects
The institutional Ethics and Research Committee of the University of Sao Paulo Medical School, as well as local ethical committees, approved the study protocol (Process N° 1.489.145/2016 and 25706913.6.0000.0066/2014). All participants signed an informed consent form.
RESULTS
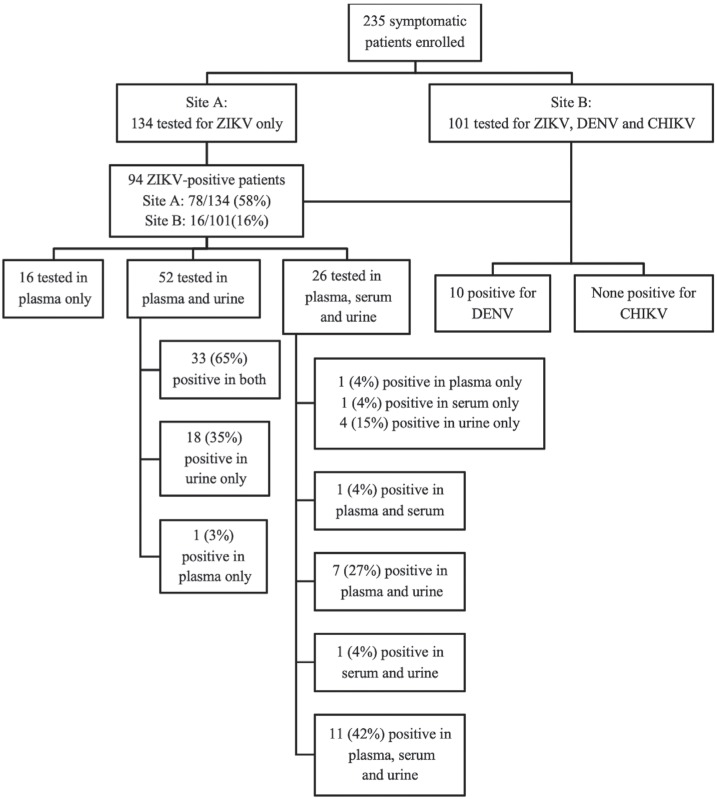
A total of 235 patients with a median age of 41 years (IQR, 31-54) were enrolled. Women comprised 69% of the sample, 24 (11%) of whom were pregnant, 23% of participants had at least one chronic comorbidity and 31% reported prior DENV infection (Table 1). Etiologic investigation of the arboviral syndrome at the time of admission included ZIKV testing only for the 134 participants of Site A, and ZIKV, DENV and CHIKV testing for the 101 participants of Site B. Overall, 94 participants (40%) had confirmed ZIKV infection by RT-PCR in either plasma, serum or urine samples, 58% (95%CI: 49-67%) in Site A and 16% (95%CI: 9-24) in Site B. In addition, in site B, prevalence of RT-PCR-positive DENV was 10%, while CHIKV or coinfections were not detected. ZIKV-positive patients were slightly older (44 versus 38 years, p=0.001) and included a higher (although not statistically significant) proportion of female participants compared with the ZIKV-negative group (76 versus 64%, p=0.058). At the time of enrollment, the median time since symptoms onset was 3 days (IQR, 1-4) for ZIKV-positive participants and 4 days (IQR, 2-5) for ZIKV-negative participants (p<0.001). Clinical presentation was broadly similar for ZIKV-positive and ZIKV-negative patients, although ZIKV-positive patients reported significantly more episodes of fever, headache, cutaneous rash, while gastrointestinal symptoms (abdominal pain, nausea, vomiting, and diarrhea) were less frequent, compared to ZIKV-negative patients.
Table 1. Demographic and clinical characteristics of the study participants according to Zika virus RT-PCR results at the time of enrollment.
| Patient characteristics | All participants N=235 | Zika-positive N=94 (40%) | Zika-negative N=141 (60%) | p-value | |
|---|---|---|---|---|---|
| Age | 41 (31-54) | 44 (36-59) | 38 (29-52) | 0.001 | |
| Female sex (%) | 161/235 (69) | 71/94 (76) | 90/141 (64) | 0.058 | |
| Pregnant (%) | 18/159 (11) | 7/70 (10) | 11/89 (12) | 0.641 | |
| Chronic comorbidities (%) | 53/226 (23) | 24/86 (28) | 29/140 (21) | 0.215 | |
| Prior dengue infection (%) | 70/229 (31) | 29/92 (32) | 41/137 (30) | 0.797 | |
| Symptoms at enrollment | |||||
| Days with symptoms | 4 (2-5) | 3 (1-4) | 4 (2-5) | <0.001 | |
| Fever (%) | 121/232 (52) | 40/92 (43) | 81/140 (58) | 0.032 | |
| Rash (%) | 201/235 (86) | 89/94 (95) | 112/141 (79) | 0.001 | |
| Pruritus (%) | 145/213 (68) | 61/93 (66) | 84/120 (70) | 0.494 | |
| Petechiae (%) | 12/131 (9) | 8/75 (11) | 4/56 (7) | 0.555 | |
| Bleeding symptoms (%) | 11/100 (11) | 4/16 (25) | 7/84 (8) | 0.073 | |
| Conjunctivitis (%) | 25/231 (11) | 9/91 (10) | 16/140 (11) | 0.713 | |
| Arthralgia (%) | 152/234 (65) | 62/94 (66) | 90/140 (64) | 0.793 | |
| Articular edema (%) | 35/134 (26) | 25/78 (32) | 10/56 (18) | 0.065 | |
| Headache (%) | 157/234 (67) | 56/94 (60) | 101/140 (72) | 0.045 | |
| Weakness (%) | 27/134 (20) | 16/78 (21) | 11/56 (20) | 0.901 | |
| Myalgia (%) | 69/129 (53) | 40/75 (53) | 29/54 (54) | 0.967 | |
| Sore throat/cough (%) | 42/129 (33) | 27/75 (36) | 15/54 (28) | 0.326 | |
| Gastrointestinal symptoms* (%) | 87/164 (53) | 33/80 (41) | 54/84 (64) | 0.003 | |
Continuous variables are presented as medians and interquartile ranges. RT-PCR: real-time polymerase chain reaction.
Abdominal pain, nauseas, vomit, diarrhea
Given the uncertainties on the ideal specimen for ZIKV diagnostic testing at the time this study was carried out, samples collected for testing at the time of enrollment included plasma, serum and urine samples in different combinations as described in Figure 1. Among participants who had a positive ZIKV RT-PCR in at least one of the specimens tested, RT-PCR was negative in 31% (24/78, 95% CI: 21-42%) of plasma samples, 46% (12/26, 95% CI: 27-67%) of serum samples, and 5% (4/78, 95% CI: 0-13%) of urine samples.
Figure 1. Investigation of arboviral diseases at enrollment.
ZIKV-positive participants were followed-up during three additional visits with medians of 13 (IQR, 11-14), 17 (IQR, 16-20) and 47 (IQR, 31-52) days after symptoms onset. Patients were invited to provide additional blood, urine, genital fluids, saliva and breast milk (if breastfeeding) samples for ZIKV RT-PCR. ZIKV was detected at follow-up visits 1 and 2 more frequently in urine than in plasma and serum samples (Table 2). On visit 3, ZIKV was still detectable in plasma samples of two patients (11%), in serum of one patient (50%) and in urine samples of three patients (19%).
Table 2. Zika virus RT-PCR detection in body fluids during follow-up of 94 patients with confirmed Zika virus infection at the time of enrollment.
| Visit | Median days since symptoms onset (IQR) | Zika virus in plasma (%) | Zika virus in serum (%) | Zika virus in urine (%) | Zika virus in genital fluids (%) | Zika virus in saliva (%) | Zika virus in breast milk (%) |
|---|---|---|---|---|---|---|---|
| Baseline | 3 (1-4) | 70/94 (74) | 14/26 (54) | 74/78 (95) | – | – | – |
| Visit 1 (≤14d) | 13 (11-14) | 2/20 (10) | 0/9 (0) | 12/18 (67) | – | – | – |
| Visit 2 (15-28d) | 17 (16-20) | 3/53 (6) | 2/4 (50) | 13/42 (31) | 1/5* (20) | 0/6 (0) | 1/1& (100) |
| Visit 3 (>28d) | 47 (31-52) | 2/18 (11) | 1/2 (50) | 3/16 (19) | 0/10 (0) | 0/12 (0) | 0/1 (0) |
IQR: interquartile range; RT-PCR: real-time polymerase chain reaction;
ZIKV positive in vaginal swab 28 days after symptoms onset;
ZIKV positive in breast milk 20 days after symptoms onset
Ten women were tested at least once for ZIKV in genital fluids, with only one woman having a positive RT-PCR result. She was a 26-year-old non-pregnant participant who was enrolled three days after the onset of rash, headache and arthralgia, and had a cervicovaginal sample collected 18 days after the initial symptoms. The vaginal fluid was her only ZIKV positive sample besides a plasma sample. There were no data available on her sexual partner's symptoms or infection status. Besides, genital fluid samples were collected from 16 women with confirmed ZIKV infection with a median of 12 months (IQR, 11-14) after enrollment and none tested positive for ZIKV RT-PCR (data not shown).
As shown in Table 2, saliva was tested in a small number of patients across two follow-up visits with no viral detection. One nursing mother who had ZIKV symptoms onset at week 36 of gestation provided breast milk samples 20, 23 and 30 days after symptoms onset. The sample collected 20 days after symptoms onset tested positive for ZIKV RT-PCR, as well as in Vero cells culture. We tested cord blood, saliva and urine samples from the infant at birth, and re-tested another urine sample one month after birth: all infant samples were negative for ZIKV RT-PCR. Unfortunately, blood samples from this infant were not tested by ZIKV serology.
ZIKV serology results at the time of enrollment and follow-up visits are presented in Table 3. At the time of enrollment, four out of 72 participants with RT-PCR-positive ZIKV infection (6%) had positive ZIKV IgM and 11 (15%) had positive ZIKV IgG results; two (3%) were positive for both IgM and IgG. Among 85 participants with negative baseline ZIKV RT-PCR, five (6%) had positive ZIKV IgM, 21 (25%) had positive ZIKV IgG and one (1%) was positive for both, IgM and IgG. Serology was performed in subsequent visits only for participants with positive baseline ZIKV RT-PCR. On visit 1, only seven participants were tested and six (86%) had positive results for both, IgM and IgG. On visit 2, 65% of participants had positive IgM and 96% had positive IgG, while on visit 3, only 4 (24%) of those tested remained IgM positive and 94% were IgG positive. On follow-up visits, most patients with positive IgM were also positive for IgG.
Table 3. Zika virus serology among 94 patients with confirmed Zika virus infection and among 85 patients with negative Zika virus RT-PCR at the time of enrollment.
| Visit | Median days since symptoms onset (IQR) | Positive IgM (%) | Positive IgG (%) | IgM/IgG dual positive (%) |
|---|---|---|---|---|
| Patients with confirmed Zika virus infection (N=94) | ||||
| Baseline | 3 (1-4) | 4/72 (6) | 11/72 (15) | 2/72 (3) |
| Visit 1 (≤14d) | 13 (11-14) | 6/7 (86) | 6/7 (86) | 6/7 (86) |
| Visit 2 (15-28d) | 17 (16-20) | 32/49 (65) | 47/49 (96) | 30/49 (61) |
| Visit 3 (>28d) | 47 (31-52) | 4/17 (24) | 16/17 (94) | 4/17 (24) |
| Patients with negative Zika virus RT-PCR at enrollment (N=85) | ||||
| Baseline | 4 (2-5) | 5/85 (6) | 21/85 (25) | 1/85 (1) |
IQR: interquartile range; RT-PCR: real-time polymerase chain reaction
Pregnancy outcomes for ZIKV-positive pregnant participants were known for six out of seven cases. One participant with first-trimester pregnancy had a miscarriage at gestational week seven, 22 days after the enrollment and 26 days after symptoms onset. She was hospitalized for curettage and discharged without additional medical complications. No investigation was performed on the fetus or on miscarriage products. Four participants with third-trimester pregnancies and one participant with a second-trimester pregnancy at the time of acute ZIKV infection gave birth to children without apparent signs of Congenital Zika Syndrome according to the initial evaluation. Eighteen to 21 months after birth, all five children were still under neuropediatric follow-up and had no signs or symptoms of neurological or developmental abnormalities.
DISCUSSION
In this hospital-based study, we enrolled 235 patients from two cities in the Sao Paulo State, Brazil, for whom investigation of acute ZIKV infection was performed by blood or urine RT-PCR. We found an overall prevalence of 40% of ZIKV-positive patients, who presented with cutaneous rash, fever, headache and less frequently with gastrointestinal symptoms when compared to ZIKV-negative patients. Few patients had detectable ZIKV RNA in plasma samples (n=2 or 11%), serum (n=1 or 50%) and urine (n=3 or 19%) up to the last follow-up visit >28 days after enrollment. We reported the presence of ZIKV in genital fluids of one female patient. We have also observed the presence of ZIKV in breast milk, and, although we were able to isolate ZIKV in culture, we cannot confirm the potential viral transmission of breast milk.
These findings corroborate those of more detailed viral kinetics studies performed in Puerto Rico after the follow-up of 150 RT-PCR-confirmed ZIKV infection patients from whom serum, urine, saliva, semen and vaginal secretion specimens were analyzed until all specimens became RT-PCR negative. They were able to determine the median time of viral shedding, which was highest for semen samples (34 days), followed by serum (14 days) and urine (8 days) samples. Interestingly, only one among 50 women tested positive in vaginal fluid15. Persistence of ZIKV in genital fluids has also been described in a smaller study conducted in Spain, which identified RT-PCR-positive samples in one of four women and one of five tested men, with viral shedding detected between days 37 and 69 for vaginal fluids and between days 23 and 107 for semen30.
Although Araraquara (Site A) and Ribeirao Preto (Site B) are only 70 km apart and enrollment in both cities took place in the same year, ZIKV prevalence was strikingly different, of 58% and 16%, respectively. Our findings and conclusions are also quite similar to those of a study conducted in another part of the Sao Paulo State31 and this disparity between ZIKV prevalences highlights the potential impact of different investigation protocols on results. Furthermore, it is essential to understand the local ZIKV epidemiology based not only on clinical definitions, which may be less useful in settings with lower epidemic intensity, but also on laboratory-based surveillance of standardized samples and time points. In 2016, 236 and 1,140 ZIKV cases were reported in Site A and Site B, respectively, corresponding to an incidence rate of 102.3 and 167.1/100,000 inhabitants, respectively22. However, it is likely that data presented in official reports are an underestimate of the actual incidence given the low availability of diagnostic tools and the frequency of asymptomatic infections.
ZIKV can be identified by RT-PCR from different body fluids and the best specimens for diagnosis are still to be defined. Our results suggest that RT-PCR testing in urine is more sensitive than in plasma, while serum was the specimen less likely to be positive. ZIKV RT-PCR testing in whole blood, has shown to be the most frequently positive fluid in a study conducted in Guadeloupe32, but was unfortunately not tested in our study. Among ten women tested for ZIKV detection in genital fluids on visits 2 or 3, only one was positive 18 days after symptoms onset, which is slightly longer frametime than the ones previously reported33–35. Among 16 women tested 12 months after the initial infection, none had detectable ZIKV suggesting that ZIKV detection in female genital fluids is probably rare and short-lived, but this has not been systematically characterized hitherto33,34. In addition, probable ZIKV female-to-male sexual transmission has also been reported36. The quantification of ZIKV transmission risk through sexual intercourse is still to be determined, and this would represent important information to characterize the potential trajectory of the ZIKV epidemic37.
Serological dynamics of ZIKV infection were analyzed in the 94 patients with confirmed ZIKV infection. We observed that 6% of patients were IgM positive and 15% were IgG positive at the time of enrollment, a median of 3 days after symptoms onset; as expected, IgM positivity increased between 14 and 28 days and decreased sharply after that, while IgG positivity increased around 14 days and remained positive in the majority of tested patients. Interestingly, among 85 patients with negative ZIKV RT-PCR at the baseline who underwent serology, 27 (32%) were positive for IgM, IgG or both. We were not able to discriminate whether these were true ZIKV infections with negative RT-PCR or false positive serological results. Although our study was not explicitly designed to address the performance of current serological tests, our results corroborate the findings from other studies that suggested a lower sensitivity of the Euroimmune IgM compared to IgG38–40 antibodies detection
Our study had some limitations. Because this study was conducted in the context of real-life routine clinical settings, we had missing observations and heterogeneous testing of specimens in different time points. Data on prior DENV infection was self-reported by participants, a procedure that is more prone to patients’ memory bias. It is possible that patients with no previously reported DENV had been exposed to the virus with asymptomatic or mild disease presentation. Unfortunately, it was not possible to perform DENV serology. This information would have been useful in the interpretation of ZIKV serological dynamics, as has been highlighted by our group and others41. Information on yellow fever vaccination was not collected from study participants and could also have potentially influenced ZIKV serology interpretation. Moreover, potential subjectivity in the identification of eligible participants by excluding those with other suspected infections based on clinical evaluation could have impacted our findings. Despite these limitations, our study provides useful information on some of the viral and serological kinetics of ZIKV in the context of an epidemic.
In conclusion, our study found critical differences in ZIKV prevalence among symptomatic patients presenting with similar arboviral syndromes in two neighboring cities in the Sao Paulo State, Brazil. In addition to a heterogeneous occurrence, nonspecific symptoms and overlapping clinical definitions for ZIKV, DENV, and CHIKV infections, highlights the need for accurate and accessible diagnostic tests, that should be timely performed using the specimens more likely to be positive. Our findings confirm the results from previous studies showing that urine ZIKV RT-PCR testing in the acute phase of infection can improve the sensitivity of diagnosis, while testing serum is probably less useful despite more sustained shedding in some cases. Higher sensitivity and specificity serological tests are needed for the diagnosis of patients who seek medical attention after the period of ZIKV detection by RT-PCR and for surveillance purposes in settings of high DENV background infection.
ACKNOWLEDGMENTS
In the memory of Aparecido Braz, who participated in data collection but sadly passed away before the study was completed. We acknowledge Mayra Sanches, Leonardo Moura and Noely Ferreira for helping in data collection and laboratory analyses. The authors wish also to acknowledge the contribution of Professor Claudio Pannuti (IMT/USP, retired) in the design, conduct of the studyand interpretation of study findings.
Funding Statement
This work was supported by the European Union's Horizon 2020 Research and Innovation Programme under ZIKAlliance Grant Agreement N° 734548, Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES – Ministry of Education, Brazil, grants N° CSF-PVE-S-88887.122640/2016-00 [VAS support] and CSF-PVE-S-88881.068160/2014-01 [ASL, ACS and PM support]) and bioMérieux Corporation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
FUNDING
This work was supported by the European Union's Horizon 2020 Research and Innovation Programme under ZIKAlliance Grant Agreement N° 734548, Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES – Ministry of Education, Brazil, grants N° CSF-PVE-S-88887.122640/2016-00 [VAS support] and CSF-PVE-S-88881.068160/2014-01 [ASL, ACS and PM support]) and bioMérieux Corporation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.World Health Organization WHO Director-general summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. [[cited 2019 Feb 4]]. Available from: https://www.who.int/news-room/detail/01-02-2016-who-director-general-summarizes-the-outcome-of-the-emergency-committee-regarding-clusters-of-microcephaly-and-guillain-barr%C3%A9-syndrome.; 1. World Health Organization. WHO Director-general summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. [cited 2019 Feb 4]. Available from: https://www.who.int/news-room/detail/01-02-2016-who-director-general-summarizes-the-outcome-of-the-emergency-committee-regarding-clusters-of-microcephaly-and-guillain-barr%C3%A9-syndrome
- 2.Bogoch II, Brady OJ, Kraemer MU, German M, Creatore MI, Kulkarni MA, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–336. doi: 10.1016/S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; 2. Bogoch II, Brady OJ, Kraemer MU, German M, Creatore MI, Kulkarni MA, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335-6. [DOI] [PMC free article] [PubMed]
- 3.Teixeira MG, Costa MC, de Oliveira WK, Nunes ML, Rodrigues LC. The epidemic of Zika virus-related microcephaly in Brazil: detection, control, etiology, and future scenarios. Am J Public Health. 2016;106:601–605. doi: 10.2105/AJPH.2016.303113. [DOI] [PMC free article] [PubMed] [Google Scholar]; 3. Teixeira MG, Costa MC, de Oliveira WK, Nunes ML, Rodrigues LC. The epidemic of Zika virus-related microcephaly in Brazil: detection, control, etiology, and future scenarios. Am J Public Health. 2016;106:601-5. [DOI] [PMC free article] [PubMed]
- 4.Li J, Li D. Epidemiological characteristics of Zika virus disease. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:329–334. doi: 10.3760/cma.j.issn.0254-6450.2016.03.007. [DOI] [PubMed] [Google Scholar]; 4. Li J, Li D. Epidemiological characteristics of Zika virus disease. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:329-34. [DOI] [PubMed]
- 5.Calvet GA, Filippis AM, Mendonça MC, Sequeira PC, Siqueira AM, Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol. 2016;74:1–3. doi: 10.1016/j.jcv.2015.11.014. [DOI] [PubMed] [Google Scholar]; 5. Calvet GA, Filippis AM, Mendonça MC, Sequeira PC, Siqueira AM, Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol. 2016;74:1-3. [DOI] [PubMed]
- 6.Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, et al. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis. 2016;10:e0004636. doi: 10.1371/journal.pntd.0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]; 6. Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, et al. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis. 2016;10:e0004636. [DOI] [PMC free article] [PubMed]
- 7.Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21:30148–30148. doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]; 7. Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21:30148. [DOI] [PubMed]
- 8.D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]; 8. D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195-8. [DOI] [PubMed]
- 9.Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387:2501–2501. doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]; 9. Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387:2501. [DOI] [PubMed]
- 10.Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-male sexual transmission of Zika virus - Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372–374. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]; 10. Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-male sexual transmission of Zika virus - Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372-4. [DOI] [PubMed]
- 11.Fréour T, Mirallié S, Hubert B, Splingart C, Barrière P, Maquart M, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill. 2016;21:30254–30254. doi: 10.2807/1560-7917.ES.2016.21.23.30254. [DOI] [PubMed] [Google Scholar]; 11. Fréour T, Mirallié S, Hubert B, Splingart C, Barrière P, Maquart M, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill.2016;21:30254. [DOI] [PubMed]
- 12.Frank C, Cadar D, Schlaphof A, Neddersen N, Gunther S, Schmidt-Chanasit J, et al. Sexual transmission of Zika virus in Germany, April 2016. Euro Surveill. 2016;21:30252–30252. doi: 10.2807/1560-7917.ES.2016.21.23.30252. [DOI] [PubMed] [Google Scholar]; 12. Frank C, Cadar D, Schlaphof A, Neddersen N, Gunther S, Schmidt-Chanasit J, et al. Sexual transmission of Zika virus in Germany, April 2016. Euro Surveill. 2016;21:30252. [DOI] [PubMed]
- 13.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011 May;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]; 13. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. May 2011;17:880-2. [DOI] [PMC free article] [PubMed]
- 14.Bonaldo MC, Ribeiro IP, Lima NS, Dos Santos AA, Menezes LS, da Cruz SO, et al. Isolation of infective Zika virus from urine and saliva of patients in Brazil. PLoS Negl Trop Dis. 2016;10:e0004816. doi: 10.1371/journal.pntd.0004816. [DOI] [PMC free article] [PubMed] [Google Scholar]; 14. Bonaldo MC, Ribeiro IP, Lima NS, Dos Santos AA, Menezes LS, da Cruz SO, et al. Isolation of infective Zika virus from urine and saliva of patients in Brazil. PLoS Negl Trop Dis. 2016;10:e0004816. [DOI] [PMC free article] [PubMed]
- 15.Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids - Preliminary report. N Engl J Med. 2018;379:1234–1243. doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]; 15. Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids - Preliminary report. N Engl J Med. 2018;379:1234-43. [DOI] [PMC free article] [PubMed]
- 16.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]; 16. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536-43. [DOI] [PubMed]
- 17.Méecharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387:1481–1481. doi: 10.1016/S0140-6736(16)00644-9. [DOI] [PubMed] [Google Scholar]; 17. Méecharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387:1481. [DOI] [PubMed]
- 18.Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, et al. Zika Virus associated with meningoencephalitis. N Engl J Med. 2016;374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]; 18. Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, et al. Zika Virus associated with meningoencephalitis. N Engl J Med. 2016;374:1595-6. [DOI] [PubMed]
- 19.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; 19. Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531-9. [DOI] [PMC free article] [PubMed]
- 20.Instituto Brasileiro de Geografia e Estatística O Brasil em síntese. [[cited 2019 Feb 4]]. Available from: https://cidades.ibge.gov.br/; 20. Instituto Brasileiro de Geografia e Estatística. O Brasil em síntese. [cited 2019 Feb 4]. Available from: https://cidades.ibge.gov.br/
- 21.São Paulo. Secretaria de Estado da Saúde Centro de Vigilância Epidemiológica “Prof Alexandre Vranjac”. Distribuição dos casos de dengue notificados e confirmados (autóctones e importados) no estado de SP, segundo o município de residência, por mês de início de sintomas, ano 2016. [[cited 2019 Feb 4]]. Available from: http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/dengue/2016/dengue16_import_autoc_res.htm.; 21. São Paulo. Secretaria de Estado da Saúde. Centro de Vigilância Epidemiológica “Prof. Alexandre Vranjac”. Distribuição dos casos de dengue notificados e confirmados (autóctones e importados) no estado de SP, segundo o município de residência, por mês de início de sintomas, ano 2016. [cited 2019 Feb 4]. Available from: http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/dengue/2016/dengue16_import_autoc_res.htm
- 22.São Paulo. Secretaria de Estado da Saúde Centro de Vigilância Epidemiológica “Prof. Alexandre Vranjac”. Distribuição dos casos de Zika Vírus notificados e confirmados (autóctones e importados), segundo o município de residência por mês de início de sintomas. Estado de São Paulo, 2016. [[cited 2019 Feb 4]]. Available from: http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/zika/zika16_autoc_import.htm.; 22. São Paulo. Secretaria de Estado da Saúde. Centro de Vigilância Epidemiológica “Prof. Alexandre Vranjac”. Distribuição dos casos de Zika Vírus notificados e confirmados (autóctones e importados), segundo o município de residência por mês de início de sintomas. Estado de São Paulo, 2016. [cited 2019 Feb 4]. Available from: http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/zika/zika16_autoc_import.htm
- 23.São Paulo. Secretaria de Estado da Saúde Centro de Vigilância Epidemiológica “Prof Alexandre Vranjac”. Distribuição dos casos de Chikungunya notificados e confirmados (autóctones e importados) no estado de São Paulo, segundo o município de residência, por mês de início de sintomas, ano 2016. [[cited 2019 Feb 4]]. Available from: http://portal.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/chikung/chikung16_import_autoc_res.htm.; 23. São Paulo. Secretaria de Estado da Saúde. Centro de Vigilância Epidemiológica “Prof. Alexandre Vranjac”. Distribuição dos casos de Chikungunya notificados e confirmados (autóctones e importados) no estado de São Paulo, segundo o município de residência, por mês de início de sintomas, ano 2016. [cited 2019 Feb 4]. Available from: http://portal.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/chikung/chikung16_import_autoc_res.htm
- 24.Avelino-Silva VI, Alvarenga C, Abreu C, Tozetto-Mendoza TR, Canto CL, Manuli ER, et al. Potential effect of Zika virus infection on human male fertility? Rev Inst Med Trop Sao Paulo. 2018;60:e64. doi: 10.1590/S1678-9946201860064. [DOI] [PMC free article] [PubMed] [Google Scholar]; 24. Avelino-Silva VI, Alvarenga C, Abreu C, Tozetto-Mendoza TR, Canto CL, Manuli ER, et al. Potential effect of Zika virus infection on human male fertility? Rev Inst Med Trop Sao Paulo. 2018;60:e64. [DOI] [PMC free article] [PubMed]
- 25.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]; 25. Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232-9. [DOI] [PMC free article] [PubMed]
- 26.Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J. 2013;10:311–311. doi: 10.1186/1743-422X-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]; 26. Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J. 2013;10:311. [DOI] [PMC free article] [PubMed]
- 27.Cecilia D, Kakade M, Alagarasu K, Patil J, Salunke A, Parashar D, et al. Development of a multiplex real-time RT-PCR assay for simultaneous detection of dengue and chikungunya viruses. Arch Virol. 2015;160:323–327. doi: 10.1007/s00705-014-2217-x. [DOI] [PubMed] [Google Scholar]; 27. Cecilia D, Kakade M, Alagarasu K, Patil J, Salunke A, Parashar D, et al. Development of a multiplex real-time RT-PCR assay for simultaneous detection of dengue and chikungunya viruses. Arch Virol. 2015;160:323-7. [DOI] [PubMed]
- 28.Huhtamo E, Hasu E, Uzcátegui NY, Erra E, Nikkari S, Kantele A, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol. 2010;47:49–53. doi: 10.1016/j.jcv.2009.11.001. [DOI] [PubMed] [Google Scholar]; 28. Huhtamo E, Hasu E, Uzcátegui NY, Erra E, Nikkari S, Kantele A, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol. 2010;47:49-53. [DOI] [PubMed]
- 29.Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill. 2016;21:30203–30203. doi: 10.2807/1560-7917.ES.2016.21.16.30203. [DOI] [PubMed] [Google Scholar]; 29. Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill. 2016;21:30203. [DOI] [PubMed]
- 30.Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92–100. doi: 10.1111/tmi.13019. [DOI] [PubMed] [Google Scholar]; 30. Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92-100. [DOI] [PubMed]
- 31.Colombo TE, Estofolete CF, Reis AF, da Silva NS, Aguiar ML, Cabrera EM, et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J Clin Virol. 2017;96:20–25. doi: 10.1016/j.jcv.2017.09.002. [DOI] [PubMed] [Google Scholar]; 31. Colombo TE, Estofolete CF, Reis AF, da Silva NS, Aguiar ML, Cabrera EM, et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J Clin Virol. 2017;96:20-5. [DOI] [PubMed]
- 32.Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200–1208. doi: 10.1016/S1473-3099(17)30444-9. [DOI] [PubMed] [Google Scholar]; 32. Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200-8. [DOI] [PubMed]
- 33.Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000–1001. doi: 10.1016/S1473-3099(16)30193-1. [DOI] [PubMed] [Google Scholar]; 33. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1. [DOI] [PubMed]
- 34.Prisant N, Breurec S, Moriniere C, Bujan L, Joguet G. Zika Virus genital tract shedding in infected women of childbearing age. Clin Infect Dis. 2017;64:107–109. doi: 10.1093/cid/ciw669. [DOI] [PubMed] [Google Scholar]; 34. Prisant N, Breurec S, Moriniere C, Bujan L, Joguet G. Zika Virus genital tract shedding in infected women of childbearing age. Clin Infect Dis. 2017;64:107-9. [DOI] [PubMed]
- 35.Visseaux B, Mortier E, Houhou-Fidouh N, Brichler S, Collin G, Larrouy L, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1220–1220. doi: 10.1016/S1473-3099(16)30387-5. [DOI] [PubMed] [Google Scholar]; 35. Visseaux B, Mortier E, Houhou-Fidouh N, Brichler S, Collin G, Larrouy L, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1220. [DOI] [PubMed]
- 36.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716–717. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]; 36. Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716-7. [DOI] [PubMed]
- 37.Kim CR, Counotte M, Bernstein K, Deal C, Mayaud P, Low N, et al. Investigating the sexual transmission of Zika virus. Lancet Glob Health. 2018;6:e24–e25. doi: 10.1016/S2214-109X(17)30419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; 37. Kim CR, Counotte M, Bernstein K, Deal C, Mayaud P, Low N, et al. Investigating the sexual transmission of Zika virus. Lancet Glob Health. 2018;6:e24-5. [DOI] [PMC free article] [PubMed]
- 38.Safronetz D, Sloan A, Stein DR, Mendoza E, Barairo N, Ranadheera C, et al. Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis. 2017;23:1577–1580. doi: 10.3201/eid2309.162043. [DOI] [PMC free article] [PubMed] [Google Scholar]; 38. Safronetz D, Sloan A, Stein DR, Mendoza E, Barairo N, Ranadheera C, et al. Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis. 2017;23:1577-80. [DOI] [PMC free article] [PubMed]
- 39.L’Huillier AG, Hamid-Allie A, Kristjanson E, Papageorgiou L, Hung S, Wong CF, et al. Evaluation of Euroimmun anti-Zika virus IgM and IgG enzyme-linked immunosorbent assays for Zika virus serologic testing. J Clin Microbiol. 2017;55:2462–2471. doi: 10.1128/JCM.00442-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; 39. L’Huillier AG, Hamid-Allie A, Kristjanson E, Papageorgiou L, Hung S, Wong CF, et al. Evaluation of Euroimmun anti-Zika virus IgM and IgG enzyme-linked immunosorbent assays for Zika virus serologic testing. J Clin Microbiol. 2017;55:2462-71. [DOI] [PMC free article] [PubMed]
- 40.Pasquier C, Joguet G, Mengelle C, Chapuy-Regaud S, Pavili L, Prisant N, et al. Kinetics of anti-ZIKV antibodies after Zika infection using two commercial enzyme-linked immunoassays. Diagn Microbiol Infect Dis. 2018;90:26–30. doi: 10.1016/j.diagmicrobio.2017.09.001. [DOI] [PubMed] [Google Scholar]; 40. Pasquier C, Joguet G, Mengelle C, Chapuy-Regaud S, Pavili L, Prisant N, et al. Kinetics of anti-ZIKV antibodies after Zika infection using two commercial enzyme-linked immunoassays. Diagn Microbiol Infect Dis. 2018;90:26-30. [DOI] [PubMed]
- 41.Felix AC, Souza NC, Figueiredo WM, Costa AA, Inenami M, da Silva RM, et al. Cross reactivity of commercial anti-dengue immunoassays in patients with acute Zika virus infection. J Med Virol. 2017;89:1477–1479. doi: 10.1002/jmv.24789. [DOI] [PubMed] [Google Scholar]; 41. Felix AC, Souza NC, Figueiredo WM, Costa AA, Inenami M, da Silva RM, et al. Cross reactivity of commercial anti-dengue immunoassays in patients with acute Zika virus infection. J Med Virol. 2017;89:1477-9. [DOI] [PubMed]