Abstract
A 17-year-old man presented to the emergency department with complaints of retro-orbital pain of the left eye and an altitudinal visual field defect for 2 weeks. Fundus examination revealed ipsilateral hyperaemic optic disc oedema, and the patient was admitted with the presumptive diagnosis of left optic neuritis. Subsequently, during follow-up, the patient developed a retinal granulomatous lesion in the superior temporal arcade with vitritis and fibrotic strands extending to the mid-periphery. Serum antibodies detection by ELISA and aqueous humour immunoblot were positive for Toxocara canis. Medical therapy with albendazole and oral steroids was instituted with satisfactory results. One year later, a new macular lesion developed with consequent vision loss.
Keywords: retina, macula
Background
Ocular toxocariasis (OT) is a parasitosis of the eye caused by incidental ingestion of Toxocara cati/canis larvae.1 It is usually unilateral, affects young patients and can have multiple presentations such as chronic uveitis, retinochoroidal granuloma, vitreoretinal traction, vitritis, endophthalmitis and/or optic disc oedema, which often lead to loss of vision in the affected eye.2 3 There is no commonly accepted treatment regimen.3
Case presentation
A 17-year-old man presented to the emergency department with left retro-orbital pain and subsequent altitudinal visual field defect for the previous 2 weeks. His medical history was otherwise unremarkable, and he only mentioned regular contact with his dog. Best-corrected visual acuities (BCVA) were 20/16 in the right eye (OD) and 20/25 in the left (OS). A left afferent pupillary defect was present and versions were smooth, painless and full in both eyes. Slit-lamp examination findings were unremarkable bilaterally. Dilated fundus examination was unremarkable OD, but left hyperaemic disc oedema with haemorrhages was documented. The patient was admitted for lumbar puncture and MRI scan, and infectious causes of optic neuritis were investigated. The patient was treated with intravenous methylprednisolone and subsequent oral methylprednisolone, with visual improvement. Few weeks after discharge, the patient complained of decreased VA OS, and dilated fundus examination revealed dense vitritis and a whitish granulomatous retinal lesion in the superior temporal retinal artery, with fibrotic vitreous strands extending towards the mid-periphery. In addition, within a few weeks, another smaller similar retinal lesion was detected in the inferior mid-periphery, with suprajacent vitritis (figure 1).
Figure 1.
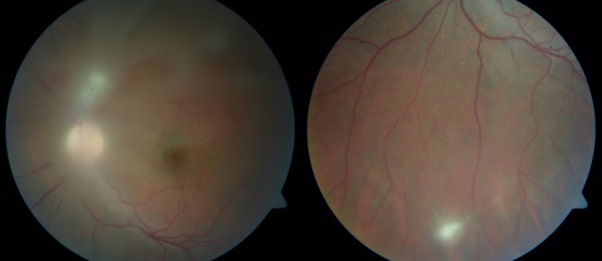
Dense vitritis, granulomatous lesion in the superior temporal retinal artery with vitreous strands extending to mid-periphery and smaller inferior retinal lesion.
Investigations
During admission, the patient underwent an extensive clinical and laboratory investigation, including complete blood count, hepatic and renal function, erythrocyte sedimentation rate, C-reactive protein, serum angiotensin-converting enzyme, antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA), and serologies of Toxoplasmosis, Cytomegalovirus, Epstein-Barr, Herpes simplex virus 1 and 2, Varicella zoster virus, Brucella, Borrelia burgdorferi, Treponema pallidum, QuantiFERON-TB Gold assay, HIV 1/2, hepatitis B and C virus. All these tests were normal. There were no oligoclonal bands on cerebrospinal fluid analysis, and MRI scan did not show any demyelinating lesions. After discharge, new relevant signs from the ophthalmological examination developed and a further ancillary investigation was conducted, including T. canis serum ELISA testing, which was positive for IgG. To confirm the diagnosis of OT, anterior chamber tap was performed and aqueous humour analysis by immunoblot was positive for T. canis antibodies (five specific bands of T. canis infection were detected).
Treatment
Treatment with an oral antihelmintic and oral steroids to control vitreous inflammation was instituted: albendazole 200 mg two times per day for 15 days and methylprednisolone 32 mg on a slow tapering regimen. Although there is no definitive evidence of the efficacy of antihelmintic drugs in parasite eradication, systemic albendazole in combination with corticosteroids appear to be a safe option for the treatment of these patients.4
Outcome and follow-up
After starting the patient on the combined treatment, left VA was stable at 20/25, and sequential fundus examinations showed a stable granulomatous lesion next to the superior temporal retinal artery with adjacent vitreous strands, a cicatricial chorioretinal lesion in the lower periphery and trace vitritis (figure 2). Following this, the patient started to avoid the dog and refrained from contact with his pet. One year later, the patient returned with sudden vision loss OS (20/80), and a new macular yellowish lesion was apparent, together with moderate vitreous inflammation (figure 3). A course of mebendazole 100 mg two times per day for another 2 weeks associated with methylprednisone 64 mg on a tapering regimen was instituted. His vision improved (20/63), the vitreous inflammation subsided, but foveal pigmentary changes persisted, with loss of subfoveal photoreceptors and external layers on optical coherence tomography (figure 4). The patient’s dog was referred to a veterinary physician for appropriate antihelminthic care.
Figure 2.
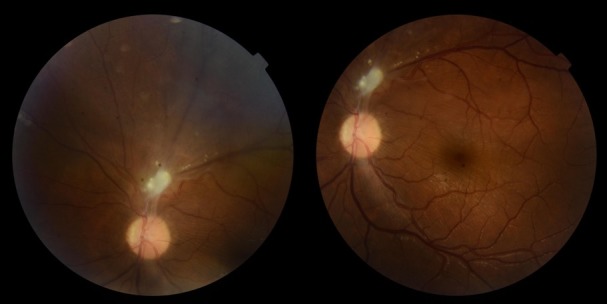
Stable granulomatous lesion in the superior temporal retinal artery with adjacent vitreous strands.
Figure 3.
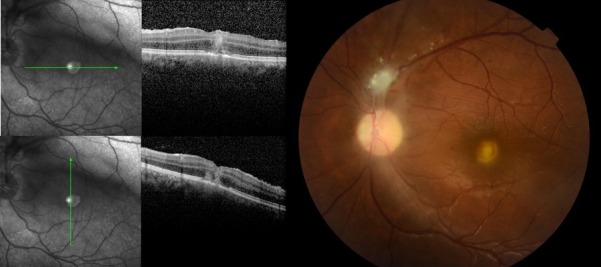
New macular yellowish lesion with moderate vitreous inflammation (right). Optical coherence tomography images showing foveal hiper-reflective lesion affecting all retinal layers (left).
Figure 4.
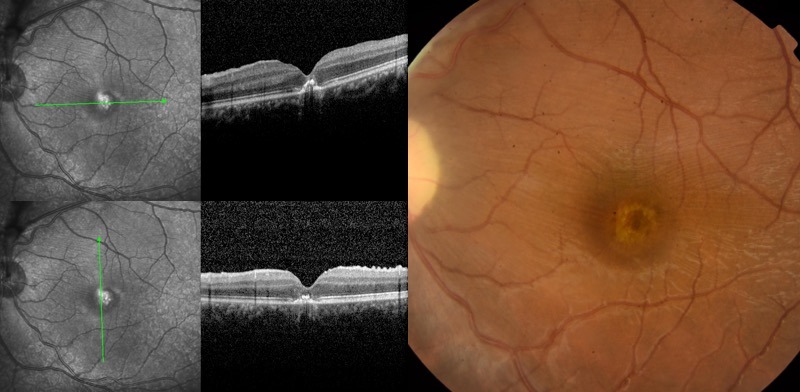
Persistent foveal pigmentary changes (right). Loss of subfoveal photoreceptors and external layers on optical coherence tomography (left).
Discussion
OT is caused by migration of the larvae through the ciliary vessels to the choroid or through the central vessels of the retina to the retina and vitreous.2 3 The clinical appearance of OT varies depending on the stage and degree of ocular involvement at the time of diagnosis. No clinical pattern is pathognomonic of OT. Classically, it occurs in one of four forms: posterior pole granuloma (most commonly), peripheral granuloma, chronic endophthalmitis or atypical presentations.2 3 5
The diagnosis of OT is presumptive because a definitive one requires actual demonstration of the larvae in the human eye.2 6 Thus, alternative investigations are used, including serum ELISA for the detection of Toxocara antibodies and western blot for the immunological diagnosis of OT.3 6 In nationwide surveys of T. canis infection in humans, the prevalence of serum antibodies is highly variable, ranging from 1% in Japan to 87% in school-aged children in the Marshall Islands.1 Although human toxocariasis seroprevalence is variable, it is higher in rural environments, particularly in communities with close contact between humans and wild or domestic canids and felids due to contamination of soils and ingestion of contaminated products.1 6 No recent data are available on human seroprevalence of T. canis in Portugal; however, an epidemiological study assessed the prevalence of parasitic infection in faecal samples from three urban dog parks in Lisbon, and showed that 0.5% of them were contaminated with Toxocara spp.7
In this case, the presentation was atypical, simulating optic neuritis, with subsequent development of a posterior pole granuloma. Serologic diagnosis from serum ELISA and aqueous humour immunoblot confirmed the diagnosis of OT.
Treatment should be guided by visual acuity, severity of ocular inflammation and presentation type. There is no widely accepted treatment regimen for OT. Treating OT with antihelmintic medication is controversial because of the possibility of severe, sight-threatening immunological reactions against the dying larvae.3 4 6 However, previous studies found that the concurrent use of antihelmintics and oral steroids was apparently effective and safe.4 8 Albendazole (10 mg/kg daily per 1–2 weeks) is a broad-spectrum antihelmintic drug and effective against the larvae. It becomes widely distributed throughout tissues when metabolized and crosses the blood–brain barrier.1 6 Other antihelmintic agents that could be used are thialbendazole (25– 50 mg/kg/day for 5–7 days) and mebendazole (20–25 mg/kg/day for 2–3 weeks).6
Regarding the outcome of this case, the development of a new lesion after proper treatment could be due to either reinfestation or intraocular larval migration. Hence, the patient avoided contact with the dog since the diagnosis of OT, another explanation for late macular lesion is larval migration. There are some previous reports of larval migration in the retina, which can present as contiguous or distant lesions.5 9 10 A retrospective cohort study from Seoul reviewed the clinical presentation and course of 101 adult patients diagnosed OT. This study reported that 12.9% and 4.3% of patients presented respectively, continuous and discontinuous migration of the lesions.5 In one case, migration resulted in a macular granuloma, with macular destruction and subsequent vision loss.5
In our case report, despite adequate treatment and stable results for 1 year, a new macular lesion later developed, with damage to foveal photoreceptors and consequent central scotoma. A new course of antihelmintic and steroid treatment was instituted and stable vision has been achieved so far, with controlled vitreous inflammation. To the best of our knowledge, there are no literature cases describing late larval migration and therapeutical approach to new lesions.
In conclusion, although OT visual prognosis is not always satisfactory, the patient maintained good BCVA with satisfactory outcomes for 1 year, followed by the development of a new macular lesion caused by presumed retinal larva discontinuous migration.
Learning points.
Ocular toxocariasis not always presents with typical clinical setting.
Close contact with pets may be a key element to make the diagnosis.
Pets should also be referred to proper veterinary care.
Larva migration can occur months after treatment with the development of new lesions.
Antihelmintic agents may be helpful when used concurrently with steroids.
Footnotes
Contributors: CF and AMS contributed to the acquisition of data, its analysis and interpretation, drafting of the manuscript and final approval. SF and RP contributed to the critical revision of the manuscript and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Ma G, Holland CV, Wang T, et al. . Human toxocariasis. Lancet Infect Dis 2018;18:e14–24. 10.1016/S1473-3099(17)30331-6 [DOI] [PubMed] [Google Scholar]
- 2. Sorribas MB, Garcia SC. Toxocariasis ocular. Rev Esp Inv Oftal 2012;2:279. [Google Scholar]
- 3. Ahn SJ, Ryoo NK, Woo SJ. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac Allergy 2014;4:134–41. 10.5415/apallergy.2014.4.3.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Despreaux R, Fardeau C, Touhami S, et al. . Ocular toxocariasis: clinical features and long-term visual outcomes in adult patients. Am J Ophthalmol 2016;166:162–8. 10.1016/j.ajo.2016.03.050 [DOI] [PubMed] [Google Scholar]
- 5. Ahn SJ, Woo SJ, Jin Y, et al. . Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis 2014;8:e2938 10.1371/journal.pntd.0002938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martínez-Pulgarín DF, Muñoz-Urbano M, Gomez-Suta LD, et al. . Ocular toxocariasis: new diagnostic and therapeutic perspectives. Recent Pat Antiinfect Drug Discov 2015;10:35–41. 10.2174/1574891X10666150410125057 [DOI] [PubMed] [Google Scholar]
- 7. Ferreira A, Alho AM, Otero D, et al. . Urban dog parks as sources of canine parasites: contamination rates and pet owner behaviours in Lisbon, Portugal. J Environ Public Health 2017;2017:1–7. 10.1155/2017/5984086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barisani-Asenbauer T, Maca SM, Hauff W, et al. . Treatment of ocular toxocariasis with albendazole. J Ocul Pharmacol Ther 2001;17:287–94. 10.1089/108076801750295317 [DOI] [PubMed] [Google Scholar]
- 9. Sivaratnam D, Subrayan V, Ali NA. Transvitreal migration of a Toxocara larva resulting in a second chorioretinal granuloma. Jpn J Ophthalmol 2008;52:416–7. 10.1007/s10384-008-0569-z [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Joko T, Akao N, et al. . Following the migration of a Toxocara larva in the retina by optical coherence tomography and fluorescein angiography. Jpn J Ophthalmol 2005;49:159–61. 10.1007/s10384-004-0157-9 [DOI] [PubMed] [Google Scholar]


